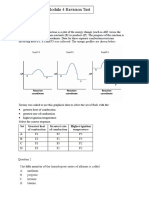
Professional Documents
Culture Documents
Solutions Manual For Chemist 2
Uploaded by
tomzhongOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Solutions Manual For Chemist 2
Uploaded by
tomzhongCopyright:
Available Formats
SECOND EDITION
HSC
Contexts
Solutions Manual
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
CHAPTER 1: ETHYLENE
Review exercise 1.1
1 The word petrochemical means any chemical that has been derived from petroleum, a
liquid fossil fuel.
2 Fossil fuels are non-renewable resources with a huge demand. They are wanted both as a
source of energy and as a source of petrochemical substances.
Review exercise 1.2
1 Ethylene (ethene) is the most widely used feedstock material derived from petroleum.
2 a Catalytic cracking produces shorter chain-length, lower-mass hydrocarbons from
high mass petroleum fractions. Thermal steam cracking converts ethane and
propane to ethylene.
b The proportions of products obtained from fractional distillation do not match the
demands for the different products. Catalytic cracking allows for specific control of
the types and amounts of products and hence more efficient use of the petroleum
feedstock and its products.
c C
9
H
20
(g) C
7
H
16
(g) + C
2
H
4
(g)
3 The catalysts used in cracking are zeolites. These solid crystalline substances adsorb the
gaseous reactants, weakening their bonds and hence lowering the activation energies.
Review exercise 1.3
1 Alkenes are more reactive than alkanes because of the presence of the double bond, a
centre of high electron density, in alkenes.
2 Place hexane and 1-hexene into separate test tubes. Add bromine water to a depth of about
1 cm, shake and allow to settle. If the bromine water is decolourised then the test tube
contained 1-hexene and the product of this addition reaction is 1,2-dibromoethane.
However, there may also be some 2-bromo-1-ethanol and hydrogen bromide as a result of
the water present. If there is no change to the colour of the bromine water then the test
tube contained hexane.
3 a C
3
H
6
+ Cl
2
C
3
H
6
Cl
2
1,2-dichloropropane
b C
7
H
14
+ HBr C
7
H
15
Br 2-bromoheptane, 1-bromoheptane. In
practice only 2-bromoheptane forms
(Markovnikovs rule).
c C
6
H
12
+ H
2
O C
6
H
13
OH 3-hexanol
4 a Addition of hydrogen chloride to 1-butene
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
b Chlorination of 2-butene
c Addition using chlorine water to 2-butene
d Hydration of 1-butene
e Oxidation of 2-pentene by reacting with cold dilute potassium permanganate
Chapter 1 Application and investigation
1 Investigation
2 a Cracking is used to produce hydrocarbons with lower molecular mass which have
greater market demand, e.g. petrol, branch-chained alkanes to improve the
performance of petrol; and to produce ethene which can be used as a starting
material for many organic compounds.
b In catalytic cracking the material to be cracked is passed over a zeolite catalyst at a
temperature of around 500
o
C. The reactants adsorb to the surface of the catalyst,
which weakens their bonds, lowering the activation energy.
c Catalytic cracking uses zeolite crystals as catalysts to lower the activation energy in
the cracking of high molecular mass hydrocarbons. Thermal cracking uses very high
temperatures (around 800900
o
C) to crack hydrocarbons such as ethane and
propane, to produce needed alkenes.
3 C
7
H
16
(g) C
5
H
12
(g) + C
2
H
4
(g)
4 Investigation
5 Alkenes contain a reactive double bond, which readily undergoes addition reactions in
order to gain a more stable single bond.
6 a 1,2-dichloropropane
b 1-chlorobutane; 2-chlorobutane. In practice only 2-chlorobutane forms
(Markovnikovs rule).
c ethanol
7 Investigation and class experimental work:
a 2-pentene with HCl
b 2-pentene with Cl
2
c propene with H
2
O in the presence of a catalyst
8 Add bromine water to samples of each of cyclohexene and cyclohexane, shake and allow
to settle. Cyclohexene undergoes an addition reaction with Br
2
across the double bond and
thus the bromine water is decolourised. There is no rapid visible reaction with
cyclohexane.
9 Investigation
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
CHAPTER 2: POLYMERS
Review exercise 2.1
1 Poymerisation is the chemical reaction whereby monomers link together to form
polymers.
2 Addition polymerisation: all atoms in the monomer are present in the polymer chain.
Unsaturated monomers join together via breaking of a C = C double bond.
Condensation polymerisation: a reaction between two monomers, which can be different,
during which a small molecule, such as water, is eliminated.
3 a Monomers have C = C double bond.
b Monomers each contain a functional group which may be different. Common
groups are COOH (carboxylic acid), OH (alcohol) and NH
2
(amine) group.
4 a i
ii
b i
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
ii
5
6
7 a Increased branching decreases hardness by making the polymer less compact and
rigid.
b Cross-linking between polymer chains increases hardness because cross-linking is
produced by covalent bonds linking chains together.
c Decreasing the length of polymer chains decreases hardness because there are less
dispersion forces between the chains.
d Increasing the orderly arrangement of polymer chains increases hardness by making
the polymer more dense and less flexible.
e Adding a plasticiser to a polymer decreases hardness because plasticisers are
generally chosen to soften a polymer.
Review exercise 2.2
1 a formation of polyethylene
b formation of polystyrene
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
c formation of polyvinylchloride
2 a chloroethylene
b phenylethylene or ethenylbenzene
3 LDPE is softer and more flexible due to greater branching of chains causing reduced
dispersion forces.
HDPE has fewer branched chains, causing greater dispersion forces and hence greater
hardness and strength.
4 1 monomer unit has molar mass of 62.5 g, so 15 000 monomer units have molar mass of
937 500 g.
5 a polystyrene because it is a cheap, lightweight insulating polymer
b low-density polyethene (LDPE) because it is mouldable, impermeable and flexible
c polypropene/nylon because it is lightweight and strong and easily formed into fibres
d LDPE because it is lightweight, cheap and flexible
e HDPE because it is tough and durable
f HDPE because it is rigid, tough and durable
g Teflon because it is tough, frictionless and resistant to heat and chemicals
h polyethene terephthalate (PET) because it is lightweight, tough and easily blown
into a shapeable film
Review exercise 2.3
1 Most of the synthetic polymers made today are derived from the petrochemical industry,
which relies on the non-renewable fossil fuel petroleum. However, these supplies will be
rapidly used up, especially if we continue to demand their use as both a fuel and a
feedstock. An alternative, renewable source of raw materials is needed.
2 Biomass is organic material derived from living organisms: plant material such as sugar
and cellulose; animal material such as dung; and domestic and industrial organic waste.
3 Cellulose is a long-chain polymer of -glucose units, C
6
H
12
O
6
, where every second
glucose unit is inverted to produce straightened chains.
4 A glucose unit loses an H from an OH group and joins to a carbon on a second glucose
unit which has lost its OH group; this links the glucose units with the loss of H
2
O and so
the formation of cellulose is an example of condensation polymerisation.
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
5 Cellulose is made from repeating units of -glucose with inversion of every second unit.
This produces long, straight chains of cellulose which are linked to each other by
hydrogen bonding. In plants, cellulose acts as a structural material. Starch (both amylase
and amylopectin) is made from long-chain repeating units of -glucose, which are also
highly branched. This results in tightly coiled, compact, insoluble starch molecules, which
are used as energy stores in plants.
6 Existing plant cellulose can be modified to produce biopolymers, such as celluloid and
rayon, or it can be broken down into smaller units that can be used to build new polymers
such as corn-starch polymers.
Review exercise 2.4
1 Silk is a biopolymer used in its natural form; rayon uses cellulose in a chemically
modified form; lactic acid can be produced from the breakdown of starch and then used to
make polylactic acid (PLA).
2 Advantages are that biopolymers come from plant material, which is a renewable
resource, and that plastics made from biopolymers are easily broken down by bacteria and
fungi.
Disadvantages include the cost of production and that they are easily biodegradable,
which is not useful in some applications.
3 a Plastic-producing bacteria are grown in fermentation vats and fed on molasses or
methanol. Extraction of plastic from the bacterium involves breaking down the
bacteriums cell walls and separating this from the cell debris.
b The bacterium used is Alcaligenes eutrophus.
c The plastic produced is of a type known as PHAspolyhydroxyalkanoateswhich
have similar properties to polypropene.
Chapter 2 Application and investigation
1 Monomer: small repeating units that join by covalent bonds to form a polymer,
e.g. ethylene.
Polymer: large-chain molecule consisting of small repeating units called monomers,
e.g. polyethylene.
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Polymerisation: the chemical reaction by which monomers link together to form
polymers, e.g. formation of polyethylene by addition reaction.
2 Investigation
3 Polyethylene can have many different molecular chain lengths. Those with around 40 000
atoms per molecule are used for food wrap films; 60 000 atoms per molecule make milk
containers; 80 000 atoms per molecule make bleach containers and 800 000 atoms per
molecule can be used in artificial ice rinks. As chain length increases, density, hardness
and melting point increase. Branching is possible in polyethylene, and as branching
increases, density, hardness and melting point decrease, increasing the uses that can be
made of polyethylene.
4 Molecular mass of 1 monomer = 28, so 84 500 28 = 3018 monomer units
5 First-hand investigation
6 Investigation based on answer to Q5
7 a
b
c
d
8 Investigation
9 Investigation
10 Investigation
CHAPTER 3: ETHANOL
Review exercise 3.1
1 a 1-propanol
b 4-chloro-2-pentanol
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
c 1,4-butanediol
d 2-methyl-2-propanol
2 a
b
c
3
2-methyl-propanol
83C
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
2-butanol
100C
2-methyl-1-propanol
108C
1-butanol
118C
4 Heat ethanol with an excess of concentrated sulfuric acid, or heat ethanol vapour over a
catalyst at 350
o
C.
5 C
2
H
5
OH(l) + 3O
2
(g) 2CO
2
(g) + 3H
2
O(l)
Review exercise 3.2
1 Methylated spirits are 95% ethanol, 5% methanol and small quantities of foul-tasting
chemicals. The additives are used to discourage people from drinking it.
2 Ethanol has a short non-polar chain, which allows it to dissolve some non-polar
substances. The OH functional group makes ethanol polar, which allows it to act a
solvent for polar substances.
3 The ethanol molecule contains the polar OH end and the non-polar hydrocarbon chain.
Hence it is able to be used as a solvent for non-polar substances such as perfumes and
aftershaves. The low boiling point ethanol evaporates easily with body heat, leaving the
heavier fragrance components on the skin.
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
4
5 Ethanol is described as renewable because it can be derived from the starch and sugars
present in various crops such as sugar cane and corn.
6 Cars using ethanol produce fewer pollutants. Ethanol is a renewable resource. However,
no commercially viable method of obtaining ethanol is available and cars will need to be
significantly modified to run on pure ethanol.
7 Mass of methanol burned = 1.10 g
Mass of water = 100 g
Mass of copper = 200.0 g
Temperature change = 5C
Specific heat capacity water = 4.18 J K
1
g
1
Specific heat capacity copper = 0.387 J K
1
g
1
Heat released per 1.10 g methanol = (mCT(copper) + mCT(water))
= (200 0.387 5 + 100 4.18 5)
= 2477 J released by 1.10 g methanol
Heat released per mole of methanol =
2477
10 . 1
32
= 72 058 J mol
1
Molar heat of combustion = 72 kJ mol
1
Review exercise 3.3
1 The acid catalyst is needed to cause the water molecule to attack the double bond in
ethylene.
2
3 a carbohydrates, e.g. glucose, sucrose, starch, plus water and yeast
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
b The yeasts produce enzymes which act as catalysts in the conversion of glucose to
ethanol and carbon dioxide.
c ethanol and carbon dioxide
d exothermic
e Once the ethanol concentration reaches 15% in the fermentation container, the yeast
is killed and the reaction ceases. Therefore naturally fermented wines have ethanol
concentrations of 1215%.
4 Fermentation of glucose occurs best in the absence of oxygen (anaerobic conditions) and
at a temperature of 3540
o
C.
5 From the equation, and using n = mass (g) molar mass (g)
mass (ethanol) = 2 mass(glucose) molar mass(glucose) molar mass(ethanol)
= 2 500 180.156 46.068
= 255.7 g
6 From the equation, and using n = mass (g) molar mass (g)
mass (ethanol) = mass (carbon dioxide lost) molar mass (carbon dioxide) molar mass
(ethanol)
= 50.0 44.01 46.068
= 52.3 g
Chapter 3 Application and investigation
1 a
b
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
c
2 a 1-propanol
b 1,4-butanediol
3
1-pentanol
2-pentanol
3-pentanol
4 Water, being polar, would not readily dissolve many esters with a non-polar component.
Hexane, being non-polar, would not readily dissolve the polar esters. Ethanol, with its
ability to dissolve both polar and non-polar components, is suitable for dissolving esters.
5 Investigation
6 Investigation
7 Investigation
8 Depends on responses to Questions 5, 6 and 7.
9 a Assume heat absorbed by copper is negligible.
Mass of fuel = 2.00 g
Mass of water = 500 g
Change in temp = 18.4C
Specific heat water = 4.18 J K
1
g
1
Methanol
Heat released by 2 g = mCT
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
= (500 4.18 18.4)
= 38 456 J
i Heat of combustion/g = 38 456 2
= 19 228 J g
1
= 19.2 k J g
1
ii Heat of combustion/mole =
38 456
32
2
= 61 5296 J mol
1
1-propanol
=
615 kJ mol
1
Heat released by 2 g = mCT
= (500 4.18 28.5)
= 59 565 J
i Heat of combustion/g = 59 565 2
= 29 782.5 J g
1
= 29.8 kJ g
1
ii Heat of combustion/mole =
= 1 789 928 J mol
1
= 1790 kJ mol
1
1-butanol
Heat released by 2 g = mCT
= (500 4.18 31.5)
= 65 835 J
i Heat of combustion/g = 65 835 2
= 32 917.5 J
= 32.92 kJ g
1
ii Heat of combustion/mole =
= 2 439 845.1 J
= 2440 kJ mol
1
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
b
10 The dehydration of ethanol to ethylene and water is carried out by heating ethanol with
concentrated sulfuric acid as a catalyst, or by heating ethanol vapour over an alumina
catalyst at 350
o
C. The hydration of ethylene to produce ethanol requires the addition of
water in the presence of a sulfuric or phosphoric acid catalyst.
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
CHAPTER 4: ELECTROCHEMISTRY
Review exercise 4.1
1 a A pink-brown deposit of copper forms on the surface of the steel wool as the copper
ions convert to elemental copper; the blue solution goes paler and slowly converts to
a green solution as the copper ions decrease in concentration and the elemental iron
converts to Fe
2+
ions with increasing concentration.
b oxidation: Fe(s) Fe
2+
(aq) + 2e
reduction: Cu
2+
(aq) + 2e
Cu(s)
c Fe(s) + Cu
2+
(aq) Fe
2+
(aq) + Cu(s)
2 a Fe(s) + Sn
2+
(aq) Fe
2+
(aq) + Sn(s); the iron would dissolve and the tin would coat
the surface of the iron; the solution would change from colourless to green
b Pb(s) + Hg
2+
(aq) Pb
2+
(aq) + Hg(l); the lead would dissolve and liquid mercury
would form
c no reaction
d 2Cr(s) + 3Fe
2+
(aq) 2Cr
3+
(aq) + 3Fe(s); the chromium would dissolve and the iron
would coat the chromium; both ions are generally green in aqueous solution
3 The iron container would dissolve as the iron was oxidised to its ions by the nickel ions,
which would convert to elemental nickel.
