Professional Documents
Culture Documents
Chem 136 - LAB F
Uploaded by
sidro123Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chem 136 - LAB F
Uploaded by
sidro123Copyright:
Available Formats
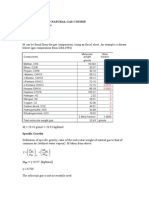
Partial Pressures of Gas Mixtures You will need the following conversion for parts B and C: 1 mm of Hg = 13.
6 mm of H2O Pre-Lab Questions 1. What is meant by the term partial pressure? The individual pressures exerted by each of the gases that make up the total atmospheric pressure. A. Partial Pressures of Oxygen and Nitrogen in Air (use Partial Pressures exercise module for a simulation of this experiment) A.1 Volume of test tube = 78.0 mL A.2 Volume of nitrogen = 62.0 mL A.3 Atmospheric pressure = 754 mm Hg Calculations: A.4 Volume of oxygen 78.0mL - 62.0mL = 16.0mL O2 A.5 Percent oxygen (show calculation) 16.0 78.0 * 100 = 20.5% O2 Percent nitrogen (show calculation) 62.0 78.0 * 100= 79.5% N2 A.6 Partial pressure of oxygen 754 mm Hg * 20.5 = 154.57 O2 100 Partial pressure of nitrogen 754 mm Hg * 79.5 = 599.43 N2 100 Questions and Problems Q.1 If you lived in the mountains where the atmospheric pressure is 685 mm Hg, what would the partial pressure of oxygen and of nitrogen be? Use your % values from A.5. (show calculation) 685 mm Hg * 20.5 = 140.43 O2 100 685 mm Hg * 79.5 = 545.37 N2 100 B. Carbon Dioxide in the Atmosphere B.1 Atmospheric pressure = 754 mm Hg
B.2 PCO2 (height of water column in tube B) = 6 mm H2O Calculations B.3 PCO2 6mm Hg * 1mm Hg = 0.44mm Hg 13.6 mm H2O B.4 Percent CO2 in the atmosphere __0.058_____ % CO2 (atmosphere) Show calculations: 0.44mm Hg * 100 = 0.058% CO2 754mm Hg C. Carbon Dioxide in Expired Air C.1 Atmospheric pressure = 754 mm Hg C.2 Height of water column = 27.0 mm H2O Calculations C.3 PCO2 Show calculations: 27.0 mm H2O * 1 mm Hg = 1.99 mm Hg 13.6 mm H20 C.4 Percent CO2 (expired air) __0.26_____% CO2 (expired air) (Show calculations) 1.99 mm Hg * 100 = 0.26% CO2 754 mm Hg Questions and Problems Q.2 Which would you expect to be higher, the percentage of CO2 in the atmosphere or in expired air? Why? Expired air because expiration occurs when the diaphragm relaxes and moves back up into the thoracic cavity to its resting position. This reduces the volume of the thoracic cavity which squeezes the lungs and decrease their volume. The pressure in the lungs is higher than the pressure of the atmosphere.
Q.3 What is the total pressure in mm Hg of a sample of gas that contains the following gases: O2 (45 mm Hg), N2 (1.20 atm), and He (825 mm Hg)? Show work or explain your thought process. 1 atm = 760 mm Hg N2= 1.2 atm * 760 mm Hg = 912 mm Hg Total Pressure = 45 mm Hg + 912 mm Hg + 825 mm Hg= 1,782 mm Hg
Q.4 A mixture of gases has a total pressure of 1650 mm Hg. The gases in the mixture are helium (215 mm Hg), nitrogen (0.28 atm), and oxygen. What is the partial pressure of the oxygen in atm? Show work or explain your thought process. 1 atm = 760 mm Hg Nitrogen= 0.28 atm * 760 mm Hg = 212.8 mm Hg Total Pressure = 215 mm Hg + 212.8 mm Hg + X = 1650 mm Hg 1650 mm Hg - 427.8 = 1222.2 mm Hg O2 Percent of O2 Partial Pressure of O2 1222.2 * 100 = 74.1% O2 1650 * 74.1 = 1222.65 1650 10
You might also like
- Chem 136-Lab G-1Document3 pagesChem 136-Lab G-1sidro123100% (14)
- Chem 136-LAB EDocument5 pagesChem 136-LAB Esidro12382% (11)
