Professional Documents
Culture Documents
Packed Column Gas Absorption Process Lab Manual Jan 2013
Uploaded by
نورسوهايلا عارفينCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Packed Column Gas Absorption Process Lab Manual Jan 2013
Uploaded by
نورسوهايلا عارفينCopyright:
Available Formats
UNIVERSITI KUALA LUMPUR MALAYSIAN INSTITUTE OF CHEMICAL BIOENGINEERING TECHNOLOGY
LABORATORY REPORT SUBMISSION FORM
To: (Lecturer) Subject: CKD 20103 Separation Technology From: (Student Name) 1. 2. 3. 4. 5. Section: Title of Experiment: PACKED COLUMN GAS ABSORPTION PROCESS Date of Experiment: Date of Submission: Student ID No. 1. 2. 3. 4. 5. Group No:
Note: If late with good reason, medical note must be submitted to the lecturer; otherwise the mark will be reduced by 20% per each day late.
CONTENT Introduction Objective Procedure Result/Data Discussion Conclusion Reference Appendix
NOTE Theoretical of process, description of equipment List of objectives of experiment Simplified procedure (start-up, analysis, experimental) Tables of results, graph and calculation Graphical explanation or discussion Principal Outcomes and Recommendation At least three (3) references; book, manual, journal, internet Raw data during experiment TOTAL
SUBMARKS 10 5 10 15 20 10 5 5 80
MARKS
Office Slip Date of Submitted: Student Name: Student ID: Chop Lecturers Name: Received Student Slip Date of Submitted: Student Name: Student ID: Experiment title:
Separation Technology/CKD 20103/Jan 2013
Vapour-Liquid Separation (Absorption) Packed Column Gas Absorption Process
1. Objective: The students should be able to: Operate Vapour Liquid Separation Experiment using a Packed Column Gas Absorption Process Unit. Analyze the sample using the method of direct Titration in order to determine the amount of unreacted NaOH in the mixture with the sample.
2. Introduction: Packed Beds are usually employed in Chemical and Process Industries for Mass Transfer operations. Here, a Gas Stream is usually interacted with a Liquid Stream flowing counter currently in order to affect the Mass Transfer. The flow rates of liquid and gas have to be carefully selected in order to achieve effective Mass Transfer and in the meantime, to avoid undesirable phenomena such as flooding of the column. It is anticipated that this experiment will impart to the students with a good understanding of the concepts mentioned above. For this particular equipment, there are three separated Packed Columns C1, C2 and C3 of equal sizes are erected vertically. The Packed Columns are arranged to operate individually. The bottom ends are connected to a line supplying Air or a mixture of CO 2 in Air depending on the requirements of the experiment. Valves V1, V2 and V3 can separately control the flow rates of the Compressed Air to each column. The Solute Gas whenever required is supplied through flow control valves FCV1 and FCV2 and flow meters FI1 and FI2 respectively. Gas outlet from the columns is vented to the atmosphere which is next to Packed Column C3. Water is fed from the mains line or pumped to the top of each column. The water flow rates can be controlled by using valves V4, V5 and V6. Provision is also available to supply water to the top of the columns through a re-circulation pump which sucks water from a Recirculation Tank which is close to Packed Column C1. The water flow rate is measured by means of a flow meter FI3. Valves V8, V9 and V10 control the outlet flow rates for each column. Valves V11 and V12 can be used to vent effluent gases and liquids and purge any liquids from the columns. By means of a Green Oil filled manometer and a multiport selection valve, Differential Pressure across the column is determined. When column dynamics are being studied, water is continuously circulated by means of the Recirculation Pump and Compressed Air is blown through the column. Plant operation is controlled from a free standing control panel fitted with a mimic diagram housing flow controllers, pressure indicators and services controllers.