4 a no reaction
b Sn(s) Sn
2+
(aq) + 2e
; Cu
2+
(aq) + 2e
Cu(s); copper would coat the tin
c Sn(s) Sn
2+
(aq) + 2e
; Ag
+
(aq) + e
Ag(s); silver would coat the tin
Review exercise 4.2
1 a CO
2
: C = +4 O = 2
O
2
: elemental state 0
NH
3
: N = 3 H = +1
H
2
S : S = 2 H = +1
HCl : H = +1 Cl = 1
b SO
2
: S = +4 O = 2
SO
3
2
: S = +4 O = 2
H
2
S
2
O
7
: H = +1 S = +6 O = 2
S
2
O
8
2
: S = +7 O = 2
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
HSO
3
: H = +1 S = +4 O = 2
S : 0
c NH
4
+
: N = 3 H = +1
ClO
4
: Cl = +7 O = 2
Cu
2
S : Cu = +1 S = 2
MgH
2
: Mg = +2 H = 1
PO
4
3
: P = +5 O = 2
2 a redox reaction: Zn oxidised (0 +2), H
+
reduced (+1 0)
b not a redox reaction
c not a redox reaction
d redox reaction: Fe
2+
oxidised (+2 +3), Cr
6+
reduced (+6 +3)
3 NO : +2 NO
2
: +4 N
2
O : +1 N
2
O
3
: +3 N
2
O
4
: +4
N
2
O
5
: +5 HNO
3
: +5 HNO
2
: +3 NO
2
: +3 NO
3
: +5
NH
2
: 3 NH
4
+
: 3 N
2
: 0 N
2
H
4
: 2 NH
3
: 3
NH
2
OH : 1 NH
4
Cl : 3
4 Br
and HBr both have bromine in 1 oxidation state.
BrO
and Br
2
O and HBrO each have bromine in +1 oxidation state.
Review exercise 4.3
1 The salt bridge allows ions to move between each half-cell.
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
2 a
b See diagram in part a.
c anode: oxidation Mg(s) Mg
2+
(aq) + 2e
cathode: reduction Cu
2+
(aq) + 2e
Cu(s)
3 a 3Pb
2+
(aq) + 6e
3Pb(s) cathode
2Cr(s) 2Cr
3+
(aq) + 6e
anode
b Cu(s) Cu
2+
(aq) + 2e
anode
2Ag
+
(aq) + 2e
2Ag(s) cathode
Review exercise 4.4
1 a Ca
2+
, Pb
2+
, Cu
2+
, Ag
+
b Ag, Sn, Cr, Mg
c strongest oxidising agent: Ag
+
strongest reducing agent: Mg
2 a H
2
O
2
, HClO, MnO
4
, Cr
2
O
7
2
b Mg, Zn, H
2
, H
2
S
3 a Cl
2
b Sn
4+
c Au
3+
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
d MnO
4
Review exercise 4.5
1 a Pb
2+
+ 2e
Pb(s) E = 0.13 V cathode
Zn(s) Zn
2+
(aq) + 2e
E = +0.76 V anode
cell potential = 0.13 + 0.76
= 0.63 V
b Cl
2
(g) + 2e
2Cl
(aq) E = +1.36 V cathode
2I
(aq) I
2
(s) + 2e
E = 0.54 V anode
cell potential = +1.36 0.54
= 0.82 V
c 2H
+
(aq) + 2e
H
2
(g) E = 0 V cathode
Mg(s) Mg
2+
(aq) + 2e
E = +2.37 V anode
cell potential = 0 + 2.37
= 2.37 V
2 Pb(s) + PbO
2
(s) + 4H
+
(aq) + 2SO
4
2
(aq) 2PbSO
4
(s) + 2H
2
O(l) E(cell) = +2.04 V
anode: Pb(s) + SO
4
2
(aq) 2PbSO
4
(s) + 2e
E = +0.36 V
cathode: PbO
2
(s) + 4H
+
(aq) + SO
4
2
(aq) + 2e
PbSO
4
(s) + 2H
2
O(l)
E = 2.04 0.36 = +1.68 V
3 a i 2I
(aq) I
2
(s) + 2e
E = 0.54 V
MnO
4
(aq) + 8H
+
(aq) + 5e
Mn
2+
(aq) + 4H
2
O(l) E = +1.51 V
Add equations.
10I
(aq) + 2MnO
4
(aq) + 16H
+
(aq) 5I
2
(s) + 2Mn
2+
(aq) + 8H
2
O(l)
ii cell potential = 0.54 + 1.51
= +0.97 V
iii Cell potential is positive, and therefore the reaction can occur spontaneously.
b i Cl
2
(g) + H
2
S(aq) + 2e
2Cl
(aq) + S(s) + 2H
+
(aq) + 2e
Cl
2
(g) + 2e 2Cl
(aq) E = +1.36 V
H
2
S(aq) S(s) + 2H
+
(aq) + 2e
E = 0.14 V
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
ii cell potential = +1.36 0.14
= +1.22 V
iii Cell potential is positive, and therefore the reaction can occur spontaneously.
c i 2Cu
+
(aq) + e
Cu
2+
(aq) + Cu(s) + e
Cu
+
(aq) Cu
2+
(aq) + e
E = 0.16 V
Cu
+
(aq) + e
Cu(s) E = +0.52 V
ii cell potential = 0.16 + 0.52
= +0.36 V
iii Cell potential is positive, and therefore the reaction can occur spontaneously.
4 a H
2
O
2
(aq) + 2H
+
(aq) + 2e
2H
2
O(l) E = +1.78 V
H
2
C
2
O
4
(aq) 2CO
2
(g) + 2H
+
(aq) + 2e
E = +0.48 V
E (cell) = +1.78 + 0.48
= +2.26 V
b A high activation energy may be necessary for this reaction to occur.
c Mix the H
2
O
2
and H
2
C
2
O
4
at a higher temperature and/or add a catalyst.
Review exercise 4.6
1 The dry cell is one of the most widely used sources of portable electricity. It has led to the
proliferation of the portable devices we see around us. Without dry cells we would be a
much less mobile society. Devices requiring electrical power would need to be in close
proximity to permanent sources such as power points. Instead of torches we could still be
using candles or kerosene lamps. However, the development of dry cells has led to landfill
pollution.
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
2
3 a If the Zn and MnO
2
were in direct contact, the following overall cell reaction would
occur:
Zn(s) + 2H
+
(aq) + 2MnO
2
(s) Zn
2+
(aq) + Mn
2
O
3
(s) + H
2
O(l)
i.e. zinc is oxidised and manganese dioxide is reduced.
b The cell would be useless because electron transfer would occur directly between
the reactants and not through an external circuit.
4 Zn(s) + 2H
+
(aq) + 2MnO
2
(s) Zn
2+
(aq) + Mn
2
O
3
(s) + H
2
O(l)
5 Pb(s) + SO
4
2
(aq) PbSO
4
(s) + 2e
(0) (+2) oxidation
PbO
2
(s) + 4H
+
(aq) + SO
4
2
(aq) + 2e
PbSO
4
(s) + 2H
2
O(l)
(+4) (+2) reduction
6 A 6V leadacid battery would have three cells, each with a cell potential of 2 V.
7 Leadacid batteries are very heavy and weight is an important issue for solar-powered
cars in the World Solar Challenge.
Chapter 4 Application and investigation
The following reactions will occur:
1 Zn(s) + CuSO
4
(aq ZnSO
4
(aq) + Cu(s)
Zn(s) + NiSO
4
(aq) ZnSO
4
(aq) + Ni(s)
2 a i Yes. Fe
2+
ions will not oxidise Ni.
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
ii No. Fe
3+
will be reduced to Fe
2+
and Ni oxidised to Ni
2+
.
b No. The steel will dissolve and displace the copper.
3 The student should determine galliums position in the order of reactivity by attempting to
oxidise it using the ions of the other metals in the list.
4 a HNO
3
(aq)
NO
2
(g) reduction
+5 +4
b Cl
2
(g)
ClO
3
(aq) oxidation
0 +5
c NH
3
(g)
N
2
(g) oxidation
3 0
d Fe
2
O
3
(s)
FeO(s) reduction
+3 +2
e SO
2
(g)
SO
4
2
(aq) reduction
+4 +2
f MnO
4
(aq)
MnO
2
(s) reduction
+7 +4
5 a Fe(s) + SO
2
(g) + O
2
(g) FeSO
4
(s)
0 +4 2 0 +2 +6 2
Fe oxidised; S oxidised; O
2
reduced
b 4FeSO
4
(s) + O
2
(g) + 6H
2
O(l) 2Fe
2
O
3
.H
2
O(s) + 4H
2
SO
4
(aq)
+2 +6 2 0 +1 2 +3 2 +1 2 +1 +6 2
Fe oxidised; O
2
reduced
6 a All the E values would be 2.93 V higher.
b Calculated e.m.f. values for cells are based on the difference in E between two
half-cell reactions. These would be unchanged.
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
7 a i
ii Cu
2+
(aq) + 2e
Cu(s) E = +0.16 V
Cr(s) Cr
3+
(aq) + 3e
E = +0.73 V
2Cr(s) + 3Cu
2+
(aq) 3Cu(s) + 2Cr
3+
(aq)
iii e.m.f. = +0.89 V
b i
Zn(s) Zn
2+
(aq) + 2e
Cl
2
(g) + 2e
2Cl
(aq)
ii Cl
2
(g) + 2e
2Cl
(aq) E = +1.36 V
Zn(s) Zn
2+
(aq) + 2e
E = +0.76 V
Zn(s) + Cl
2
(g) Zn
2+
(aq) + 2Cl
(aq)
iii e.m.f = 1.36 + 0.76
= 2.12 V
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
8 a The half-cells are separated in order to generate a flow of current in the external circuit.
b A salt bridge allows ions to move between each half-cell and complete the circuit.
9 a anode: Zn(s) Zn
2+
(aq) + 2e
E = +0.76 V
cathode: Cu
2+
(aq) + 2e
Cu(s) E = +0.34 V
cell: Zn(s) + Cu
2+
(aq) Zn
2+
(aq) + Cu(s) E(cell) = +1.10 V
b As the cell operates, the concentration of Cu
2+
decreases and the Zn
2+
concentration
increases. Both changes would decrease the tendency of the forward reaction to take
place and hence the cell e.m.f. would decrease.
c i Transfer of electrons cannot occur, and therefore the reaction between the two
half-cells will cease.
ii Transfer of charged ions between half-cells cannot occur.
10 The reduction potential of +1.7 V for Au
+
is higher than that for the reduction of O
2
, and
therefore O
2
would not oxidise the gold. The reduction potential for [Au(CN)
2
]
(aq) is
lower than that for O
2
, and therefore in the presence of cyanide ion, gold can be oxidised.
11 a Ni
2+
(aq) or Co
2+
(aq) or Cd
2+
(aq)
b Mn(s) or Al(s) or Zn(s)
12 a i Fe
2+
Fe
3+
+ e
E
= 0.77 V
MnO
4
+ 8H
+
+ 5e
Mn
2+
+ 4H
2
O
E
= +1.51 V
E(cell) = +0.74 V
ii 2Cl
Cl
2
+ 2e
E
= 1.36 V
MnO
4
+ 8H
+
+ 5e
Mn
2+
+ 4H
2
O
E
= +1.51 V
E(cell) = +0.15 V
iii Fe
2+
Fe
3+
+ e
E
= 0.77 V
Cr
2
O
7
2
+ 14H
+
+ 6e
2Cr
3+
+ 7H
2
O
E
= +1.33 V
E(cell) = +0.56 V
iv 2Cl
Cl
2
+ 2e
E
= 1.36 V
Cr
2
O
7
2
+ 14H
+
+ 6e
2Cr
3+
+ 7H
2
O
E
= +1.33 V
E(cell) = 0.03 V
Thus a redox reaction should occur in i, ii and iii.
b iv above shows that Cr
2
O
7
2
will not oxidise the Cl
ion of HCl, but ii shows that
MnO
4
will. Hence, it is inappropriate to use HCl to acidify solutions involving
permanganate titrations.
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
13 a An anode is the electrode at which oxidation takes place. A cathode is the electrode
at which reduction takes place.
b A salt bridge allows a flow of ions between two half-cells. An external circuit
allows a flow of electrons from anode to cathode.
c A secondary cell can be recharged, whereas a primary cell cannot.
14 a i anode: Pb(s) + SO
4
2
(aq) PbSO
4
(s) + 2e
cathode: PbO
2
(s) + 4H
+
(aq) + SO
4
2
(aq) + 2e
PbSO
4
(s) + 2H
2
O(l)
ii anode: PbSO
4
(s) + 2H
2
O(l) 2e
+ SO
4
2
(aq) + 4H
+
(aq) + PbO
2
(s)
cathode: PbSO
4
(s) + 2e
Pb(s) + SO
4
2
(aq)
b i On discharging, the density decreases.
ii On recharging, the density increases.
c Chemical potential energy is transformed into electrical energy and some heat
energy as the battery is discharged. On charging, electrical energy is transformed
into chemical potential energy and some heat energy.
15 a Anode: Al(s) Al
3+
(aq) + 3e
Cathode: O
2
(g) + 2H
2
O(l) + 4e
4OH
(aq)
b Al(s) Al
3+
(aq) + 3e
E = +1.66 V
O
2
(g) + 2H
2
O(l) + 4e
4OH
(aq) E = +0.40 V
E(cell) = +2.06 V
Cell e.m.f. would be 2.06 V.
c It would weigh much less.
It avoids use of corrosive H
2
SO
4
.
It uses O
2
from the air as a cathode.
1618 Investigation
CHAPTER 5: NUCLEAR CHEMISTRY
Review exercise 5.1
1 a Atoms are not conserved in nuclear reactions.
b Chemical reactions involve changes in electrons between atoms. Nuclear reactions
involve the particles in the nucleus.
c Temperature, pressure/concentration and catalysts have no effect on nuclear
reactions.
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
d Far more energy is obtained from nuclear reactions.
2 a i
17
O : 8 protons; 9 neutrons
ii
52
Fe : 26 protons; 26 neutrons
iii
137
I : 53 protons; 84 neutrons
iv
258
Md : 101 protons; 157 neutrons
b i
ii Nb
93
41
iii Pb
208
82
3 a large mass; very small mass; no mass
b 2+ charge; 1 charge; no charge
c least penetrating, about 5 cm through air
about 1 m through air, stopped by aluminium
can pass through several cm of lead
4 a He
4
2
b e
0
1
c n
1
0
d p
1
1
e e
0
1
5 Photographic film: becomes dark on exposure. Darkness increases with length of
exposure and greater intensity of radiation.