- Chem 136-Lab HDocument3 pagesChem 136-Lab Hsidro12325% (4)
- Nuclear Radiation Lab CHEM136Document3 pagesNuclear Radiation Lab CHEM136NatNo ratings yet
- Chem 136-LAB DDocument2 pagesChem 136-LAB Dsidro12375% (4)
- Chem 136-LAB CDocument3 pagesChem 136-LAB Csidro12371% (7)
- Chem 136-LAB ADocument3 pagesChem 136-LAB Asidro12388% (8)
- Lab 3 Atomic Structure (Chem 136)Document5 pagesLab 3 Atomic Structure (Chem 136)NatNo ratings yet
- Suspensions, Colloids and SolutionsDocument2 pagesSuspensions, Colloids and SolutionsJim Goetz88% (8)
- Conversion FactorsDocument4 pagesConversion FactorsJim Goetz75% (4)
- Chem 136-LAB BDocument2 pagesChem 136-LAB Bsidro12380% (5)
- Solutions, Electrolytes and ConcentrationDocument3 pagesSolutions, Electrolytes and ConcentrationJim Goetz90% (10)
- Density and Specific Gravity: Jenna Voigt Professor Boyke CH 136 8 July 2022Document3 pagesDensity and Specific Gravity: Jenna Voigt Professor Boyke CH 136 8 July 2022KailaNo ratings yet
- Lab9 IndicatorsDocument6 pagesLab9 IndicatorsjpraanggreniNo ratings yet
- 2 GasDocument14 pages2 GasSSNo ratings yet
- Draft of Chimney CalculationDocument7 pagesDraft of Chimney Calculationshani5573No ratings yet
- DepEd Cebu Lilo-an National High School Gases Module 5 Answer SheetDocument12 pagesDepEd Cebu Lilo-an National High School Gases Module 5 Answer SheetJeston Mar BayogNo ratings yet
- Biomass Combustion ManojDocument16 pagesBiomass Combustion Manojsugandaraj522No ratings yet
- Chapter 10 Powerpoint - Student VersionDocument95 pagesChapter 10 Powerpoint - Student VersionAnj LTNo ratings yet
- Termodinamika Yunus Changel Chapter 15Document45 pagesTermodinamika Yunus Changel Chapter 15Silvi Wildia Hariadi PribadiNo ratings yet
- Chem HelpDocument29 pagesChem HelpRobyn KentNo ratings yet
- The Gas Laws ExplainedDocument11 pagesThe Gas Laws ExplainedBenson Anselmo0% (1)
- Chapter 5 Gases and The Kinetic-Molecular Theory: H D H DDocument33 pagesChapter 5 Gases and The Kinetic-Molecular Theory: H D H DGregNo ratings yet
- SH 5107 Principles of Airflow 2021 Version 1Document126 pagesSH 5107 Principles of Airflow 2021 Version 1Shuyuan LuNo ratings yet
- De Lina - Gas Laws Worksheet 3Document2 pagesDe Lina - Gas Laws Worksheet 3Jana De LiñaNo ratings yet
- Sample ProblemsDocument18 pagesSample ProblemsEggy ThreekingsNo ratings yet
- CH 05 WEDocument43 pagesCH 05 WEBeauponte Pouky MezonlinNo ratings yet
- Mass Balance With ExcelDocument22 pagesMass Balance With ExcelZakiyah Kamto Irfin50% (2)
- L36 - Combustion ReactionsDocument25 pagesL36 - Combustion ReactionsGermano Menzel100% (1)
- Universal Gas Law Constant LabDocument3 pagesUniversal Gas Law Constant LabDan FerenceNo ratings yet
- Combust Part 1Document12 pagesCombust Part 1Philip Anthony MasilangNo ratings yet
- Lec 6 Combustion of Liquid and Solid FuelsDocument51 pagesLec 6 Combustion of Liquid and Solid FuelsEli EliNo ratings yet
- Satuan KOnversi PPMVDocument16 pagesSatuan KOnversi PPMVpurwakaadiNo ratings yet
- 101 GasesDocument6 pages101 GasesQaz Zaq100% (1)
- EPA METHOD 5 CALCULATIONSDocument42 pagesEPA METHOD 5 CALCULATIONSDipeshBardoliaNo ratings yet
- Atk2 1 2014Document34 pagesAtk2 1 2014Zakiyah Kamto IrfinNo ratings yet
- Introduction To Air Pollution - WorkbookDocument10 pagesIntroduction To Air Pollution - Workbookrocky21stNo ratings yet
- GasesDocument38 pagesGaseshNo ratings yet