3. Chemicals and Ancillary Equipments Required: a) Chemicals required: 0.01 M HCl 0.01 M NaOH Phenolpthalein Indicator
2
Separation Technology/CKD 20103/Jan 2013
b) Ancillary Equipments required: 50 ml Burette and Stand 10 ml Graduated Cylinder 50 ml Pippette 100 ml or 250 ml Conical Flask Beaker A Process Flow Diagram for Packed Column Gas Absorption Process Unit is as shown below.
4. Operating Instructions:
4.1 Safety Considerations The flow meters should be operated smoothly in order to avoid pressure surges within the equipment. The flow rates of liquids and gases should not exceed the maximum of the flow meter. The CO2 supply pressure to the column should not exceed approximately 2 bar. The maximum flow rates should be controlled so that the pressure should be within the manometer range particularly when the column is operated near flooding conditions as shown by DP1 ( Height of Indicator is about 5 cm ). Once adjustments are made on parameters such as flow rates etc., sufficient time should be allowed for the process to attain steady state. [Allow at least 15 minutes for this operation.]
Separation Technology/CKD 20103/Jan 2013
4.2 General Start-up Procedures a) Chemicals Preparation: 1. 0.01 M HCl Standard Solution 1 litre. Withdraw 10 ml solution of HCl 1 M from the container. Pour 10 ml solution of HCl 1 M into 1000 ml Volumetric Flask. Add Distilled Water up to marked level at Volumetric Flask. Shake the solution for a while.
2. 0.01 M NaOH Standard Solution 1 litre Weigh 0.4 g of 40 M NaOH pellets. Pour NaOH pellets into 100 ml Beaker. Add Distilled Water into the 100 ml Beaker. Use Magnetic Stirrer to stir the mixture. Pour the mixture into 1000 ml Volumetric Flask. Add Distilled Water up to marked level at Volumetric Flask. Shake the solution for a while.
b) Pre-startup checks: Inspect the equipment visually for any damaged components or glass breakage. Ensure that the Cylindrical Re-circulation Vessel is charged with water and that the liquid level is satisfactory (Refer to your lecturer or technician). Ensure valves FCV1, FCV2, FCV3, FCV4, V7, V12 and V13 are closed. Ensure valve V11 are open. Choose the column to be operated and adjust the following valves:
Valve V1 V2 V3 V4 V5 V6 V8 V9 V10 SV1
Column 1 Open Closed X X X X X X X X X X
Column 2 Open Closed X X X X X X X X X X
Column 3 Open Closed X X X X X X X X X X
Ensure the manometer is reading zero. Switch the selector valve SV1 to connect to the column chosen.
c) Setting Up Of Liquid Flow: Check valves V11, V12 and FCV3 is closed and open valve V7. Turn on the power switch of recirculation pump.
4
Separation Technology/CKD 20103/Jan 2013
Slowly open valve FCV4 to give the required flowrate of 0.5 L/min as indicated by FI3. Ensure that the liquid overflow from the unit is connected to a suitable drain. Open valve V11 and V13 while close V7 when the water has entered the column and when the flowrate has reached 1.5 L/min. The unit is now ready to be used for experiment when the water has come down from the column.
4.3 Experiment: Absorption of CO2 into Water from Air / CO2 Mixture Open the water feed valve gradually until the desired flow rate 1.5 l/min is achieved as indicated in FI3. Ensure that the water flow rate is allowed to stable for about 10 minutes after each change until the desired value is reached. Open the Compressed Air Valve and ensure that the pressure is 1.5 bar gauge. Depending on the column to be operated, OPEN the valve V1, V2, or V3. Gradually OPEN the feed valve FCV1 for Air flow until the desired flow rate about 60 L/min is indicated in the flow meter FI1. Allow flow rate of 40 L/min and increase to 60 L/min after time elapse between 10 to 15 minutes. Ensure that you keep on monitoring the Indicator from the manometer or DP1. Ensure that there is no sudden jump of the level. (Consult your lecturer or technician). Ensure that the water flow rate is allowed to stable for about 10 minutes after each change until the desired value is reached. After steady state conditions are reached, OPEN valve FCV2 GENTLY until CO2 rate is 2L/min is achieved. Allow further period of 15 minutes for the Absorption process to attain steady state (Consult your lecturer or technician). Draw 10 ml sample from the outlet valve, V12 for every 15 minutes. Add 30 ml of prepared 0.01 M NaOH solution to the sample. (Consult your lecturer or technician concerning how to prepare this solution). The volume of NaOH is in excess to ensure that all CO2 has reacted with NaOH in the mixture. Add a few drops of an indicator (Phenolphthalein) to the solution. Titrate the mixture with already prepared 0.01 M HCl solution. (Consult your lecturer or technician on how to prepare this solution). This is meant to determine the amount of unreacted NaOH with CO2 in the sample mixture. Repeat the measurements every 15 minutes and carry out the titration with FRESH samples until CONSTANT concentration of NaOH has been achieved. Record your results in the provided table in the next page.