Geiger counter: The argon gas inside a metal tube is ionised into positive ions and
electrons. These move towards electrodes which conduct a current measured by a
recording device.
Scintillation counter: Electrons in substances such as zinc sulfide are excited by radiation
and emit photons of light as they return to lower energy states. Radiation is measured by
counting flashes of light.
Review exercise 5.2
1 a H
3
1
He
3
2
+ e
0
1
b
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
c
2 a
b Pu
239
94
U
235
92
+ He
4
2
c U
234
92
Th
230
90
+ He
4
2
3 a N
12
7
C
12
6
+ e
0
1
b B
8
5
Be
8
4
+ e
0
1
c
4 a Be
7
4
+ e
0
1
Li
7
3
b Np
232
93
+ e
0
1
U
232
92
c
5 a beta emission
b positron emission
c alpha emission
d positron emission
6 a positron or electron capture
b decay
c decay
7 a No, the neutron : proton ratio is too high and outside the zone of stability.
b No, the neutron : proton ratio is too low and outside the zone of stability.
c Yes, the neutron : proton ratio is in the zone of stability.
Review exercise 5.3
1 After 1st 2.6 hours: 0.1/2 = 0.05 g
After 5.2 hours: 0.025 g
After 7.8 hours: 0.0125 g
2 1st half-life: 0.120/2 = 0.06 g
2nd half-life: 0.06/2 = 0.03 g
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
3rd half-life: 0.03/2 = 0.015 g
3 half-lives in 24 days
I-131 has a half-life of 8 days.
3 a 2.1 g = 21%
b 164 g 20.5 g, i.e. 12.5% left = 3 half-lives, 84 years
c Half-life is 28 years.
4 Thallium-201 and gallium-67: suitable, as have a short half-life; sulfur-35 and
hydrogen-3: unsuitable as half-life is too long.
Review exercise 5.4
1 To overcome the electrostatic repulsion alpha particles and protons will encounter in the
nuclide. Neutrons do not carry a charge and therefore do not need to be accelerated.
2 They are produced synthetically.
3 a N
15
7
+ p
1
1
C
12
6
+ He
4
2
b Fe
56
26
+ H
2
1
He
4
2
+ Mn
54
25
c Li
6
3
+ n
1
0
H
3
1
+ He
4
2
d Co
59
27
+ H
2
1
Co
60
27
+ p
1
1
e Cr
53
24
+ He
4
2
Mn
57
25
+ n
0
1
4 The nuclear transformations in a, b, d and e would all take place in a cyclotron, as the
charged particles are used to bombard the nucleus.
Nuclear transformation c would take place in a nuclear reactor, as it involves
bombardment by neutrons.
5 Fe
56
26
+ H
2
1
Mn
54
25
+ He
4
2
Chapter 5 Application and investigation
1 a 90 protons; 140 neutrons
b 96 protons; 148 neutrons
c 77 protons; 115 neutrons
2 Chemical reactions involve changes in the arrangements of electrons around the nucleus.
Nuclear reactions involve changes within the nucleus.
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
3 a Ionisation counter: uses a tube filled with an inert gas. As radiation enters the tube it
causes the gas to be ionised, forming cations and free electrons. These particles are
attracted to electrodes of the opposite charge, causing a current to flow, which is
heard as an audible click and can be recorded as a digital readout.
b Scintillation counter: electrons in some substances, such as zinc sulfide, are excited
by radiation and emit photons of light when the electrons return to a lower energy
state. The flashes of light are counted electronically to measure the amount of
radiation.
4 a decay
b decay, positron emission, electron capture
5 a Pu
241
94
U
237
92
+ He
4
2
b Rn
210
86
Fr
210
87
+ e
0
1
c V
48
23
Ti
48
22
+ e
0
1
d Cd
107
48
+ e
0
1
Ag
107
47
e U
234
92
Th
230
90
+ He
4
2
f Na
26
11
Mg
26
12
+ e
0
1
g N
12
7
C
12
6
+ e
0
1
h
6 Uranium and thorium undergo alpha emission spontaneously. Therefore helium is found
within the ore.
7 Elements with high neutron-to-proton ratios emit -particles, thereby raising the number
of protons and lowering the number of neutrons. Elements with low neutron-to-proton
ratios undergo either positron emission or electron capture, thereby raising the number of
neutrons and lowering the number of protons.
8 a unstable; decay
b unstable; positron emission or electron capture
c unstable; positron emission or electron capture
d unstable; decay
e stable
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
f unstable; decay
9 a 3 half-lives mg 1 . 0
2
0.2
; 2 . 0
2
0.4
; 4 . 0
2
8 . 0
= = =
b 2 half-lives 12.3 2 = 24.6 years
10 a 35%
b 20% 500 g = 100 g remaining
c 84 days
d 25 days
11 a 32 days is 4 half-lives
0.625 mg remaining after 32 days
b 5 half-lives drop to 0.312 g, so < 0.5 g in 36 days
12
13 Investigation
14 a Co
59
27
+ n
1
0
Co
60
27
b
60
Co would be made in a nuclear reactor as it involves the capture of a neutron. In a
nuclear reactor, atoms are bombarded with neutrons. A cyclotron is used to
bombard atoms with charged particles.
15 Investigation
16 a Cu
63
29
+ H
2
1
Zn
65
30
+
b N
14
7
+ He
4
2
O
17
8
+ H
1
1
c Ca
40
20
+ H
2
1
K
38
19
+ He
4
2
d Pu
239
94
+ He
4
2
Cm
242
96
+ n
1
0
17 Investigation
18 particles can cause serious damage to cells.
19 Investigation
20 Investigation
21 Research based on information gathered in Questions 19 and 20
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Module 1
REVIEW
1 D
2 A
3 D
4 D
5 D
6 A
7 D
8 B
9 B
10 A
11 a Ethylene is more reactive due to the presence of the double bond.
b addition
c polyethylene
d HI
12 a
b vinyl chloride
i chloroethene
ii polyvinylchloride (PVC)
iii PVC is a thermoplastic with long chains with weak dispersion forces between
them. This allows the polymer to be heated and remoulded. With additives,
PVC can be used as a rigid plastic (e.g. guttering, water pipes) or as a flexible
plastic (e.g. garden hoses).
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
13 a LDPE has a greater degree of branching between its polymer chains, reducing the
dispersion forces between the chains. HDPE has less branching, resulting in a
stronger and more dense plastic.
b cling wrap, insulation for wires
c LDPE: Organic peroxides are used as catalysts to attack the ethene double bond and
form a covalent bond with a carbon atom. This new molecule then attacks another
ethene molecule, causing the chain to grow (propagate). Branches are formed when
the chain curls back and removes a hydrogen from a carbon in the chain, which will
in turn react with another ethene molecule. The reaction terminates when two
polymer radicals react together.
HDPE: Ionic catalysts (ZieglerNatta catalysts) are used. Ethene molecules are
added to the polymer on the surface of the catalyst, which reduces the amount of
branching.
d The polymer chains in HDPE are packed tightly together due to less branching of
the chains. This gives HDPE greater strength and toughness but reduces its
flexibility.
14 a Biomass is organic material derived from living organisms, such as: plant material
(e.g. sugar and cellulose); animal material (e.g. dung); and domestic and industrial
organic waste.
b -glucose
c Cellulose is a formed as a condensation polymer when -glucose monomers
combine with the removal of a water molecule. Polyethylene forms as an addition
polymer from ethylene monomers as the double bond breaks.
15 Use spirit burners containing each fuel, weigh mass before and after use (to give mass of
fuel burnt), heat same volume of water, record temperature rise. Fuels are volatile and can
easily ignite if care is not taken.
16 a i C
6
H
12
O
6
(aq) 2C
2
H
5
OH(l) + 2CO
2
(g)
ii anaerobic (no oxygen); temperature 3540
o
C
b Harvest cane, extract sugars, ferment to ethanol using yeast, warmth and anaerobic
conditions.
c C
2
H
5
OH(g) C
2
H
4
(g) + H
2
O(g)
The reaction requires a catalyst, e.g. concentrated H
2
SO
4
17 No commercially viable method of producing ethanol from crops is yet available. Fuel
mixtures containing greater than 15% ethanol require some modification to the car before
being used.
Ethanol is a renewable fuel and petroleum is not.
Solutions Manual: Module 1
Production of materials
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
18 a
b Cell potential = +0.76 0.13 = 0.63 V
c Zinc
19 Uranium-238 is bombarded with neutrons to form einsteinium.
U
238
92
+ n 15
1
0
Es
253
99
+ e 7
0
1
20 a
b Sr is in the same periodic group as calcium and therefore is chemically similar.
c 85.3 years is three half-lives > 0.125 g remains at 85 years.
21 a Iodine-131 is used in the diagnosis of thyroid misfunction and in treatment of
thyroid tumours.
b I-131 is produced by neutron bombardment in a reactor.
c Benefit: short half-life and therefore is only in the body for a short time.
Problem: I-131 can destroy some healthy cells when used for radiation.
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
CHAPTER 6: ACIDS, BASES AND INDICATORS
Review exercise 6.1
1 a acetic acid, CH
3
COOH
citric acid, 2-hydroxypropane-1,2,3-tricarboxylic acid
b sulfuric acid, H
2
SO
4
nitric acid, HNO
3
c magnesium hydroxide, Mg(OH)
2
2 Own answers
3 Own answers
4 a Zn(s) + 2H
+
(aq) Zn
2+
(aq) + H
2
(g)
b CO
3
2
(aq) + 2H
+
(aq) CO
2
(g) + H
2
O(l)
c 2H
+
(aq) + K
2
O(s) 2K
+
(aq) + H
2
O(l)
d H
+
(aq) + OH
(aq) H
2
O(l)
5 a 2Cr(s) + 2OH
(aq) + 6H
2
O(l) 2[Cr(OH)
4
]
(aq) + 3H
2
(g)
b Cr(OH)
3
(s) + OH
(aq) [Cr(OH)
4
]
(aq)
6 Acid solutions have excess hydrogen ions and base solutions have excess hydroxide
ions. These ions are free to move and carry a current.
Review exercise 6.2
1 a K
2
O ionic bonding; Ga
2
O ionic; Br
2
O
7
covalent
b K
2
O basic; Ga
2
O amphoteric; Br
2
O
7
acidic
2 CO
2
(g) + H
2
O(l) H
2
CO
3
(aq)
3 Natural sources of sulfur dioxide:
bacteria decomposing organic matter to make H2S, which is then oxidised
atmospherically.
volcanic gases
bushfire smoke.
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Sources due to human activities:
burning of fossil fuels
smelting of sulfide ores such as CuFeS
2
and ZnS.
4 SO
2
H
2
SO
3
; SO
3
H
2
SO
4
; NO
2
HNO
3
; CO
2
H
2
CO
3
5 Increased incidence of low pH in lakes and rivers and continued damage to metal and
sandstone buildings.
6 The sulfur oxides in the atmosphere usually come from burning fossil fuels in power
stations or smelting sulfide ores in smelting plants. Nitrogen oxide and carbon
emissions usually come from motor vehicles. Therefore acid rain usually occurs in
densely populated areas or industrial areas.
Review exercise 6.3
1 a Acidic. The solution has a pH between 4 and 6.
b The pH range is 4 to 8. This solution could be neutral, acidic or basic.
c The solution is slightly basic. The pH is between 7.5 and 8.
2 a Bromothymol blue: yellow; phenolphthalein: colourless; methyl orange: red
b Bromothymol blue: blue; phenolphthalein: pink; methyl orange: yellow
c Bromothymol blue: blue; phenolphthalein: colourless; methyl orange: yellow
Chapter 6 Application and investigation
1 Not all acids are dangerous. Acids are present in foods we eat, e.g. ethanoic acid in
vinegar, lactic acid in milk. Also, acids are found in our bodies, e.g. lactic acid,
hydrochloric acid. The concentration of acids should be considered.
2 a 2Al(s) + 6HCl(aq)
2AlCl
3
(aq) + 3H
2
(g)
b KHCO
3
(s) + HNO
3
(aq)
KNO
3
(aq) + CO
2
(g) + H
2
O(l)
c Fe
2
O
3
(s) + 3H
2
SO
4
(aq)
Fe
2
(SO
4
)
3
(aq) + 3H
2
O(l)
d Ba(OH)
2
(aq) + 2HF(aq)
BaF
2
(aq) + 2H
2
O(l)
3 a 2Al(s) + 2NaOH(aq) + 6H
2
O(l)
2Na[Al(OH)
4
](aq) + 3H
2
(g)
b KOH(aq) + Fe(OH)
3
(s)
no reaction
c 2KOH(aq) + Zn(OH)
2
(s)
K
2
[Zn(OH)
4
](aq)
d 2NaOH(aq) + SO
3
(g)
Na
2
SO
4
(aq) + H
2
O(l)
4 a KHC
4
H
4
O
6
+ NaHCO
3
KNaC
4
H
4
O
6
+ CO
2
(g) + H
2
O(l)
b Fe
2
O
3
(s) + 6HCl(aq)
2FeCl
3
(aq) + 3H
2
O(l)
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
c Al(OH)
3
(s) + 3HCl(aq)
AlCl
3
(aq) + 3H
2
O(l)
d H
2
SO
3
(aq) + CaCO
3
(s)
CaSO
3
(aq) + CO
2
(g) + H
2
O(l)
5 Al(OH)
3
reacts with both acids and bases, and therefore is amphoteric.
Reacting with acid: Al(OH)
3
(aq) + 3HCl(aq) AlCl
3
(aq) + 3H
2
O(l)
Reacting with base: Al(OH)
3
(aq) + NaOH(aq) Na [Al(OH)
4
](aq)
6 Investigation
7 Investigation
8 A useful indicator is one whose colour change occurs over a selected pH range. Methyl
orange changes colour too far outside the narrow 7.27.6 range for swimming pools.
Litmus changes colour over this range, but the colour difference in the 7.27.6 range
may be too subtle to detect easily. Universal indicator undergoes many colour changes
and the change over the 7.27.6 range, from yellow-green to green may also be too
subtle to detect easily. Bromothymol blue could be a useful indicator. The change for
phenolphthalein is above a pH of 7.6 and so this indicator would not be useful.
9 Investigation
10 Acid rain has a pH less than 5; methyl orange when yellow means pH is greater than
4.5, and litmus when red indicates a pH of less than 7. Therefore the pH of the sample
of rain water lies between 4.5 and 7, and so is unlikely to be classified as acid rain.
