Professional Documents
Culture Documents
Chem Mole Lab
Uploaded by
api-239474550Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chem Mole Lab
Uploaded by
api-239474550Copyright:
Available Formats
Chemistry Name ___Litsa Sursock_____ Period ___1 Date ___/___/___
Moles, Molecules, and Grams Lab
T h e M o l e C o n c e p t U n i t 4
As weve already discussed in class, its easy to make conversions between moles, molecules/formula units, and grams. For example, if we want to go from moles to grams, we use the molar mass to make this conversion. If we want to go from moles to molecules, we use Avogadros number, or 6.02 x 1023. Finally, if we want to go from grams to molecules/formula units, we just use a two-step process where we first convert from grams to moles, and then from moles to molecules/F.U. Visually, it looks like this: Molar mass Molecules/Formula Units In this lab, we will be weighing out five different substances then finding out how many moles and molecules/F.U. of each one we have. Pre-lab: If you measure out 25.0 grams of NaOH in this lab, how many moles of NaOH would you have? How many formula units? 25 grams NaOH ( 1 Mol/39.997 grams) = 0.63 Mol NaOh
6.02 x 1023 Grams
Moles
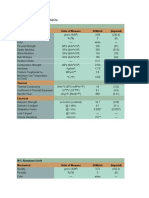
Lab: In this lab, there are five different balances set up, each of which has a labeled substance in a little jar next to it. Your job is to find out how many moles and how many molecules of each substance are in the canister. Some information you might find useful: sand baking soda (NaHCO3) chalk (CaCO3) table salt (NaCl) sugar: sucrose (C12H22O11) Make sure that your lab has a data table that contains the following: The name of each of these substances The formula for each of these substances The molar mass of each of these substances The mass in grams of each of these substances. When you are done weighing each material, calculate the number of moles of each material, and the number of molecules of each one, and put them in the right space. All calculations must be shown on lined paper with all conversion factors and unit cancelling. Data Table:
DATA TABLE FORMULA
BAKING SODA NaHCO3
SAND SiO2 60.08 grams 7.60 grams
TABLE SALT NaCl 58.44 grams 5.92 grams
SUGAR C12H22O11 342.2965 grams 5.46 grams
CHALK CaCO3 100.0869 grams 1.92 grams
MOLAR MASS 84.007 grams MASS OF SAMPLE FORMULA FOR MOLES 7.86 grams
7.866 g x 1 Mol / 84.007 g 0.0936 Mol
7.60 g x 1 5.92 g x 1 Mol / 60.08 g Mol / 58.44g
5.46 g x 1 Mol / 342.2965 grams 0.0160 Mol
1.92 g x 1 Mol / 100.0869 g 0.0192 Mol
NUMBER OF MOLES FORMULA FOR MOLECULES NUMBER OF MOLECULES
0.126 Mol
0.101 Mol
0.0936 Mol x 6.02 x 10^23 Molecules / 1 Mol 5.63 x 10^22 Molecules
0.126 Mol x 6.02 x 10^23 Molecules / 1Mol 7.59 x 10^22 Molecules
0.101 Mol x 6.02 x 10^23 Molecules / 1 Mol 6.08 x 10^22 Molecules
0.0160 Mol x 6.02 x 10^23 Molecules /1 Mol 9.63 x 10^22 Molecules
0.0192 Mol x 6.02 x 10^23 Molecules / 1 Mol 1.16 x 10^22 Molecules
Calculations: Using your data, find the following values. Make sure to show all work and write all numbers with the correct significant figures. 1a. Number of moles of sand: 0.126 Mol
1b. Number of molecules of sand: 7.59 x 10^22 Molecules
2a. Number of moles of baking soda: 0.094 Mol
2b. Number of formula units of baking soda: 5.63 x 10^22 Formula Units
3. Number of formula units of chalk: 1.16 x 10^22 Formula Units
4. Number of formula units of salt: 6.08 x 10^22 Formula Units
5. Number of molecules of sugar: 9.63 x 10^22 Molecules
Post Lab Questions:
1. Which of the materials we worked with had the largest number of molecules/formula units?
Was this the material that had the largest weight? Why? We found that sugar had the largest number of molecules, though it also seemed to have the lowest mass. Its molar mass, on the other hand, was very large, which shows that it would have the greatest number of molecules.
2. Water has a molecular formula of H2O. If I have 50.0 g of water, how many moles of water do I
have? How many molecules? 50.0g H2O x 1Mol / 18.01528 grams = 2.78 Mol H2O 2.78 Mol x 6.02 x 10^23 Molecules / 1Mol = 1.67 x 10^24 Molecules
3. Butane has a molecular formula of C4H10. If I have 50.0 grams of butane, how many moles of
natural gas do I have? How many molecules? 50.0g C4H10 x 1 Mol / 58.12 grams = 0.860 Mol C4H10 0.860 Mol x 6.02 x 10^23 Molecules / 1 Mol = 5.18 x 10^24 Molecules
4. I had the same weight of water and butane in problems 3 and 4. Why didnt the answer come
out the same? Explain.