4.4 General Shut Down Procedures Slowly close CO2 supply valve. Slowly close the compressed air flow at FCV1. Open Valve 7. Turn off the power switch of recirculation pump. Turn off the air supply and CO2 supply (green valve). Open Valve V11 and allow Fresh Water to enter the column for a few minutes to drain off the water.
Separation Technology/CKD 20103/Jan 2013
5. Result:
Complete the following data of results:
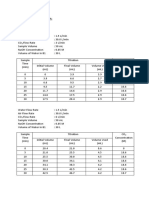
Table 1: Volume and Moles of HCI Titrated/Used
Time ( Minutes ) 0 10 20 30 40 50 60 70 80 90 100 110 120
Volume Of HCl Titrated/Used ( ml )
Moles of HCl Titrated/Used (mol)
Note: Stoichiometry equation: 2 NaOH + CO2 Na2CO3 + H2O Na2CO3 + HCl CO2 + H2O+ NaCl 2 moles of NaOH results with 1 mole of CO2 Let, x = moles of NaOH added = 0.01V1 y = moles of HCl used = 0.01V2 Then, g moles of NaOH reacted = (x-y) g moles of CO2 reacted = 0.5 (x-y) Hence, CO2 concentration = 0.5 (x-y)/ (VCO2 sample) = gmol/Liter
You might also like
- Building MaterialsDocument16 pagesBuilding MaterialsCleo Buendicho100% (1)
- Modification of LV Panels at Dubai International AirportDocument14 pagesModification of LV Panels at Dubai International AirportPushpakumara KarunadasaNo ratings yet
- Computational Techniques for Chemical Engineers: International Series of Monographs in Chemical EngineeringFrom EverandComputational Techniques for Chemical Engineers: International Series of Monographs in Chemical EngineeringNo ratings yet
- Method Statement For Installation of SwitchboardsDocument5 pagesMethod Statement For Installation of SwitchboardsDimitris NikouNo ratings yet
- LAB REPORT-Gas AbsorptionDocument16 pagesLAB REPORT-Gas Absorptionmizizasbonkure90100% (1)
- Quality Metrics For Aerospace: Tim Robertson PQA Nasa/JplDocument20 pagesQuality Metrics For Aerospace: Tim Robertson PQA Nasa/Jplnikhil jNo ratings yet
- Chemical Injection Unit Datasheet PDFDocument1 pageChemical Injection Unit Datasheet PDFMahesh DivakarNo ratings yet
- Lab Report Aspen Hysis UiTMDocument12 pagesLab Report Aspen Hysis UiTMAhmad SiddiqNo ratings yet
- Production of Aniline by Hydrogenation of NitrobenzeneDocument15 pagesProduction of Aniline by Hydrogenation of Nitrobenzeneananya srivastavaNo ratings yet
- PK - FKK.PPM - Manual Makmal Che565: Chemical Engineering Laboratory IiiDocument21 pagesPK - FKK.PPM - Manual Makmal Che565: Chemical Engineering Laboratory Iiibedirtupak92% (12)
- Tray Distillation Column With RefluxDocument26 pagesTray Distillation Column With RefluxMelvin MoorNo ratings yet
- Chemical Engineering Questions and AnswersDocument28 pagesChemical Engineering Questions and AnswersbabulubalaNo ratings yet
- Physical and Chemical Equilibrium for Chemical EngineersFrom EverandPhysical and Chemical Equilibrium for Chemical EngineersRating: 5 out of 5 stars5/5 (1)
- Gas AbsorptionDocument24 pagesGas AbsorptionShalini Krishnan100% (1)