11 a Na
2
O, Al
2
O
3
, SiO
2
, P
4
O
10
, Cl
2
O
7
b K
2
O, Ga
2
O
3
, GeO
2
, As
2
O
3
, Br
2
O
7
12 CO
2
(aq) + H
2
O(l) H
2
CO
3
(aq) carbonic acid
4NO
2
(g) + 2H
2
O(l) + O
2
(g) 4HNO
3
(aq) nitric acid
SO
2
(g) + H
2
O(l) H
2
SO
3
(aq) sulfurous acid
Cl
2
O
7
(l) + H
2
O(l) 2HClO
4
perchloric acid
13 a SO
2
(g) + H
2
O(l) H
2
SO
3
(aq)
b H
2
SO
3
(aq) + CaCO
3
(s) CaSO
3
(aq) + CO
2
(g) + H
2
O(l)
14 Investigation
15 Investigation
16 Investigation
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
CHAPTER 7: CHEMICAL EQUILIBRIUM
Review exercise 7.1
1 a NH
3
(g) + HCl(g) NH
4
Cl(s)
b 2SO
2
(g) + O
2
(g) 2SO
3
(g)
c Ag
+
(aq) + Fe
2+
(aq) Ag(s) + Fe
3+
(aq)
d 2NH
3
(g) N
2
(g) + 3H
2
(g)
2 a Ammonia molecules are reacting with hydrogen chloride molecules to form
ammonium chloride at the same rate at which the ammonium chloride is
decomposing to ammonia and hydrogen chloride.
b Sulfur dioxide reacts with oxygen to form sulfur trioxide at the same rate at which
the sulfur trioxide decomposes to produce sulfur dioxide and oxygen.
c Silver ions are reduced to silver at the same rate at which the iron(III) is reduced
to iron(II).
d Ammonia decomposes to nitrogen and hydrogen at the same rate at which
hydrogen and nitrogen combine to form ammonia.
3 a If 0.21 mol L
1
H
2
remains, 0.79 mol L
1
reacted.
Molar ratio of H
2
: HI is 1 : 2
0.79 2 = 1.58 moles HI formed
equilibrium conc HI = 1.58 mol L
1
b i and ii
c Forward and reverse reactions will occur at equal but opposing rates. The
concentrations of reactants and products remain constant.
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
4 In an open container, the CO
2
would escape and the reverse reaction would not occur. In
a sealed container, increasing the temperature would increase the pressure until
equilibrium was established.
Review exercise 7.2
1 a i [NO], [H
2
O], [NH
3
]
ii Will increase rate of forward reaction until equilibrium is re-established.
b i [NH
3
], [O
2
], [H
2
O]
ii Will increase rate of forward reaction until equilibrium is re-established.
2 a i Concentration of H
2
O will decrease, concentration of CO, H
2
will increase.
No change to [C(s)].
ii Will increase rate of forward reaction. This increases concentration of CO
and H
2
, decreases [H
2
O].
b i Concentration of H
2
O will increase, concentration of CO, H
2
will decrease.
No change to [C(s)].
ii Will increase rate of reverse reaction. [CO] , [H
2
] and [H
2
O] until
equilibrium is re-established.
3 a i [CO], [O
2
], [CO
2
]
ii Will increase rate of reverse reaction.
b i [CO], [O
2
], [CO
2
]
ii Will increase rate of forward reaction.
4 a The system would reach equilibrium more quickly.
b Both forward and reverse rates would increase equally, therefore no change.
Review exercise 7.3
1 CO
2
(g) CO
2
(aq)
CO
2
(aq) + H
2
O(l) H
2
CO
3
(aq)
H
2
CO
3
(aq) H
+
(aq) + HCO
3
(aq)
HCO
3
(aq) H
+
(aq) + CO
3
2
(aq)
2 a When the bottles are opened, the pressure in the system is decreased, causing a
shift in the equilibrium to the left, where there are more moles of gas and so the
change is counteracted. This results in bubbles of CO
2
(g).
b Cooling the drink shifts the equilibrium to the right (exothermic direction),
meaning that more carbon dioxide will be dissolved into the solution. When the
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
drink is served cold, the rate at which CO
2
(g) escapes to the atmosphere is
decreased.
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Chapter 7 Application and investigation
1 a Changes in concentration
b Rate of reaction
2 No further change in the colour of the mixture would be detected.
3 At equilibrium, the concentration of all species remains constant, therefore there will be
no change in any observable properties. The dynamic process refers to the equal but
opposite reactions occurring at the molecular level.
4 concentration, pressure, temperature
5 a [NO]; [O
2
]; [NO
2
]
b Concentration of all species will double.
c [O
3
]; [O
2
]; [NO
2
]
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
6 a [HBr]; [O
2
]; [H
2
O]; [Br
2
]
b [HBr]; [O
2
]; [H
2
O]; [Br
2
]
7 a i Shift equilibrium to the right.
ii Shift equilibrium to the left.
b i Shift equilibrium to the left.
ii Shift equilibrium to the right.
8 a Ca(HCO
3
)
2
(s); CaO(s); [H
2
O]; [CO
2
] after initial increase of CO
2
b Ca(HCO
3
)
2
(s); CaO(s); [CO
2
]; [H
2
O] after initial decrease of H
2
O
c Ca(HCO
3
)
2
(s); CaO(s); [CO
2
]; [H
2
O]
d no change
e Ca(HCO
3
)
2
(s); CaO(s); [CO
2
]; [H
2
O]
9 a i Equilibrium will shift right to counteract the change: [NO
2
]; [SO
3
];
[NO].
ii The forward reaction rate will increase.
b i There are equal numbers of gaseous molecules on each side, so pressure will
increase the concentration of all species, but the system will remain at
equilibrium.
ii Forward and reverse reactions increase equally.
c i Concentrations of all species will decrease, but the system will remain at
equilibrium.
ii Forward and reverse reaction rates decrease equally.
d i The equilibrium will shift left in an endothermic direction: [SO
2
],
[NO
2
], [SO
3
], [NO].
ii Reverse reaction is favoured.
e i No change as a catalyst does not affect the position of equilibrium.
ii Both forward and reverse reaction rates will increase.
10 a Pressure is decreased, so equilibrium will shift to counteract the change.
Therefore CO
2
(g) is produced and bubbles form.
b It is an exothermic reaction, so warm temperatures cause the equilibrium to shift
to the left and form CO
2
(g).
c The H
+
in the orange juice causes increased production of carbonic acid.
HCO
3
(aq) + H
+
(aq) H
2
CO
3
(aq)
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
The [H
2
CO
3
] causes the production of more CO
2
(aq):
H
2
CO
3
(aq) CO
2
(aq) + H
2
O(l)
Therefore the [CO
2
(aq)] results in the production of more CO
2
(g) causing drinks
to go flat, as follows:
CO
2
(aq) + 19kJ CO
2
(g)
11 Investigation
CHAPTER 8: THE HISTORICAL DEVELOPMENT OF IDEAS OF ACIDS AND
BASES
Review exercise 8.1
1 a SO
2
(g) + H
2
O(l) H
2
SO
3
(aq)
b Na
2
O(s) + H
2
O(l) 2NaOH(aq)
2 Hydrochloric acid, HCl; hydrobromic acid, HBr; cyanic acid, HCN
3 Arrhenius theory states that bases are substances which deliver hydroxide ions in water.
NaCN is a substance that acts as a base but does not contain a hydroxide group.
4 a H
2
SO
4
(aq) 2H
+
(aq) + SO
4
2
(aq)
or H
2
SO
4
(aq) HSO
4
(aq) + H
+
(aq), then HSO
4
(aq) SO
4
2
+ H
+
(aq)
b HNO
3
(aq) H
+
(aq) + NO
3
(aq)
5 a 1
b 2
c 3
d 1
e 1
f 1
6 a 1
b 3
c 2
7 HOOCCOOH(aq)
HOOCCOO
(aq) + H
+
(aq)
HOOCCOO
(aq)
OOCCOO
(aq) + H
+
(aq)
Review exercise 8.2
1 a A BL acid is a proton donor (or has a proton pulled from it).
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
b A BL base is a proton acceptor (or takes a proton).
2 a HCl, HCO
3
, NH
4
+
, HClO
4
, HSO
4
b F
, SO
4
2
, NH
3
, PO
4
3
, H
2
O
3 a An amphiprotic substance can either accept or donate a proton, and therefore it
can act as both an acid and a base.
b HPO
4
2
(aq) + H
2
O(l)
H
2
PO
4
(aq) + OH
(aq)
HPO
4
2
(aq) + H
2
O(l)
PO
4
3
(aq) + H
3
O
+
(aq)
4 Acid HSO
4
(aq) + OH
(aq)
SO
4
2
(aq) + H
2
O(l)
Base HSO
4
(aq) + H
3
O
+
(aq)
H
2
SO
4
(aq) + H
2
O(l)
5 a A conjugate base is the species that results when a BL acid has lost its proton
b A conjugate acid is the species that results when a BL base accepts a proton
6 a HCO
3
/SO
4
2
: HF/F
b HSO
4
/SO
4
2
: NH
4
+
/NH
3
c HF/F
: H
3
O
+
/H
2
O
Review exercise 8.3
1 a i HCl
ii HCl
iii HCl
b i H
2
SO
4
ii H
2
SO
4
iii H
2
SO
4
2 a i NaOH
ii NaOH
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
iii NaOH
b i Ba(OH)
2
ii Ba(OH)
2
iii Ba(OH)
2
3 a 5 mol L
1
HCl
b 0.1 mol L
1
NaOH
c 5 mol L
1
NH
3
d 0.1 mol L
1
CH
3
COOH
4 a HBr(aq)
H
+
(aq) + Br
(aq)
b H
2
SO
3
(aq)
HSO
3
(aq) + H
+
(aq)
c RbOH(aq)
Rb
+
(aq) + OH
(aq)
d NaF(aq)
Na
+
(aq) + F
(aq)
5 a small extent
b large extent
c small extent
Review exercise 8.4
1 a S
2
(aq) + H
2
O(l) HS
(aq) + OH
(aq)
b CO
3
2
(aq) + H
2
O(l) HCO
3
(aq) + OH
(aq)
c NH
4
+
(aq) + H
2
O(l) NH
3
(aq) + H
3
O
+
(aq)
d [Fe(H
2
O)
6
]
3+
(aq) + H
2
O(l) [Fe(OH)(H
2
O)
5
]
2+
(aq) + H
3
O
+
(aq)
e F
(aq) + H
2
O(l) HF(aq) + OH
(aq)
f HSO
4
(aq) + H
2
O(l) SO
4
2
(aq) + H
3
O
+
(aq)
g ClO
+ H
2
O HClO + OH
h CH
3
COO
(aq) + H
2
O(l) CH
3
COOH(aq) + OH
(aq)
2 Use Table 8.5.
a neutral
b acidic due to reaction of NH
4
+
ion with water
c basic due to reaction of ClO
ion with water
d basic due to reaction of PO
4
3
ion with water
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
e acidic due to reaction of Al
3+
ion (actually will be aquated aluminium ion
[Al(H
2
O)
6
]
3+
with water)
f acidic due to reaction of H
2
PO
4
ion with water
g neutral
h basic due to reaction of CO
3
2
ion with water
Chapter 8 Application and investigation
1 According to Arrhenius, acids are substances that release hydrogen ions in solution.
HCl(aq) completely ionises in water to produce H
+
and Cl
, and therefore HCl(aq) is an
Arrhenius acid. HCl(l) cannot release hydrogen ions and so is not an Arrhenius acid.
2 a HCl(aq)
H
+
(aq) + Cl
(aq)
b Ca(OH)
2
(aq)
Ca
2+
(aq) + 2OH
(aq)
c H
2
SO
4
(aq)
HSO
4
(aq) + H
+
(aq)
HSO
4
(aq)
SO
4
2
(aq) + H
+
(aq)
d H
2
SO
4
(aq) + 2NaOH(aq)
Na
2
SO
4
(aq) + 2H
2
O(l)
e Al(OH)
3
(aq) + 3HCl(aq)
AlCl
3
(aq) + 3H
2
O(l)
3 a 4
b 2
c 2
d CH
2
(COOH)
2
(aq) + 2NaOH(aq) CH
2
(COONa)
2
(aq) + 2H
2
O(l)
4 a HCl(aq) + H
2
O(l)
H
3
O
+
(aq) + Cl
(aq)
b CH
3
COOH(aq) + H
2
O(l)
H
3
O
+
(aq) + CH
3
COO
(aq)
c NH
3
(aq) + H
2
O(l)
NH
4
+
(aq) + OH
(aq)
d H
2
SO
4
(aq) + H
2
O(l)
H
3
O
+
(aq) + HSO
4
(aq)
HSO
4
(aq) + H
2
O(l)
H
3
O
+
(aq) + SO
4
2
(aq)
e HCl(aq) + NaOH(aq)
NaCl(aq) + H
2
O(l)
5 a ClO
3
, S
2
, NH
3
, OH
b H
2
CO
3
, H
2
S, [Fe(H
2
O)
6
]
3+
, N
2
H
5
+
6 a As acid: HCO
3
(aq) + H
2
O(l)
CO
3
2
(aq) + H
3
O
+
(aq)
As base: HCO
3
(aq) + H
2
O(l)
H
2
CO
3
(aq) + OH
(aq)
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
b The extent to which the HCO
3
ion reacts as a base with water exceeds the degree
to which it reacts with water as an acid. (K value is greater for HCO
3
as a base.)
Hence more OH
ions are produced than H
3
O
+
ions.
7 a i H
2
C
2
O
4
/HC
2
O
4
, H
3
O
+
/H
2
O
ii small extent
b i H
2
O/OH
, HCN/CN
ii small extent
c i CH
3
COOH/CH
3
COO
, HS
/S
2
ii large extent
d i HCl/Cl
, HF/F
ii large extent
8 a HClO
4
(l) + H
2
O(l)
ClO
4
(aq) + H
3
O
+
(aq)
b HCOOH(l) + H
2
O(l)
HCOO
(aq) + H
3
O
+
(aq)
c LiOH(s) + H
2
O(l)
Li
+
(aq) + OH
(aq)
d N
2
H
4
(l) + H
2
O(l)
N
2
H
5
+
(aq) + OH
(aq)
9 a concentrated solution of a strong acid
b diluted solution of a strong acid
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
c concentrated solution of a weak acid
d diluted solution of a weak acid
10 a CH
3
COO
(aq) + H
2
O(l) CH
3
COOH(aq) + OH
(aq)
b The hydroxide ions produced raise the pH above 7. The ethanoic acid molecule
formed has only a very small degree of dissociation.
11 a neutral
b basic PO
4
3
(aq) + H
2
O(l)
HPO
4
2
(aq) + OH
(aq)
c basic CO
3
2
(aq) + H
2
O(l)
HCO
3
(aq) + OH
(aq)
d acidic NH
4
+
(aq) + H
2
O(l)
NH
3
(aq) + H
3
O
+
(aq)
e acidic [Cr(H
2
O)
6
]
3+
(aq) + H
2
O(l)
[Cr(OH)(H
2
O)
5
]
2+
(aq) + H
3
O
+
(aq)
f basic SO
4
2
(aq) + H
2
O(l)
HSO
4
(aq) + OH
(aq)
g basic CN
(aq) + H
2
O(l)
HCN(aq) + OH
(aq)
h acidic NH
4
+
(aq) + H
2
O(l)
NH
3
(aq) + H
3
O
+
(aq)
12
Acid Base Neutralisation
Arrhenius produces H
+
ions
in water
produces OH
ions
in water
H
+
+ OH
H
2
O
BrnstedLowry proton donor proton acceptor NH
3
(aq) + H
2
ONH
4
+
+ OH
proton transfer
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
13 The reaction: HCl(g) + NH
3
(g) NH
4
Cl(s) can be viewed as a BrnstedLowry acid
base reaction because a proton is transferred. However, the reaction is not happening in
solution and there are no hydroxide ions released, so the reaction cannot be viewed as
an Arrhenius acidbase reaction. When nitric acid is mixed with sulfuric acid, for the
manufacture of nitroglycerine, the reaction that occurs is
H
2
SO
4
+ HNO
3
HSO
4
+ H
2
NO
3
+
This is a BL acidbase reaction because a proton is transferred but it is not an
Arrhenius acidbase reaction.