- Model Calculation NKarthickDocument24 pagesModel Calculation NKarthickSeenu Hassan100% (2)
- A Fixed Quantity of Gas at 21Document8 pagesA Fixed Quantity of Gas at 21nonoytagupa3No ratings yet
- Comprehensive Problem: Laurito, E. R. (N.D.) - Stoichiometry of Fuel Combustion andDocument20 pagesComprehensive Problem: Laurito, E. R. (N.D.) - Stoichiometry of Fuel Combustion andVilma GaelaNo ratings yet
- Neraca Massa FIXsDocument15 pagesNeraca Massa FIXsmega fratiwiNo ratings yet
- Air Liquefaction using Linde Single-Column SystemDocument2 pagesAir Liquefaction using Linde Single-Column SystemNaiduJagarapuNo ratings yet
- Understanding Gas Laws and the Kinetic Molecular TheoryDocument27 pagesUnderstanding Gas Laws and the Kinetic Molecular Theorysidra89No ratings yet
- Azucar HojaDocument9 pagesAzucar HojaJorge PerezNo ratings yet
- Gaseous FuelDocument20 pagesGaseous FuelCaguioa Mark Anthony G.100% (3)
- Determining Molar Volume and Absolute ZeroDocument4 pagesDetermining Molar Volume and Absolute ZeroJeanine Bianca LastinoNo ratings yet
- CheCal 2 Solved ProblemsDocument10 pagesCheCal 2 Solved ProblemsJames Laurence RavizNo ratings yet
- Basic Pneumatic SystemDocument95 pagesBasic Pneumatic SystemRoyal Ritesh SharmaNo ratings yet
- UAEU Phthalic Anhydride ProjectDocument78 pagesUAEU Phthalic Anhydride ProjectminumcincauNo ratings yet
- Week 11 Solutions - ENB222Document11 pagesWeek 11 Solutions - ENB222Don Wook WonNo ratings yet
- Gases Sample Questions PDFDocument25 pagesGases Sample Questions PDFKhay Nochefranca100% (1)
- Air Dispersion Modeling Conversions and FormulasDocument11 pagesAir Dispersion Modeling Conversions and FormulaskitorzaNo ratings yet
- TPG 4140 CALCULATIONSDocument6 pagesTPG 4140 CALCULATIONSpatrickandreas77No ratings yet
- Anggi Bagus FixDocument23 pagesAnggi Bagus FixAbdul QodirNo ratings yet
- Humidity Unit Conversions-General PDFDocument10 pagesHumidity Unit Conversions-General PDFMachineryengNo ratings yet
- MIDTERM2023Document3 pagesMIDTERM2023Anders Rojas Coa.No ratings yet
- Fuels and Heat Power: Engr. Roxanne R. NavarroDocument13 pagesFuels and Heat Power: Engr. Roxanne R. NavarroDhonna ManigbasNo ratings yet
- ISO 23210 2009 Calculation Program EDocument9 pagesISO 23210 2009 Calculation Program ETri SulyonoNo ratings yet
- Bab 3 Behavior of Ideal GasDocument12 pagesBab 3 Behavior of Ideal GasYosua Ferian OlgaNo ratings yet
- Lab 10 - Organization of Nervous TissueDocument6 pagesLab 10 - Organization of Nervous Tissuesidro123No ratings yet
- Solutions, Electrolytes and ConcentrationDocument3 pagesSolutions, Electrolytes and ConcentrationJim Goetz90% (10)
- Lab 9 - Gross Anatomy of The Muscular SystemDocument13 pagesLab 9 - Gross Anatomy of The Muscular Systemsidro12380% (5)
- Chem 136-LAB BDocument2 pagesChem 136-LAB Bsidro12380% (5)
- Chem 136-LAB CDocument3 pagesChem 136-LAB Csidro12371% (7)
- Lab 11 - Gross Anatomy of The Central Nervous SystemDocument10 pagesLab 11 - Gross Anatomy of The Central Nervous Systemsidro123100% (3)
- Chem 136-LAB ADocument3 pagesChem 136-LAB Asidro12388% (8)
- Histology LabDocument9 pagesHistology Labsidro123100% (1)
- Lab 8 - Joints and Body MovementsDocument4 pagesLab 8 - Joints and Body Movementssidro123100% (1)
- Lab 7 - The Axial and Appendicular SkeletonDocument5 pagesLab 7 - The Axial and Appendicular Skeletonsidro123100% (3)