- 1.0 Summary: CLB 20804 Exp 3: Gas AbsorptionDocument12 pages1.0 Summary: CLB 20804 Exp 3: Gas AbsorptionFaez Fikri MoitNo ratings yet
- CSTR FinalDocument36 pagesCSTR FinalMuhammad Yar KhanNo ratings yet
- Universiti Teknologi Mara Fakulti Kejuruteraan Kimia Chemical Engineering Laboratory Ii CHE523Document14 pagesUniversiti Teknologi Mara Fakulti Kejuruteraan Kimia Chemical Engineering Laboratory Ii CHE523Heather Jarvis100% (2)
- 154 Loesche Mills For Cement Raw Material E 2016Document28 pages154 Loesche Mills For Cement Raw Material E 2016faheemqcNo ratings yet
- Continuous Stirred Tank Reactor ExperimentDocument25 pagesContinuous Stirred Tank Reactor ExperimentChristopher Emeka Ominyi100% (1)
- Absorption in PackedDocument21 pagesAbsorption in PackedfreakameNo ratings yet
- Experiment 4: Gas Diffusion Coefficient: KeywordsDocument9 pagesExperiment 4: Gas Diffusion Coefficient: KeywordsMuhd Mukhrizan100% (3)
- Comparing Drainage Elements in PLAXIS 2D and 3D for Consolidating ClayDocument8 pagesComparing Drainage Elements in PLAXIS 2D and 3D for Consolidating ClayWilliam ChongNo ratings yet
- Modeling in Transport Phenomena: A Conceptual ApproachFrom EverandModeling in Transport Phenomena: A Conceptual ApproachRating: 3 out of 5 stars3/5 (2)
- Gas Absorption Theory, Apparatus, ProcedureDocument16 pagesGas Absorption Theory, Apparatus, Proceduresolehah misni100% (1)
- Tubular Flow Reactor Sample UiTM Lab ReportDocument20 pagesTubular Flow Reactor Sample UiTM Lab ReportNur AqilahNo ratings yet
- Cement Additives Improve High-Performance BindersDocument7 pagesCement Additives Improve High-Performance Bindersimsurender87No ratings yet
- Fluidized Bed Pressure Drop ExperimentDocument8 pagesFluidized Bed Pressure Drop Experimentlovelygirl_256No ratings yet
- EXP 2 - Plug Flow Tubular ReactorDocument18 pagesEXP 2 - Plug Flow Tubular ReactorOng Jia YeeNo ratings yet
- Gas Absorption: Determining Drag and Flooding FlowsDocument5 pagesGas Absorption: Determining Drag and Flooding FlowsDean Joyce AlborotoNo ratings yet
- Oil Distillation ReportDocument10 pagesOil Distillation ReportnisasoberiNo ratings yet
- Exp - 2 Bubble Cap Distillation ColumnDocument13 pagesExp - 2 Bubble Cap Distillation ColumnAdawiyah Al-jufri100% (1)
- Gas AbsorptionDocument7 pagesGas AbsorptionAnnerlynn Solano0% (1)
- Experiment 2 - Study of Packed Column DistillationDocument7 pagesExperiment 2 - Study of Packed Column DistillationAdawiyah Az-zahra100% (1)
- Unit Operation Laboratory 2 (CCB 3062)Document7 pagesUnit Operation Laboratory 2 (CCB 3062)Carl Erickson100% (1)
- Gas Absorption LabDocument8 pagesGas Absorption Labsolehah misni100% (1)
- Gas AbsorptionDocument19 pagesGas AbsorptionAnonymous NyvKBW100% (3)
- Lab 1Document12 pagesLab 1JoeJeanNo ratings yet
- Packed Distillation Column ExperimentDocument20 pagesPacked Distillation Column ExperimentChan Chun ChenNo ratings yet
- Lab Report Gas AbsorptionDocument14 pagesLab Report Gas AbsorptionM Asrar SidonNo ratings yet