14 Investigation
CHAPTER 9: HYDROGEN ION CONCENTRATION AND THE pH SCALE
Review exercise 9.1
1 a K
W
= [H
+
] [OH
]
1.0 10
14
= [H
+
] 1.6 10
7
[H
+
] = 6.2 10
8
mol L
1
b [H
+
] is less than 1 10
7
mol L
1
pool is slightly basic
2 a [H
+
] = 5.0 10
3
mol L
1
[NO
3
] = 5.0 10
3
mol L
1
[OH
] = 1 10
14
5.0 10
3
= 2 10
12
mol L
1
b [H
+
] = 1.5 mol L
1
[Cl
] = 1.5 mol L
1
[OH
] = 1 10
14
1.5
= 6.7 10
15
mol L
1
c [K
+
] = 0.25 mol L
1
[OH
] = 0.25 mol L
1
[H
+
] = 4 10
14
mol L
1
d [Ba
2+
] = 6.0 10
2
mol L
1
[OH
] = 0.12 mol L
1
[H
+
] = 8.3 10
14
mol L
1
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Review exercise 9.2
1 a pH = log[H
+
]
= log[5.0 10
1
]
= 0.30
b [H
+
] = 1 10
14
0.0065
= 1.54 10
12
mol L
1
pH = log[1.54 10
12
]
= 11.81
c pH = log[3.6 10
3
]
= 2.44
d [H
+
] = 1 10
14
(6.5 10
4
2)
= 7.7 10
12
mol L
1
pH = log[7.7 10
12
]
= 11.1
2 a pH = 3.50; [H
+
] = 3 10
4
mol L
1
; [OH
] = 3.16 10
11
mol L
1
b pH = 11.90; [H
+
] = 1.3 10
12
mol L
1
; [OH
] = 7.9 10
3
mol L
1
c pH = 0.80; [H
+
] = 0.16 mol L
1
; [OH
] = 6.3 10
14
mol L
1
3 pH = 7 so [H
+
] = 1 10
7
pH = 5 so [H
+
] = 1 10
5
pH changes by factor of 100
Review exercise 9.3
1 a HClO
2
b HClO
2
c HClO
2
, HF, HCN
d No. Complete ionisation of 0.1 mol L
1
gives pH = 1.
Review exercise 9.4
1 a A buffer is a species that resists changes to pH despite small additions of acid or
base.
b A buffer contains approximately equal amounts of a weak acid and its conjugate
base (some are a mixture of weak bases and their conjugate acids).
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
2 a Buffered; contains approximately equal amounts of the weak acid HSO
4
and its
conjugate base SO
4
2
.
b Not buffered; HNO
3
is a strong acid.
c Buffered; contains approximately equal amounts of the weak acid HClO and its
conjugate base ClO
.
3 a HPO
4
(aq) + H
3
O
+
(aq) H
2
PO
4
(aq) + H
2
O(l)
When excess hydrogen ions are added, the system shifts to the right and thus
decreases the H
+
concentration.
b H
2
PO
4
+
(aq) + OH
(aq) HPO
4
(aq) + H
2
O(l)
When excess hydroxide is added, the system shifts to the right and thus decreases
the OH
concentration.
4 The concentration of the acid and conjugate base ions is limited. If too much H
+
or OH
is added then the system is unable to provide sufficient acid or conjugate base particles
to counteract their effect.
5 Lactic acid in the blood produces lactate ions and hydrogen ions. The bloods buffering
system H
2
CO
3
(aq) + H
2
O(l) HCO
3
(aq) + H
3
O
+
(aq) responds by combining the extra
hydrogen ions with the hydrogencarbonate ions, thus shifting the system to the left and
decreasing their concentration.
Chapter 9 Application and investigation
1 a [H
+
] = 0.5 mol L
1
[OH
] = 1 10
14
0.5
= 2 10
14
mol L
1
pH = log[0.5]
= 0.30
b [OH
] = 3.0 10
3
2
= 6.0 10
3
mol L
1
[H
+
] = 1 10
14
0.006
= 1.67 10
12
mol L
1
pH = log[1.67 10
12
]
= 11.8
2 a [H
+
] = 1.0 10
3
mol L
1
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
[OH
] = 1 10
14
0.001
= 1 10
11
mol L
1
b [H
+
] = 3.9 10
3
mol L
1
[OH
] = 2.51 10
12
mol L
1
c [H
+
] = 3.2 10
9
mol L
1
[OH
] = 3.2 10
6
mol L
1
d [H
+
] = 7.9 10
13
mol L
1
[OH
] = 1.3 10
2
mol L
1
3 a i [H
+
] = 0.20 mol L
1
[NO
3
] = 0.20 mol L
1
[HNO
3
] = 0 mol L
1
ii pH = log
10
[0.20]
= 0.70
b As HNO
2
is a weak acid, it will not dissociate completely. Therefore [H
+
] will be
less than 0.20 mol L
1
and pH will be higher than 0.70.
4 H
2
CO
3
, H
3
PO
4
, HClO
2
, H
2
SO
3
, HCl
5 Investigation
6 Investigation
CHAPTER 10: VOLUMETRIC ANALYSIS AND ESTERIFICATION
Review exercise 10.1
1 a NaOH(aq) + HCl(aq)
NaCl(aq) + H
2
O(l)
b 2NaOH(aq) + H
2
SO
4
(aq)
Na
2
SO
4
(aq) + 2H
2
O(l)
c 2NaOH(aq) + H
2
CO
3
(aq)
Na
2
CO
3
(aq) + H
2
O(l)
d 3NaOH(aq) + H
3
PO
4
(aq)
Na
3
PO
4
(aq) + H
2
O(l)
2 a 2HCl(aq) + Ca(OH)
2
(aq)
CaCl
2
(aq) + 2H
2
O(l)
b 3HCl(aq) + Al(OH)
3
(s)
AlCl
3
(aq) + 3H
2
O(l)
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
c 2HCl(aq) + MgO(s)
MgCl
2
(aq) + H
2
O(l)
d 2HCl(aq) + Na
2
CO
3
(s)
2NaCl(aq) + CO
2
(g) + H
2
O(l)
3 Neutralisation is the result of an acidbase interaction. In BrnstedLowry terms this
refers to proton transfer from the acid to the base.
4 Neutralisation results in the formation of a new bond as the proton bonds to its base.
Bond formation is an exothermic process, and thus neutralisation is an exothermic
process.
5 a Add solid sodium hydrogencarbonate until the fizzing stops. The reaction
produces carbon dioxide, so when the fizzing stops, this indicates that the
neutralisation is complete.
b Rinse with copious amounts of water. A base should not be used, as the
neutralisation reaction is exothermici.e. it releases a lot of heat.
Review exercise 10.2
1 2OH
(aq) + H
2
SO
4
(aq) 2H
2
O(l) + SO
4
2
(aq)
Calculate moles of OH
n(OH
) = cV
= 1.90 0.02368
= 0.045 moles
moles of H
2
SO
4
: n(H
2
SO
4
) = 0.5n(OH
) (from equation)
= 0.0225 moles
concentration of H
2
SO
4
n(H
2
SO
4
) = cV
0.0225 = c 0.005
c = 4.50 mol L
1
2 NaOH(aq) + HCl(aq) NaCl(aq) + H
2
O(l)
Calculated moles of HCl
n = cV
= 0.02052 0.952
= 0.019535 moles
moles of HCl = moles NaOH (from equation)
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
concentration of NaOH =
V
n
= 0.019535 / 0.005
c = 3.91 mol L
1
NaOH
3 a Pipettes are more accurately calibrated than a measuring cylinder; this accuracy is
important in volumetric analysis.
b If rinsed with water, any drops left in the pipette or burette will alter the
concentration of the solution and this will affect the results. Rinsing with the
solution will not have this effect.
c Volume may change slightly once the solid is dissolved; the customary unit of
concentration is mol/L, where the volume refers to litres of solution, not water.
4 a No. moles Na
2
CO
3
= 0.250 0.1
= 0.025 moles
moles =
M
m
so 0.25 =
99 . 105
m
m = 2.65 g of Na
2
CO
3
is dissolved in water in a 250 mL
volumetric flask with solution made up to the
calibrated mark
b n = cV
nH
2
C
2
O
4
.2H
2
O = 0.5 0.015
= 7.5 10
3
moles
n =
M
m
7.5 10
3
=
07 . 126
m
m = 0.95 g oxalic acid is dissolved in water in a 500 mL
volumetric flask with the solution made up to the
calibrated mark
5 a n =
M
m
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
=
07 . 126
3162 . 0
= 0.0025 moles
n = cV
0.0025 = c 0.25
c = 0.010 mol L
1
H
2
C
2
O
4
.2H
2
O
b 2NaOH(aq) + H
2
C
2
O
4
(aq) Na
2
C
2
O
4
(aq) + 2H
2
O(l)
n(H
2
C
2
O
4
) = cV
n = 0.010 0.02061
= 2.068 10
4
moles H
2
C
2
O
4
n(OH
) = 2 nH
2
C
2
O
4
(from equation)
n(OH
) = 4.136 10
4
mol
n(NaOH) = cV
4.136 10
4
= c 0.020
c = 0.0207 mol L
1
NaOH
c NaOH(aq) + HCl(aq) NaCl(aq) + H
2
O(l)
n(NaOH) = cV
n = 0.0207 0.020
= 4.14 10
4
mol NaOH
volume of HCl used = 20.26 1.83
= 18.43 mL
= 0.01843 L
nHCl = nNaOH from equation
n(HCl) = 4.14 10
4
moles
nHCl = cV
4.14 10
4
= c 0.01843
c = 2.24 10
2
HCl mol L
1
Review exercise 10.3
1 a i NaF(aq)
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
ii basic
iii phenolphthalein
b i KNO
3
(aq)
ii neutral
iii methyl orange/phenolphthalein
c i NaCl(aq)
ii acidic
iii methyl orange
2 i a Phenolphthalein; as a strong acid/strong base titration and endpoint will be
around 7.
b Colourless in acid changing to pink as base added, with pale pink at
equivalence point.
ii a Methyl orange; as strong acid/weak base titration and endpoint will be less
than pH 7.
b Yellow in base and changing to orange at equivalence point, and to red as
point is passed.
iii a Bromothymol blue; weak acid/strong base titration and endpoint will be
greater than pH 7.
b Yellow in acid changing to blue as base added, with pale yellow at
equivalence point.
3 This is a weak acid/strong base titration with an equivalence point above pH 7. If
methyl orange was used as an indicator, a colour change would be observed at around
pH 3.14.4 and an equivalence point assumed, so the volume of hydroxide recorded
would be too low.
4 a Running this strong base into the weak acid.
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
b Running this weak base into the strong acid.
c Running this strong base into the strong acid.
Review exercise 10.4
1 a n =
71 . 22
V
=
71 . 22
5 . 2
= 0.11 mol H
2
b n =
M
m
0.11 =
016 . 2
m
= 0.22 g hydrogen gas
2 a n =
M
m
=
32
0 . 48
= 1.5 mol
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
n =
71 . 22
V
1.5 =
71 . 22
V
v = 34.07 L at 0C and 100 kPa
b n =
79 . 24
V
1.5 =
79 . 24
V
v = 37.19 L at 25C and 100 kPa
3 Zn(s) + 2HCl(aq) ZnCl
2
(aq) + H
2
(g)
1 2 1 2
a nH
2
79 . 24
V
n =
79 . 24
5 . 2
= 0.1 moles H
2
From equation nZn = 0.1 (nH
2
)
= 0.1 moles
nZn =
M
m
0.1 =
4 . 65
m
= 6.54 g Zn required
b From equation nHCl = 2 nH
2
= 2 0.1
= 0.2 moles
nHCl = cV
0.2 = 1.5 V
V = 0.13 L HCl required
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
4 CaCO
3
(s) + 2HNO
3
(aq) Ca(NO
3
)
2
(aq) + CO
2
(g) + H
2
O(l)
1 2 1 1 1
a nCaCO
3
=
M
m
=
1 . 100
10
n = 0.10 moles
From equation nCaCO
3
= nCO
2
nCO
2
= 0.10 moles
nCO
2
=
71 . 22
V
0.1 =
71 . 22
V
V = 2.271 L CO
2
at 0C and 100 kPa
b nCO
2
=
79 . 24
V
0.1 =
79 . 24
V
V = 2.48 L CO
2
at 25C and 100 kPa
c From equation nHNO
3
= 2 nCaCO
3
nHNO
3
= 2 0.1
= 0.2 moles
nHNO
3
= cV
0.2 = 1.00 V
V = 0.2 L HNO
3
required
d From equation nCaCO
3
= nCa(NO
3
)
2
nCa(NO
3
)
2
= 0.10 moles
nCa(NO
3
)
2
=
M
m
0.1 =
1 . 164
m
m = 16.41 g of Ca(NO
3
)
2
produced
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Review exercise 10.5
1 a 1-propanol
b 1-butanol
c 2-butanol
2 a
b
c
3 a pentanoic acid
b 5-methylhexanoic acid
4 a
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
b
5 They form more H-bonds because they have a C=O group as well as an OH group,
making two H-bonds possible between two molecules.
6 a methyl butanoate
b ethyl methanoate
7 a
b
8 a
b
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
c
d
e
f
9 a C
3
H
7
OH + CH
3
COOH
conc. H
2
SO
4
CH
3
COOC
3
H
7
+ H
2
O
The ester is propyl ethanoate.
b C
5
H
11
OH + C
3
H
7
COOH
conc. H
2
SO
4
C
3
H
7
COOC
5
H
11
+ H
2
O
The ester is pentyl butanoate.
10 a H
2
SO
4
acts as a catalyst.
b Refluxing prevents volatile reactants from escaping before equilibrium is reached
and allows the reaction to be carried out at temperatures lower than would
normally be possible.