The molar mass of C4H10 and the molar mass of H2O is different, therefore the answer did not come out the same, and the answers that result in dimensional analysis will result differently .
You might also like
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Fundamentals of Electronics - Awais YasinDocument185 pagesFundamentals of Electronics - Awais YasinAsif HameedNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5795)
- 1 s2.0 S0306261918311140 MainDocument11 pages1 s2.0 S0306261918311140 MainAllal BouzidNo ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Home AssignmentDocument2 pagesHome AssignmentEffecure HealthcareNo ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Mass Volume Density Notes PDFDocument17 pagesMass Volume Density Notes PDFNadya PutriNo ratings yet
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- 1.experiment On Single Rotor SystemDocument8 pages1.experiment On Single Rotor SystemRahul Kumar DwivediNo ratings yet
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Complete Overview of Lightning Arresters Part 3Document7 pagesComplete Overview of Lightning Arresters Part 3Robert GalarzaNo ratings yet
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Fundamentals of Physics Sixth Edition: Halliday Resnick WalkerDocument4 pagesFundamentals of Physics Sixth Edition: Halliday Resnick WalkerAhmar KhanNo ratings yet
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- 182590-615ASTRO-N7 CHSM72NDGF-BH 2382x1134x30 EN 20230802Document2 pages182590-615ASTRO-N7 CHSM72NDGF-BH 2382x1134x30 EN 20230802Gilberto SousaNo ratings yet
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Mass Transfer 1 CLB 20804Document54 pagesMass Transfer 1 CLB 20804KumaranNo ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Kinematics 1Document6 pagesKinematics 1Shiva Ram Prasad PulagamNo ratings yet
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- BS 7354Document63 pagesBS 7354Abu Monsur Ali67% (3)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- E101 Lab Report Group 4Document8 pagesE101 Lab Report Group 4Julia LovinoNo ratings yet
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Engr302 - Lecture 6 - Capacitance and Laplace's EquationDocument64 pagesEngr302 - Lecture 6 - Capacitance and Laplace's EquationNitin KathuriaNo ratings yet
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- DB - Pefy P VMHS eDocument26 pagesDB - Pefy P VMHS eAndi IrawanNo ratings yet
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Engineering Properties (Al O) : 94% Aluminum Oxide Mechanical Units of Measure SI/Metric (Imperial)Document7 pagesEngineering Properties (Al O) : 94% Aluminum Oxide Mechanical Units of Measure SI/Metric (Imperial)Hendy SetiawanNo ratings yet
- Problem Set 1Document2 pagesProblem Set 1Lovedeep SinghNo ratings yet
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Data Sheet - GBL Tag No. P309A P-ADocument7 pagesData Sheet - GBL Tag No. P309A P-ARajendra GuptaNo ratings yet
- Ild1, Ild2, Ild5, Ilq1, Ilq2: Vishay SemiconductorsDocument10 pagesIld1, Ild2, Ild5, Ilq1, Ilq2: Vishay SemiconductorsTan Hung LuuNo ratings yet
- Interference of Light Waves: ObjectivesDocument25 pagesInterference of Light Waves: Objectivesjay singhviNo ratings yet
- BTS443P Smart Highside Power SwitchDocument15 pagesBTS443P Smart Highside Power SwitchLuis N. Montiel V.No ratings yet
- Olson - Elements of Acoustical Engineering 1940Document368 pagesOlson - Elements of Acoustical Engineering 1940Corina MariaNo ratings yet
- MOSO Driver 24V 150WDocument9 pagesMOSO Driver 24V 150WMuhammad SholehNo ratings yet
- Tutorial Material Data InputDocument6 pagesTutorial Material Data InputJavier TrujillanoNo ratings yet
- Eddy PiezoDocument19 pagesEddy Piezoshrish ukhalkarNo ratings yet
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Fan Systems: Fan and System MatchingDocument28 pagesFan Systems: Fan and System Matchingvalerio.garibayNo ratings yet
- Fig. 1: Clap Switch Block DiagramDocument26 pagesFig. 1: Clap Switch Block DiagramDiljot Singh 236No ratings yet
- Module 3 TorsionDocument13 pagesModule 3 TorsionJay LopezNo ratings yet
- Energy Transfer in TurbomachinesDocument60 pagesEnergy Transfer in TurbomachinesBasavaraja K M Kotyal83% (6)
- 9709 w15 QP 41Document4 pages9709 w15 QP 41yuke kristinaNo ratings yet
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Modul 2.3 Physics Form 4 - AnswerDocument6 pagesModul 2.3 Physics Form 4 - AnswerNURSHAFAZLEEN BINTI AK DAMIT KPM-GuruNo ratings yet
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)