- CSTR 40LDocument17 pagesCSTR 40LMuhammad Affifudin100% (1)
- CHE504 - Lab Report On Gas Absorption L8 PDFDocument23 pagesCHE504 - Lab Report On Gas Absorption L8 PDFRakesh KumarNo ratings yet
- Experiment: Batch Reactor Unit Operations Lab I (CHEGR3787L) Fall 2004Document5 pagesExperiment: Batch Reactor Unit Operations Lab I (CHEGR3787L) Fall 2004Janice YanNo ratings yet
- Experiment 1B - Tubular ReactorDocument14 pagesExperiment 1B - Tubular ReactorNajmul Puda PappadamNo ratings yet
- Heat Transfer Lab Manual 2015-16Document99 pagesHeat Transfer Lab Manual 2015-16Harshit Sinha100% (1)
- Experiment: Packed Distillation ColumnDocument4 pagesExperiment: Packed Distillation Columnnhalieza1067No ratings yet
- Plate Column Distillation EfficiencyDocument7 pagesPlate Column Distillation EfficiencyVijay PrasadNo ratings yet
- Intro CSTRDocument6 pagesIntro CSTREmmanuel PlazaNo ratings yet
- Results and Discussion of CSTR in SeriesDocument3 pagesResults and Discussion of CSTR in SeriesleenzalalNo ratings yet
- Gas Absorption Lab ReportDocument3 pagesGas Absorption Lab ReportNur Shaffikha Azmi100% (1)
- Group A5 - EXP 5 Batch Packed DistillationDocument35 pagesGroup A5 - EXP 5 Batch Packed DistillationKabilashini Mana Mohan100% (3)
- Plug FlowDocument17 pagesPlug FlowNurshahirahSapianNo ratings yet
- MT4 Lab FinalDocument19 pagesMT4 Lab FinalAmelia MaharajNo ratings yet
- Distillation Column Pressure Drop & Refractive IndexDocument18 pagesDistillation Column Pressure Drop & Refractive IndexAmir Al-AimanNo ratings yet
- GAS ABSORPTION - ReportDocument6 pagesGAS ABSORPTION - Reportgzairene8762No ratings yet
- 4.3. Consider The Flowsheet For The Manufacture of Vinyl Chloride in Figure 4.8Document2 pages4.3. Consider The Flowsheet For The Manufacture of Vinyl Chloride in Figure 4.8Anonymous QwUTQlAO100% (1)
- CHE506 - Lab Report On Continuous Stirre PDFDocument29 pagesCHE506 - Lab Report On Continuous Stirre PDFMuhammad AimanNo ratings yet
- Refrigeration Unit (DONE)Document33 pagesRefrigeration Unit (DONE)Eimint Mansor Applez100% (1)
- L9-Tubular Flow ReactorDocument20 pagesL9-Tubular Flow ReactorCik Tiem Ngagiman82% (11)
- Absorption in Packed Bed Lab ManualDocument5 pagesAbsorption in Packed Bed Lab ManualAshish Verma100% (1)
- Modeling AbsorptionDocument57 pagesModeling AbsorptionAbdul MalikNo ratings yet
- Batch ReactorDocument4 pagesBatch ReactorFoo Xiao BingNo ratings yet
- Series and Parallel Pumps: Flow Rate & PressureDocument11 pagesSeries and Parallel Pumps: Flow Rate & PressureKevin Devastian100% (1)
- Optimized Solids Suspension: Achieving Uniform Dispersion is Critical to Product QualityDocument7 pagesOptimized Solids Suspension: Achieving Uniform Dispersion is Critical to Product QualitymichsantosNo ratings yet
- Design of An Absorption Tower For The Separation of Acrylonitrile in - IndustryDocument9 pagesDesign of An Absorption Tower For The Separation of Acrylonitrile in - IndustryLouell Nikki HipulanNo ratings yet