Chapter 10 Application and investigation
1 a 2HCl(aq) + CuO(s)
CuCl
2
(aq) + H
2
O(l)
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
b 3H
2
SO
4
(aq) + 2Fe(OH)
3
(s)
Fe
2
(SO
4
)
3
(aq) + 6H
2
O(l)
c 2HNO
3
(aq) + CaCO
3
(s)
Ca(NO
3
)
2
(aq) + CO
2
(g) + H
2
O(l)
d HCl(aq) + NaHCO
3
(aq)
NaCl(aq) + H
2
O(l) + CO
2
(g)
2 a i Na
+
(aq), SO
4
2
(aq), OH
(aq), H
+
(aq), H
2
O(l)
ii H
+
(aq), Na
+
(aq), OH
(aq) all at 2 mol L
1
b i H
+
(aq), SO
4
2
(aq), H
2
O(l)
ii H
+
(aq)
c i Na
+
(aq), SO
4
2
(aq), H
2
O(l)
ii Na
+
(aq), SO
4
2
(aq)
3 a i Pipette: used to place an accurate volume of solution into a conical flask.
ii Burette: used to deliver variable volumes into a conical flask.
iii Conical flasks: used to mix two solutions.
b i citric acid
ii NaOH
iii deionised water
4 Sodium hydroxide cannot be made as a primary standard and hence must be
standardised by titration with an acid of accurately known concentration.
5 a A good primary standard has a relatively high molecular weight to minimise
weighing errors, does not react with any component of the atmosphere such as
oxygen, moisture or carbon dioxide, and is available in a very pure form.
b Investigation
6 a nNaHCO
3
= cV = 2 1
= 2 moles
n =
M
m
2 =
008 . 84
m
m = 168 g
b nNa
2
CO
3
= cV
n = 0.05 0.5
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
= 0.025 moles
n =
M
m
0.025 =
m = 2.65 g
c nBa(OH)
2
.8H
2
O = cV
n = 2 10
3
2
= 0.004
n =
M
m
m = 0.004 315.444
= 1.26 g
d nH
2
C
2
O
4
.2H
2
O = cV
n = 0.150 0.25
= 0.0375 moles
n =
M
m
m = 0.0375 126.068
= 4.728 g
7 a NaOH(aq) + HNO
3
(aq) NaNO
3
(aq) + H
2
O(l)
nHNO
3
= cV
= 0.0961 0.02
= 1.9 10
3
From equation nNaOH = nHNO
3
= 1.9 10
3
nNaOH = cV
1.9 10
3
= 0.118 V
V = 1.62 10
2
L
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
b 2NaOH(aq) + H
2
SO
3
(aq) Na
2
SO
3
(aq) + 2H
2
O(l)
nH
2
SO
3
= cV
= 0.0531 0.02
= 1.062 10
3
moles
nNaOH = 2 nH
2
SO
3
from equation
2 1.062 10
3
= 0.118 V
V = 18 10
3
L
c 2NaOH(aq) + H
2
C
2
O
4
.2H
2
O(s) Na
2
C
2
O
4
(aq) + H
2
O(l)
nH
2
C
2
O
4
.2H
2
O
=
M
m
=
126
134 . 0
= 1 10
3
moles
2 n(oxalic acid) = n(NaOH)
n = cV
2 10
3
= 0.118 V
V = 16.95 10
3
L
8 a H
2
SO
4
(aq) + 2NaOH(aq) Na
2
SO
4
(aq) + 2H
2
O(l)
nNaOH = cV
= 0.5 0.02
= 0.01 moles
nH
2
SO
4
= 0.5nNaOH
= 0.005 moles H
2
SO
4
n = cV
V =
202 . 0
005 . 0
= 2.48 10
2
L
b H
2
SO
4
(aq) + Ba(OH)
2
BaSO
4
(aq) + 2H
2
O(l)
nBa(OH)
2
= 0.02 0.165
= 0.0033 moles
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
nBa(OH)
2
= nH
2
SO
4
nH
2
SO
4
= 0.0033 = 0.202 V
V = 1.63 10
2
L
c Na
2
CO
3
(s) + H
2
SO
4
(aq) Na
2
SO
4
(aq) + CO
2
(g) + H
2
O(l)
nNa
2
CO
3
=
M
m
=
106
343 . 0
= 3.24 10
3
moles
nNa
2
CO
3
= nH
2
SO
4
nH
2
SO
4
: 3.24 10
3
= 0.202 V
V = 1.60 10
2
L
d total nOH
= nOH
in Ca(OH)
2
+ nOH
in NaOH
nCa(OH)
2
=
M
m
=
1 . 74
0746 . 0
= 1 10
3
moles
moles OH
= 1 10
3
2
= 2 10
3
moles
nNaOH =
M
m
=
40
247 . 0
= 6.2 10
3
moles OH
Total nOH
= 2 10
3
+ 6.2 10
3
= 8.18 10
3
moles
nH
2
SO
4
= nOH
0.5
= 4.08 10
3
moles
n = cV
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
= 4.08 10
3
= 0.202 V
VH
2
SO
4
= 2.02 10
2
L
9 a HCl(aq) + NaOH(aq) NaCl(aq) + H
2
O(l)
nHCl = cV
= 0.121 0.02565
= 3.1 10
3
moles
nHCl = nNaOH
nNaOH = cV
3.1 10
3
= c 0.020
c = 0.16 mol L
1
b Ba(OH)
2
(aq) + 2HCl(aq) BaCl
2
(aq) + 2H
2
O(l)
nHCl = cV
= 0.0503 0.01523
= 7.66 10
4
moles
nBa(OH)
2
= 0.5nHCl
= 0.5 7.66 10
4
= 3.83 10
4
moles
Ba(OH)
2
= 3.83 10
4
0.02
= 1.92 10
2
mol L
1
10 a H
2
SO
4
(aq) + CuO(s) CuSO
4
(aq) + H
2
O(l)
nCuO =
M
m
=
5 . 79
05 . 1
= 0.013 moles
nCuO = nH
2
SO
4
nH
2
SO
4
= 0.13 moles = 2.0 V
V = 6.6 10
3
L
b 1 mole H
2
SO
4
1 mole CuSO
4
.5H
2
O
nCuSO
4
.5H
2
O = 0.013 moles
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
n =
M
m
0.013 =
6 . 249
m
m = 3.24 g
11 Investigation
12 A known mass of the tablets could be dissolved in 20 mL of water. This solution could
be titrated against HCl of known concentration to determine the concentration of
NaHCO
3
.
13 nNaOH = cV
= 0.119 0.02053
= 2.4 10
3
moles
n vitamin C = 2.44 10
3
moles
n =
M
m
2.44 10
3
=
176
m
m = 0.4294 g
% in capsule = 100
450 . 0
4294 . 0
= 95.4%
14 a NaOH + HCl NaCl + H
2
O
1 1
nHCl = cV
= 0.125 0.01759
= 0.0022 moles
nNaOH = nHCl
= 0.0022 moles
c =
020 . 0
0022 . 0
=
V
n
= 0.11 mol L
1
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
NaOH + CH
3
COOH NaCH
3
COO + H
2
O
nNaOH = cV
= 0.11 0.03812
= 4.2 10
3
moles
nNaOH = nCH
3
COOH
= 4.2 10
3
nCH
3
COOH = 4.2 10
3
=
M
m
4.2 10
3
=
60
m
m = 0.25 g in 5.00 mL sample vinegar
b % mass = 0.25/5.00 100
= 5% ethanoic acid in vinegar
15 NH
3
(aq) + HCl(aq) NH
4
Cl(aq)
nHCl = cV
= 0.110 0.02462
= 2.7 10
3
moles
nNH
3
= 2.7 10
3
= cV
2.7 10
3
= c 0.02
c = 0.135 mol L
1
ammonia
Number of moles NH
3
present in 250 mL
n = cV
= 0.135 0.25
= 0.339 moles
n =
M
m
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
0.339 =
17
m
m = 0.575 g ammonia in 9.97 g
% mass ammonia in household ammonia
= 100
97 . 9
575 . 0
= 5.77%
16 Na
2
CO
3
+ H
2
O + 2HCl(aq) 2NaCl(aq) + 2H
2
O(l) + CO
2
(g)
nHCl = cV
= 0.104 0.02520
= 2.62 10
3
moles
nNa
2
CO
3
= 0.5nHCl
nNa
2
CO
3
= 0.5 2.62 10
3
= 1.31 10
3
moles
nNa
2
CO
3
=
M
m
1.31 10
3
=
106
m
m = 0.1389 g
Mass of water in washing soda = 0.374 0.1389
= 0.235 g H
2
O
nH
2
O =
M
m
n =
18
235 . 0
n = 0.0131 moles
Ratio of nNa
2
CO
3
: nH
2
O =
1.31 10
3
: 1.31 10
2
=
1 : 10
Number of molecules of water of crystallisation = 10
i.e. Na
2
CO
3
.10H
2
O
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
17 The pH at the equivalence point is below 7 due to the reaction of NH
4
+
produced at the
equivalence point reacting with H
2
O to form H
3
O
+
. Phenolphthalein changes colour
above 7, and therefore it would not be appropriate.
18 a i Na
+
, CH
3
COO
, H
2
O, CH
3
COOH, OH
ii basic
iii phenolphthalein
b i KCl(aq), H
2
O
ii neutral
iii bromothymol blue
c i NH
4
+
, NO
3
, NH
3
, H
3
O
+
ii acidic
iii methyl orange
d i BaCl
2
(aq), H
2
O
ii neutral
iii bromothymol blue
e i H
2
CO
3
(aq), NaCl, H
3
O
+
, HCO
3
ii acidic
iii methyl orange
19 a i nO
2
=
71 . 22
V
0.500 =
71 . 22
V
V = 11.36 L O
2
at 0C and 100 kPa
ii nO
2
=
79 . 24
V
0.500 =
79 . 24
V
V = 12.4 L O
2
at 25C and 100 kPa
b i nN
2
=
71 . 22
V
=
71 . 22
2
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
= 0.09 moles N
2
at 0C and 100 kPa
ii nN
2
=
79 . 24
V
=
79 . 24
10
= 0.40 moles N
2
at 25C and 100 kPa
20 a i nCl
2
=
71 . 22
V
=
71 . 22
3
= 0.13 moles
nCl
2
=
M
m
0.13 =
71
m
m = 9.5 g Cl
2
at 0C and 100 kPa
ii nCl
2
=
79 . 24
V
79 . 24
3
= 0.12 moles
n =
M
m
0.12 =
71
m
m = 8.7 g Cl
2
at 25C and 100 kPa
b i nCO
2
=
M
m
=
44
55
= 1.25 mol
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
n =
71 . 22
V
1.25 =
71 . 22
V
V = 28.39 L CO
2
at 0C and 100 kPa
ii nCO
2
= 1.25 mol
n =
79 . 24
V
1.25 =
79 . 24
V
V = 30.99 L CO
2
at 25C and 100 kPa
21 a nFeS
2
=
M
m
=
120
1000
= 8.33 mol
From equation, FeS
2
:O
2
equals 1:2.75
nO
2
= 2.75 nFeS
2
= 2.75 8.33
= 22.92 mol O
2
nO
2
=
71 . 22
V
22.92 =
71 . 22
V
V = 520.5 L O
2
at 0C and 100 kPa
b i nSO
2
= 2 nFeS
2
= 2 8.33
= 16.67 moles
At 0C and 101.3 kPa:
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
16.67 =
71 . 22
V
V = 378.6 L SO
2
produced at 0C and 100 kPa
ii At 25C and 101.3 kPa:
16.67 =
79 . 24
V
V = 413.3 L SO
2
produced at 25C and 100 kPa
22 a CH
3
CH
2
CH
3
; CH
3
CH
2
CH
2
OH; CH
3
CH
2
COOH
b propane 41C
1-propanol 97C
propanoic acid 141C
Propane has only weak dispersion forces between molecules lowest boiling
point. 1-propanol has strong H-bonds between molecules but propanoic acid has
stronger intermolecular forces due to two H-bonds between acid molecules.
23 a
b
24 a Reflux ethanol with butanoic acid with concentrated H
2
SO
4
as a catalyst as an
esterification process
b Reflux pentanol with propanoic acid with concentrated H
2
SO
4
as a catalyst as an
esterification process
Refluxing is necessary so that none of the volatile components of the reaction are lost
while this slow, low-yielding reaction proceeds.
25 Investigation
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Module 2
REVIEW
1 A
2 D
3 D
4 B
5 D
6 A
7 B
8 C
9 A
10 B
11 a CH
3
COOH(aq) + NaOH(aq) CH
3
COONa(aq) + H
2
O(l)
b nNaOH = 0.095 0.02
= 1.9 10
3
moles
Average =
3
0 . 19 8 . 18 9 . 18 + +
= 18.9 mL
nCH
3
COOH = 1.9 10
3
= c 0.0189
c = 0.1005 mol L
1
acetic acid
c c
1
V
1
(original) = c
2
V
2
(volumetric flask)
c 0.02 = 0.1005 0.2
c = 1.005 mol L
1
d Phenolphthalein; pH at end point will be above 7.
e Distilled water. The number of moles of vinegar added will not be affected by
water in the flask.
12 NH
3
(g) + H
2
O(l) NH
4
+
(aq) + OH
(aq)
The OH
causes the solution to be basic.
NH
4
+
(aq) + H
2
O(l) NH
3
(aq) + H
3
O
+
(aq)
The H
3
O
+
causes the solution to be acidic.
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
13 a HCl is a strong acid which dissociates completely and therefore has high
conductivity and low pH. Acetic acid is weak and therefore only slightly
dissociates, causing a higher pH and lower conductivity.
b The CH
3
COO
reacts with H
2
O to form some OH
ions.
c Greater number of ions are present in the solution in Beaker 3.
14 a Bacteria can decompose organic matter to produce H
2
S, which then oxidises to
SO
2
. Burning of fossil fuels and smelting of sulfide ores results in sulfur
compounds which are oxidised to SO
2
.
b 2CuFeS
2
(s) + 5O
2
(g) + 2SiO
2
(s) 2Cu(l) + 4SO
2
(g) + 2FeSiO
3
(l)
c i SO
3
(g) + H
2
O(l) H
2
SO
4
(aq) sulfuric acid
ii Lakes and waterways become acidic, killing aquatic life. Soil can become
acidic, affecting plant growth. Limestone can be dissolved by the action of
acid rain. These are all of concern if we want to protect our environment.
iii Student opinion
15 a Both molecules have an OH group but ethanoic acid contains a C=O group,
which increases the intermolecular forces. Even though 1-butanol has more
dispersion forces than acetic acid, acetic acid has more H-bonding and so the
intermolecular forces are very nearly the same in each substance.
b i CH
3
COOH(aq) + C
4
H
9
OH(l) CH
3
COOC
4
H
9
(aq) + H
2
O(l)
Catalyst is conc. H
2
SO
4
.
ii H
2
SO
4
acts as a catalyst.
c Refluxing allows the reaction mixture to be boiled without losing the volatile
reactants and increases the time over which this quite slow reaction can occur.
d Acetic acid would be more soluble than 1-butanol. Compound X would be least
soluble.
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
e
16 a i Equilibrium shifts to the left and more CO
2
(g) is produced
the drink goes flat.
ii More space above the liquid means that pressure is lower and CO
2
(aq)
CO
2
(g) and the drink will be flat.
b The soft drink could be carefully poured into a beaker and gently heated. If
bubbles of CO
2
are observed forming in the solution (which they should be), this
would indicate that the equilibrium is shifting to the left (CO
2
(g) CO
2
(aq)). As
the addition of heat favours the reverse reaction, it follows that this reaction must
be endothermic. Therefore the forward reaction must be exothermic.