- Lab 6 - Overview of The Skeletal SystemDocument6 pagesLab 6 - Overview of The Skeletal Systemsidro123No ratings yet
- Lab 5 - Integumentary SystemDocument7 pagesLab 5 - Integumentary Systemsidro123No ratings yet
- Using The MicroscopeDocument4 pagesUsing The Microscopesidro123100% (1)
- Classification of Body Membranes LabDocument6 pagesClassification of Body Membranes Labsidro123No ratings yet
- An Overview of AnatomyDocument7 pagesAn Overview of Anatomysidro123100% (3)
- Test Paper 12 TH GradeDocument2 pagesTest Paper 12 TH GradecoroleamNo ratings yet
- Ancient Egypt WwtbamDocument61 pagesAncient Egypt WwtbamJayme FigueroaNo ratings yet
- Gurpreet 10900898Document2 pagesGurpreet 10900898Jatinder SinghNo ratings yet
- Six Sigma DMAICDocument2 pagesSix Sigma DMAICjaviermzNo ratings yet
- ToyoIto Tod's OmotesandoDocument23 pagesToyoIto Tod's OmotesandoMehmet Akif CiritNo ratings yet
- Appendix D 2008Document14 pagesAppendix D 2008Daniel LiawNo ratings yet
- Sherpas of NepalDocument18 pagesSherpas of Nepalprincess_d5526100% (1)
- L111 SMCDocument0 pagesL111 SMCozy1979No ratings yet
- Aisc 34Document3 pagesAisc 34Stephanie ScottNo ratings yet
- Hazardous Area Classification PresentationDocument9 pagesHazardous Area Classification PresentationAdeel Afzal100% (1)
- Apartment Hunting ChecklistDocument1 pageApartment Hunting ChecklistTime Warner Cable NewsNo ratings yet
- 5594VET - Topic 10 - Inbreeding and Genetic RelationshipsDocument8 pages5594VET - Topic 10 - Inbreeding and Genetic Relationshipsاحمد البزورNo ratings yet
- Ganga Roto Tanks1Document2 pagesGanga Roto Tanks1Deepakkmrgupta786No ratings yet
- Pressure Reducing ValveDocument3 pagesPressure Reducing ValveSasa JadrovskiNo ratings yet
- ME 338 Manufacturing Processes II HW#1 Vibration and Grinding AnalysisDocument2 pagesME 338 Manufacturing Processes II HW#1 Vibration and Grinding AnalysisAditya DaveNo ratings yet
- Dosificacao de Ingles 9a ClasseDocument2 pagesDosificacao de Ingles 9a ClasseJames Conjo100% (1)
- My Sister Works Downtown.: Unit 6, Exercise 3Document1 pageMy Sister Works Downtown.: Unit 6, Exercise 3Wilber RivasNo ratings yet
- Expanding Oralcare in IndiaDocument1 pageExpanding Oralcare in IndiaBala Meenakshi0% (1)
- Nursery ChartingDocument3 pagesNursery ChartingRI NANo ratings yet
- Bolt On Acoustic Guitar NeckDocument1 pageBolt On Acoustic Guitar NeckJayMorgan50% (2)
- Basics of Electric MotorsDocument16 pagesBasics of Electric Motorshodeegits9526No ratings yet
- Polavaram OrdinanceDocument2 pagesPolavaram OrdinanceRakesh Reddy Dubbudu100% (1)
- Genetic Concepts & Terms ExplainedDocument9 pagesGenetic Concepts & Terms ExplainedMante Cros NalzaroNo ratings yet
- Mr. Jagdish Singh: Scientific Officer Gd-IIDocument2 pagesMr. Jagdish Singh: Scientific Officer Gd-IIPrasad DNo ratings yet
- Hdv100a1 3903dsDocument1 pageHdv100a1 3903dsAlex AlanisNo ratings yet
- BGS Relationship of Birla GroupDocument7 pagesBGS Relationship of Birla GroupManan DesaiNo ratings yet
- FM Dipol AntenaDocument2 pagesFM Dipol AntenaNebojsa_28100% (1)
- Press Release - VW of Marion Ground BreakingDocument2 pagesPress Release - VW of Marion Ground Breakingashlee87No ratings yet
- SAOUDocument3 pagesSAOUelectronico69No ratings yet
- Irregular Plural Nouns PDFDocument2 pagesIrregular Plural Nouns PDFelenaflorinNo ratings yet