- Quartz ToleranceDocument36 pagesQuartz Tolerancenick10686No ratings yet
- Disassembly of The Parts (Indoor Unit) : LS-K1823/1863/1867/2465/2665/2669CL/CM/CNDocument6 pagesDisassembly of The Parts (Indoor Unit) : LS-K1823/1863/1867/2465/2665/2669CL/CM/CNmanuelNo ratings yet
- Floor ReportDocument4 pagesFloor ReportStephen Gallagher0% (1)
- Evaluation of Mechanical and Thermal Properties of Al 6360 Alloy Reinforced With Sic ParticulatesDocument13 pagesEvaluation of Mechanical and Thermal Properties of Al 6360 Alloy Reinforced With Sic ParticulatesSripad ANo ratings yet
- Poly (Vinyl Chloride) (PVC) Plastic Drain, Waste, and Vent (DWV) Pipe and Fittings Having Post-Industrial Recycle ContentDocument7 pagesPoly (Vinyl Chloride) (PVC) Plastic Drain, Waste, and Vent (DWV) Pipe and Fittings Having Post-Industrial Recycle Contentastewayb_964354182No ratings yet
- Sealbond Etl-100: Epoxy Tank Lining (100% Solids) Solvent Free System Food GradeDocument2 pagesSealbond Etl-100: Epoxy Tank Lining (100% Solids) Solvent Free System Food GradegregNo ratings yet
- Synthesis of LiCoO2 Prepared by Sol-Gel MethodDocument4 pagesSynthesis of LiCoO2 Prepared by Sol-Gel Methodمصطفى محمودNo ratings yet
- PWHT ProcedureDocument12 pagesPWHT ProcedureDaengkulle Firmansyah PuteraNo ratings yet
- Raven 2350 Ultra Carbon Black Technical Data SheetDocument2 pagesRaven 2350 Ultra Carbon Black Technical Data SheetCoopertiva PlastcooperNo ratings yet
- 08 - TDS - Emaco S66 TDocument4 pages08 - TDS - Emaco S66 TaahtagoNo ratings yet
- Valve Regulated Lead Acid Battery ManualDocument22 pagesValve Regulated Lead Acid Battery Manualevanelizan100% (1)
- A Case Study To Bottle The Biogas in Cylinders As Source ofDocument4 pagesA Case Study To Bottle The Biogas in Cylinders As Source ofSamir VahoraNo ratings yet
- King Saud University Mass Transfer ExamDocument7 pagesKing Saud University Mass Transfer ExamAnnisa RahmaditaNo ratings yet
- AURORA KN95 Face Mask Introduction 2020Document23 pagesAURORA KN95 Face Mask Introduction 2020victorcamposNo ratings yet
- 423-Renolin CLP-1Document1 page423-Renolin CLP-1Moutaz IsmailNo ratings yet
- Salari A Tabarsa T Khazaeian A, Saraeian A. Effect of Nanoclay On Some AppliedDocument12 pagesSalari A Tabarsa T Khazaeian A, Saraeian A. Effect of Nanoclay On Some AppliedArif AnsariNo ratings yet
- 2a. Specs For Valves - IADocument4 pages2a. Specs For Valves - IAPraveen KasrottaranNo ratings yet
- FN Steel Datasheet Spring Steels ENG-V2Document2 pagesFN Steel Datasheet Spring Steels ENG-V2ariNo ratings yet
- Multistage Air Compressor FinalDocument21 pagesMultistage Air Compressor FinalJames TheeNo ratings yet
- The Introduction of Sterilization PouchesDocument3 pagesThe Introduction of Sterilization PouchesjarzloniezNo ratings yet
- Gujarat Milk ProductionDocument25 pagesGujarat Milk ProductionvizindiaNo ratings yet