Alternatively:
Take an unopened bottle of soft drink. Immediately upon opening it, insert a
thermometer (at the same initial temperature as the drink) and observe any change
in temperature. Bubbles of CO
2
gas should be observed forming in the solution,
indicating that the equilibrium is shifting to the left (as written). If a temperature
decrease is recorded (which it should), this will indicate that the reverse reaction
is endothermic, as heat is being absorbed from the surroundings (the drink itself).
Therefore the forward reaction must be exothermic.
17 a An acid loses an H
+
to become its conjugate base,
e.g. C
6
H
5
N
2
O
5
(aq) C
6
H
4
N
2
O
5
(aq).
b Yellow. The CN
would react with the H
3
O
+
, causing the equilibrium to shift to
the right.
CN
(aq) + H
3
O
+
H
2
O(l) + HCN(aq)
Solutions Manual: Module 2
The acidic environment
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
c i Yes: strong acidstrong base titration. pH = 7 at end point and the pH
changes from 11 to 3 very rapidly.
ii Yes: weak basestrong acid; pH < 7 at end point.
iii No: weak acidstrong base; pH > 7 at end point.
18 a [H
+
] = 0.12 mol L
1
pH = log
10
[H
+
] = log
10
[0.12]
pH = 0.92
b Mg(OH)
2
(aq) + 2HCl(aq) MgCl
2
(aq) + 2H
2
O(l)
The antacid reacts with excess acid to produce salt and water.
19 a Curve 1
b The acetate ion reacts with water to form OH
and CH
3
COOH, raising the pH at
equivalence point. There is no such reaction in the NaCl(aq) produced at the
equivalence point where pH = 7.
c Starting pH is higher for acetic acid due to less dissociation.
20 a CH
3
COOH(aq) CH
3
COO
(aq) + H
+
(aq)
If CH
3
COO
is added then the equilibrium will shift left, resulting in less H
+
and
a higher pH.
Also CH
3
COO
(aq) + H
2
O(l) CH
3
COOH(aq) + OH
(aq). The presence of OH
ions raises the pH.
b Buffering systems result from the action of a weak acid in approximately equal
concentration with its conjugate base. Acetic acid is a weak acid and the acetate
ions from the sodium acetate would be close in concentration to the acetic acid.
Thus the resulting solution could act as a buffering system.
21 n (SO
2
) = 2 n (CuFeS
2
)
= 2 mass (CuFeS
2
) molar mass (CuFeS
2
)
Vol (SO
2
) = 2 mass (CuFeS
2
) molar mass (CuFeS
2
) molar vol
SATP
= 2 500 10
3
183.54 24.79
= 135066 L
22 a Decreased pressure would cause the equilibrium to shift to the left. This would
result in the formation of CaCO
3
(s) and CO
2
(g).
b Change in temperature. Increased temperature would cause this exothermic
reaction to shift to the left to counteract the change. This would cause a decrease
in [Ca
2+
].
Solutions Manual: Module 3
Chemical monitoring and management
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
CHAPTER 11: THE ROLE OF CHEMISTS AND CHEMICAL ANALYSIS
Review exercise 11.1
1 Most products used in the home, car etc, e.g. shampoo, medications, food additives,
laundry detergents
2 Investigation
3 Energy industry, mineral industry, petrochemical industry
Review exercise 11.2
1 Qualitative analysis identifies substances present in a sample.
Quantitative analysis determines the amount of each substance present.
2 Treating substances with electromagnetic radiation and measuring the amount of
absorption or emission.
3 a AAS is most useful for determining concentrations of cations in samples. The
sample to be tested is vaporised in a flame. A beam of light emitted by a cathode
lamp of the element being investigated is shone through the vaporised sample.
The element absorbs that wavelength of radiation and the remaining light is
analysed to determine the intensities remaining. By comparing to known samples
gathered under the same conditions, concentrations can be identified.
b AAS is a relatively cheap and rapid process that can detect very low
concentrations (ppm and ppb) of elements, and therefore is useful in detecting
trace elements.
4 Concentration of Pb in solution = 5.5 mg kg
1
(from the graph)
Mass of Pb in solution = 5.5 0.1
= 0.55 mg
Concentration of Pb in soil =
002 . 0
55 . 0
= 275 ppm
Review exercise 11.3
1 Their electrons occupy different energy levels and will absorb then emit light of
different frequencies when they fall back from higher to lower energy levels.
2 a i Ba
2+
ii Add to H
2
SO
4
solution; a white precipitate of BaSO
4
(s) will form.
Solutions Manual: Module 3
Chemical monitoring and management
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
b Add HCl to the solution. Ca
2+
ions will have no reaction if a precipitate forms,
Pb
2+
is present.
3 a i CO
3
2
ii Add HCl to form CO
2
(g) and confirm CO
2
(g) by turning limewater milky.
b Add AgNO
3
. AgCl(s) is white, Ag
3
PO
4
(s) is yellow. When HNO
3
is added,
Ag
3
PO
4
will dissolve.
Review exercise 11.4
1 nAl
2
O
3
=
M
m
=
102
153 . 0
= 1.5 10
3
moles
nAl = 2 nA1
2
O
3
= 2 1.5 10
3
= 3 10
3
moles
mass Al = 3 10
3
27
= 0.081 g
% mass Al in sample = 100
516 . 0
081 . 0
= 15.7%
2 a nBaSO4 =
M
m
=
4 . 233
01 . 2
= 8.6 10
3
moles
nSO
4
2
= nS = nBaSO
4
= 8.6 10
3
moles
mass SO
4
2
= 8.6 10
3
96.1
= 0.83 g
% mass SO
4
2
= 100
23 . 1
83 . 0
= 67.3%
Solutions Manual: Module 3
Chemical monitoring and management
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
mass S = 8.6 10
3
32.1
= 0.28 g
% mass S = 100
23 . 1
28 . 0
= 22.5%
b % NH
4
+
= 100 67.3 10.1
= 22.6%
22.6% of 1.23 g = 0.28 g
nNH
4
+
=
= 1.5 10
2
moles
mass N = 1.5 10
2
14
= 0.22 g
% mass N = 100
23 . 1
22 . 0
= 17.6%
3 n(NaOH) = [HCl] vol
HCl
= 0.1690 30.15 10
3
= 0.00509525 mol in 10 mL after dilution
= 0.00509525 mol in 1 mL before dilution
[NaOH] = 0.00509525 1 10
3
= 5.1 mol/L
Chapter 11 Application and investigation
1 The industrial process would involve a much larger scale using a great deal more
reactants. It would require monitoring to ensure production was economical. The
process would be continually reviewed and refined.
2 Investigation
3 Investigation
4 Investigation
5 Investigation
Solutions Manual: Module 3
Chemical monitoring and management
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
6 a If chemists are asked, for example, to participate in an environmental impact
study for a particular area then they will need to collaborate to present a
comprehensive report, as no one chemist can be a specialist in all the areas of
analysis that would be required.
b A chemist might not collaborate if asked to do a simple analytical task in which
they have great expertise.
7 a
b 3.25 mg L
1
8 Absorbance = 0.720
Concentration of mercury from curve = 0.290 ppm = 0.290 mg/kg
Mass of mercury in 100 mL
(assume 1 mL = 1 g)
= 0.290 0.1 = 0.029 mg
Concentration of mercury in tuna =
002 . 0
029 . 0
mg/kg
= 14.5 ppm
9 a nAgC1 =
4 . 143
276 . 0
n = 1.9 10
3
moles
nAgC1 = nNaC1
nNaC1 = 1.9 10
3
moles
mass NaCl = 1.9 10
3
58.5
= 0.113 g
Solutions Manual: Module 3
Chemical monitoring and management
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
b % mass of salt in chips =
100
5 . 7
113 . 0
= 1.5%
10
6.25 ppm
11 The cation is most likely Pb
2+
. This can be confirmed by adding potassium iodide
solution. If a bright yellow precipitate forms, then the cation is Pb
2+
.
12 a Anion could be CO
3
2
. Confirm by adding acidif a gas is produced that turns
limewater cloudy, then can conclude that carbonate is present.
b CO
3
2
and SO
4
2
. Confirm presence of carbonate by bubbling gas through
limewater; positive test if limewater turns cloudy.
13 Investigation
14 2MnO
4
(aq) + 5C
2
O
4
2
(aq) + 16H
+
(aq) 2Mn
2+
(aq) + 10CO
2
(g) + 8H
2
O(l)
nMnO
4
= cV
= 0.05 0.03820
= 1.91 10
3
moles
nC
2
O
4
2
= 1.91 10
3
2.5
= 4.78 10
3
moles
c C
2
O
4
2
=
02 . 0
10 78 . 4
3
= 0.239 mol L
1
nC
2
O
4
2
= cV
in 100 mL = 0.239 0.1
Solutions Manual: Module 3
Chemical monitoring and management
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
= 0.0239 moles
nCa
2+
= 0.0239 moles
mass CaCO
3
= 0.0239 100.1
= 2.39 g
% mass CaCO
3
in limestone =
05 . 4
39 . 2
100
= 59.01 %
15 Investigation
CHAPTER 12: MONITORING AND MANAGING CHEMICAL INDUSTRIES
Review exercise 12.1
1 Investigation
2 a Advantages: labour supply is close; transport costs are lower.
b Disadvantages: waste disposal; social and safety issues.
Review exercise 12.2
1 a CH
4
(g) + 2O
2
(g) CO
2
(g) + 2H
2
O(l)
b CH
4
(g) +
2
3
O
2
(g) CO(g) + 2H
2
O(l)
2 Closing windows and doors can limit the supply of oxygen in the room. Incomplete
combustion could occur, producing pollutants such as carbon monoxide and soot.
3 To ensure that complete combustion occurs; to ensure that engine efficiency is
maintained; to ensure that harmful pollutants are reduced.
Review exercise 12.3
1 Raising the temperature; increasing concentration of reactants; using catalysts.
2 High temperatures are expensive to maintain (catalysts are preferable).
3 Reaction yield is the proportion of product formed compared to the maximum amount
that could be formed by complete reaction of the reactants.
4 Batch processing involves set amounts of reactants forming a product, which is
removed when the reaction is complete. Continuous processing involves a production
line method where reactants are added and products removed continuously.
Solutions Manual: Module 3
Chemical monitoring and management
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Review exercise 12.4
1 a Increasing temperature would cause the equilibrium to shift left (endothermic
direction) will decrease yield.
b Greater pressure would cause a shift to the side with fewer gaseous molecules
equilibrium will shift right and increase yield.
c Removal of NH
3
will cause the equilibrium to shift right to counteract the change,
thus increasing yield.
2 Temperature 500C, high pressures (35 000 kPa), ironiron oxide catalyst, removal of
ammonia. These conditions are a compromise between rate, equilibrium yield and
economic factors.
3 fertilisers, metal extraction, cleaning agents, manufacture of synthetic fibres,
manufacture of explosives.
Chapter 12 Application and investigation
1 Investigation
2 It has been argued that if the industrial synthesis of ammonia had not been developed by
the Germans during the early 1900s then they would have been unable to produce
sufficient munitions to support their troops and fertilisers to feed their population and
they would have surrendered sooner.
3 High pressure will favour the products and increase yield of NO
2
.
High temperature will favour reactants and decrease yield of NO
2
.
Catalyst will maintain a higher rate of reaction.
Conditions of moderate temperature, high pressure and a catalyst would be used.
4 CH
4
(g) + H
2
O(g) 3H
2
(g) + CO(g) + H
Low pressure and high temperature.
Low pressure would shift equilibrium to the right to the side with more gaseous
molecules increase yield of H
2
.
High temperature would shift equilibrium to the right as reaction is endothermic
increase yield of H
2
.
Additional aspects include the use of a catalyst, transport and storage of gases under
high pressure, the explosive nature of hydrogen gas and flammability of methane gas.
The source of methane should be nearby and the poisonous by-product, CO,
released/treated appropriately.
5 Investigation
6 Investigation
Solutions Manual: Module 3
Chemical monitoring and management
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
7 a At 300C the rate of reaction is too slow and the yield is not produced quickly
enough. With the use of a catalyst, the reaction rate is increased despite increasing
temperature (500C) decreasing the yield.
b High pressures favour the production of NH
3
according to Le Chateliers
principle. Therefore yield is increased at 35 000 kPa.
c Continuous removal of small quantities of ammonia ensures that the reaction rate
to products is preferred. Equilibrium between products and reactants is not
attained.
8 Fast reaction rates are generally the most economical, and for the Haber process
reactions can be speeded up by the use of a catalyst, especially as this allows lower
temperatures to be used, thus increasing the yield. The Haber process does not allow
equilibrium to be reached, as products are continually being removed and reactants
added, so there is a constant drive to the right and a continuous yield.
9 Investigation
10 Investigation
CHAPTER 13: MONITORING AND MANAGING OUR ATMOSPHERE
Review exercise 13.1
1 The temperature falls throughout the troposphere to 50C then rises throughout the
stratosphere to 10C. Temperature drops to about 100C throughout the mesosphere
then rises to over 25C in the thermosphere.
2 Gases in the atmosphere trap heat being radiated into space from Earth. Therefore more
gases in the atmosphere will cause a temperature increase on Earth.
3 Nitrogen is used by plants and animals to manufacture protein. Oxygen is used in
respiration, the breakdown of sugars to produce energy in all living things. Carbon
dioxide is used in plants to make glucose by photosynthesis.
4 lead, hydrocarbons, carbon monoxide, carbon dioxide, nitrogen oxides
5 Nitrogen combines with oxygen in high temperature combustion conditions to form
NO(g). This combines with more oxygen to produce nitrogen dioxide. Given approriate
environmental UV conditions, the colourless nitrogen dioxide converts to brown
dinitrogen tetroxide, which gives photochemical smog its characteristic colour.
Review exercise 13.2
1 a O
2
: double covalent bond between the two atoms.
O
3
: a V-shaped molecule that can be viewed as having a double covalent bond
between two atoms and a coordinate covalent bond between two atoms. However,
it appears to actually exist as a resonance structure with delocalised electrons.
Solutions Manual: Module 3
Chemical monitoring and management
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
b O
2
is a highly reactive oxidising agent.
O
3
is a very highly reactive oxidising agent.
2 O
3
occurs naturally in the troposphere to a limited extent but is found in photochemical
smog and used as an indicator for this. Concentrations above these low levels are
regarded as polluting and can cause biological harm.
O
3
in the stratosphere absorbs damaging UV radiation. It is formed from the reaction of
O
2
with UV.
3 no difference
4 a
b
c
d
Solutions Manual: Module 3
Chemical monitoring and management
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
5
These molecules do not have even numbers of valence electrons, so all electrons cannot
form pairs. The octet rule is not followed.
Review exercise 13.3
1 Haloakanes: Any hydrocarbon with one or more halogen attached.
CFCs: All the hydrogens are replaced with chlorine and fluorine.
HCFCs: All but one hydrogen is replaced with chlorine and fluorine.
HFCs: Hydrocarbon with fluorine and chlorine attached.
Halons: CFCs with bromine atoms.
Examples:
haloalkane: CH
3
I iodomethane
CFC: CC1
3
F freon-11
HCFC: CHC1F
2
HFC: C
2
H
2
F
4
halon: CBrC1F
2
2 a i 1-chlorooctane
ii 2-bromo-3-fluoropentane
iii 1,1-dichloropropane
iv 5-bromo-1,4-dichloro-2,2-difluorohexane
b i
ii
Solutions Manual: Module 3
Chemical monitoring and management
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
iii
iv
3 Production of ozone from O
2
and oxygen atoms, and decomposition of ozone, occur
continuously in the stratosphere using UV radiation.
4 a The ozone layer prevents harmful ultraviolet radiation from reaching Earth and
causing damage to living things and delicate ecosystems.
b The decomposition of ozone is catalysed by substances like NO and Cl. Pollution
containing such substances causes greater decomposition of O
3
and therefore
depletion of the ozone layer.
Solutions Manual: Module 3
Chemical monitoring and management
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Chapter 13 Application and investigation
1 Troposphere, stratosphere, mesosphere, thermosphere
The troposphere and stratosphere contain gases that are essential for life, CO
2
and O
2
,
and play a role in the water cycle. They absorb UV radiation before it reaches Earth and
infrared radiation from Earth.
2 CO
2
is used by plants in the making of glucose (photosynthesis).
O
2
is used in respiration, i.e. breakdown of sugars to give energy.
N
2
is used in the manufacture of protein in living things.
3 Investigation
4 The Earth has gases which contribute to warming due to natural causes, e.g. H
2
O, CH
4
,
CO, CO
2
. These gases absorb UV radiation and reradiate it as heat. Human activities
have increased the concentrations of some of these gases, thereby enhancing the
warming effect.
5 Investigation
6 a i
ii
iii
Solutions Manual: Module 3
Chemical monitoring and management
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
iv
b i
ii
iii
iv
7 a Decreases in the ozone concentration of the stratosphere over Antarctica,
particularly in spring, have been recorded.
b CFCs are unreactive molecules which eventually break down in the stratosphere
to produce chlorine atoms. Chlorine atoms catalyse the decomposition of ozone.
8 Investigation
9 a Oxygen O
2
: Used in the breakdown of glucose to produce energy in living things.
Ozone O
3
: Absorbs UV radiation in the stratosphere.
Solutions Manual: Module 3
Chemical monitoring and management
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
b O
2
Double covalent bond
between two oxygen
atoms
O
3
Central oxygen atom joined
on one side to another
oxygen atom by a double
covalent bond, and on the
other by a coordinate
covalent bond.
O
2
Oxygen ion has two negative
charges due to an extra two
electrons in the valence shell of
the atom.
10 a O
2
(g) + UV
2O(g)
O
2
(g) + O(g)
O
3
(g)
b O
2
(g)
(electrical energy) 2O(g)
O
2
(g) + O(g)
O
3
(g)
c NO
2
(g) + UV radiation
NO(g) + O(g)
O
2
(g) + O(g)
O
3
(g)
d O
2
(g) + UV
2O(g)
O
2
(g) + O(g)
O
3
(g)
11 A coordinate covalent bond forms when one atom provides both electrons for the shared
pair between atoms forming a bond. In this example both electrons are provided by N.
12 Investigation
13 a Warm, sunny day with still air. Oxides of nitrogen. Hydrocarbons.
b UV radiation is absorbed by NO
2
. A series of reactions leads to the production of
ozone. Then, unburned hydrocarbons react with ozone to produce organic
compounds such as aldehydes, e.g. ethanal.
14 Investigation
15 Investigation
Solutions Manual: Module 3
Chemical monitoring and management
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
CHAPTER 14: MONITORING AND MANAGING OUR WATERWAYS
Review exercise 14.1
1 When the top of the lake freezes, the warmer water below is more dense, and so it stays
lower.
2 Ions in sea water are the result of leaching from rocks and soils into rivers and then into
seas.
3 The concentration of ions in sea water is higher than that found in fresh water, as most
rivers run into seas. The concentrations are determined by:
prevalence in the Earths crust
solubility
the degree to which they are extracted by living things.
The ions found in fresh water are dependent on the source of the water and the geology
of the catchment area. Therefore their concentrations will vary considerably.
Review exercise 14.2
1 a Evaporate a filtered sample of water and weigh the remaining solids, or measure
electrical conductivity of the solution.
b To determine levels of Mg
2+
and Ca
2+
ions, the sample is titrated against
ethylenediaminetetraacetic acid (EDTA). A simple lather test using soap flakes
will give an indication of the relative hardness of water.
c A turbidity disk is lowered into the water until it can no longer be seen. This
measures the suspended solids in the sample. Turbidity can also be determined by
shining a beam of light through a sample and detecting the amount of transmitted
light.
d Acidity is determined using indicator paper or a pH meter, or by titration.
2 Dissolved oxygen measures the solubility of O
2
in the sample. Low levels indicate
pollution.
Biochemical oxygen demand assesses the capacity of the organic matter in the sample to
use the oxygen present.
Review exercise 14.3
1 Fertiliser run-off, animal waste and sewage.
2 Excessive forest clearing, irrigation.
3 While most heavy metals are essential to life as trace elements, at higher concentrations
they pose a substantial health risk, particularly as they are frequently cumulative
poisons and may give few symptoms at a low level of poisoning.
Solutions Manual: Module 3
Chemical monitoring and management
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Review exercise 14.4
1 Flocculation, clarification, filtering, chlorination and fluoridation.
2 To strengthen the enamel on childrens teeth and help protect against tooth decay.
3 Gravity, vacuum or pressure pumps force water through a membrane containing small
pores. The membranes block the passage of particles, therefore purifying the water.
4 Water is forced under pressure to move from a more concentrated solution into a less
concentrated one.
5 The stearate ion in soap bonds with the Ca
2+
and Mg
2+
ions in hard water, forming
scum. This means that the soap does not work effectively at cleaning.
6 Temporary hard water can be boiled to remove hardness. Permanent hard water needs
to be treated with softeners to reduce its hardness.
Review exercise 14.5
1
2 Acute water shortages would necessitate the use of tertiary treatment of waste water for
recycling.
Solutions Manual: Module 3
Chemical monitoring and management
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Chapter 14 Application and investigation
1 Investigation
2 They are prevalent in the Earths crust; they are highly soluble and are not extracted
from the water by living things.
3 Salinity of the oceans is increased by dissolved salts being added by rivers. The excess
salts are removed by formation of coral and limestone reefs and nutrient and waste
exchange in living things. Therefore salinity level remains constant.
4 Mass of Cl
in 3.5 g = 60% 3.5
= 2.1 g Cl
ions in 100 g
water
nCl
=
M
m
=
5 . 35
1 . 2
= 5.92 10
2
moles in 100 g
nCl
in 1 litre = 5.92 10
2
10
= 0.59 moles in 1 litre sea
water.
5 1.35 g = 1350 mg NaCl
ppm (mg/kg) =
3
1350
= 450 ppm
6 Oxygen in waterways is provided by photosynthesis in aquatic plants and from the
atmosphere. It is reduced by respiration in marine plants and animals and in
decomposition of organic matter. Low levels of oxygen can indicate organic wastes,
heat pollution or eutrophication caused by excessive growth of plants.
7 Excessive nutrients can have a serious impact on natural aquatic ecosystems, and
therefore natural waterways need to be monitored. High levels of nutrients can lead to
the serious problems of algal blooms and eutrophication.
8 Investigation
9 Chlorine kills microorganisms, including bacteria and algae. Fluoride is added to help
prevent tooth decay in children.
10 The ongoing good health of water consumers and the well-being of natural aquatic
systems is essential for current and future generations. Monitoring these systems is the
only way to ensure government-determined standards are maintained.
11 pH: to ensure drinking water is not too acidic or basic.
Solutions Manual: Module 3
Chemical monitoring and management
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Heavy metals: e.g. Cd, Pb, as these are toxic.
Turbidity: amount of suspended solids in water.
12 a Hard water is water that contains high concentrations of Ca
2+
and Mg
2+
ions in
addition to hydrogencarbonate, sulfate and chloride ions.
b
scum soap
(aq) 2Na (s) COO) ) (CH Ca(CH (aq) Ca COO(aq) ) (CH 2NaCH
2 16 2 3
2
16 2 3
+ +
+ +
13 Na
2
[Na
4
(PO
3
)
6
] combines with Ca
2+
and Mg
2+
ions, forming soluble ions.
Na
2
CO
3
.10H
2
O reacts with Ca
2+
ions only and forms a precipitate.
14 Deionised water has positive and negative ions removed by using ion exchange resins.
Non-ionic or insoluble ionic materials could still be present in the water. Distilling
removes all ionic/dissolved/suspended impurities but is much more expensive.
15 Investigation
Module 3
REVIEW
1 B
2 C
3 A
4 A
5 B
6 C
7 C, D
8 B
9 Friend: in the stratosphere, ozone absorbs UV radiation.
Foe: in the troposohere, ozone is a component of photochemical smog, a pollutant that
is poisonous over 20 ppm.
10 a SO
2
: burning fossil fuels, mineral smelters
CH
4
: ruminants, anaerobic decomposition of organic matter
NO: high temperature combustion
CO
2
: burning fossil fuels, deforestation
b Pass exhaust gases (e.g. CO gas) through catalytic converters.
Solutions Manual: Module 3
Chemical monitoring and management
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
11 a haloalkanes
b dichloro-difluoromethane
c CFCs are unreactive molecules which eventually break down in the stratosphere
to produce chlorine atoms. Chlorine atoms catalyse the decomposition of ozone.
UV radiation acting on CFCs is believed to be one of the major causes of world
ozone depletion.
12 a nitrogen, hydrogen
b Moderate temperature, high pressure, catalyst, removal of ammonia.
13 a Scale (CaCO
3
(s) and/or CaSO
4
(s)) forms inside the iron, which blocks the steam
holes and reduces its efficiency.
b High phosphate levels in aquatic systems can result in algal blooms and
eutrophication in our waterways.
c Lead, which is poisonous, especially to children, is released through the exhaust
to the atmosphere.
14 a Q, as solubility of a gas in water will decrease at higher temperatures.
b Evaporate the water to obtain the ionic solid.
15 a Lead carbonate is insoluble in water acid is necessary.
Acid + carbonate salt + water + CO
2
(g)
b Add Na
2
SO
4
to solution. A precipitate will form if lead is present.
c No, as Ba
2+
could also be present and cause the precipitate.
16 a calcium ion: orange/red
copper ion: green
b As the ion is heated, the electrons in the atom move to a higher energy level. As
these electrons drop to lower energy levels, energy is emitted as colour.
17 NaCl(aq) could be used to precipitate all the Ag
+
ions as AgCl(s). The precipitate would
be filtered, dried and weighed. The moles of silver could be determined using the mass
and hence the molarity of the AgNO
3
(aq) solution.
18 a carbon dioxide, CO
2
; methane, CH
4
b Increased numbers of molecules of greenhouse gases in the atmosphere result in
re-radiated infrared energy from Earth being trapped in the atmosphere. This
causes a greenhouse effect and raises the Earths temperature.
c Increasing demand for energy has resulted in greater burning of fossil fuels and
thus increasing levels of CO
2
in the atmosphere.
19 a Concentrations of chlorine, fluorine and iron.
Solutions Manual: Module 3
Chemical monitoring and management
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
b Chlorine: excessive chlorine adversely affects the taste and smell of water.
Chlorination destroys any bacteria present.
c Chemical analysis by titration with AgNO
3
solution or gravimetric analysis by
precipitation as AgCl.
20 a biochemical oxygen demand
b BOD measures the capacity of the organic matter in a sample to consume oxygen.
If there is too much organic matter, the water is polluted.
c Aerobic organisms are used to decompose organic matter in a sample over five
days. Dissolved oxygen is measured before and after the five-day period.
21 Primary treatment: filtration and settling to remove large objects, solids, oil and scum.
Secondary treatment: treatment of organic matter with aerobic bacteria via sludge
method. About 90% of the organic matter is removed.
Tertiary treatment: removal of contaminants such as nitrates, phosphates and metal
ions.
22 a CuCO
3
(s) + H
2
SO
4
(aq) CuSO
4
(aq) + CO
2
(g) + H
2
O(l)
b green
c Barium will form a white precipitate with H
2
SO
4
. Calcium may form a white
precipitate with H
2
SO
4
but will form a white precipitate with NaF.
23 a carbon monoxide, carbon dioxide, nitrogen dioxide
b CO is formed when incomplete combustion occurs due to a lack of oxygen.
e.g. )(l) 9H 8CO(g) (g) O (l) H C
2 2 2
17
18 8
+ +
c Production of CO can be reduced by getting cars tuned regularly to ensure
efficient combustion.
24 a Germany was dependent on overseas supplies of nitrates as a source of NH
3
for
fertilisers and manufacture of explosives (during WWI) and its shipping routes
were blocked by the Allies.
b Distillation of liquid air.
c Carbon monoxide produced while forming H
2
is converted to CO
2
and used by
soft drink manufacturers. Unreacted N
2
and H
2
in the process are recirculated and
re-used.
d To keep the reaction rate high at temperatures of 500C in order to obtain a
reasonable yield.
e No, as equipment for temperatures of 500C and pressures of 35 000 kPa would
be prohibitively expensive in a small laboratory.
Solutions Manual: Module 3
Chemical monitoring and management
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
25 a C and D. Compare temperature of river before and after steel mill location.
b The solubility of gases decreases as the temperature increases, and therefore
dissolved oxygen levels in the river would fall.
c i Nitrogen and phosphorus.
ii Nitrogen could come from excessive use of fertilisers on farmland, and
phosphorus from sewage containing phosphates from detergents.
d pH meter or indicator paper.
e Chlorination to destroy any bacteria present.
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
CHAPTER 15: THE CHEMICAL INDUSTRY AND THE ROLE OF EQUILIBRIUM
REACTIONS
Review exercise 15.1
1 Synthetic dyes, celluloid and superphosphate have been used to replace Tyrian purple,
ivory and sodium nitrate. The alternative synthetic chemicals are more readily available,
more economically produced and often have superior properties to the naturally
occurring substances.
2 Teflon
: non-stick cookware, greaseless bearings, chemically resistant liners
Kevlar
: bulletproof vests, radial tyres, flame-resistant garments
Zincalume
: roofing and fencing materials
Aspirin: analgesic, anti-inflammatory drug
Vitamin C: in humans prevents scurvy, essential in collagen synthesis, maintenance of
structural strength in blood vessels
Sodium stearate: soap
3 a
b
4 Natural rubber is formed by enzymes that produce a polymer with all the methyl groups
arranged on the same side of the double bonds within the polymer chains, and this was
initially very difficult to duplicate synthetically.
Review exercise 15.2
1 a Equilibrium shifts to the right of the equation to counteract decrease in [H
2
].
b Increase in volume equals a decrease in pressure. Equilibrium shifts to right of the
equation as more molecules are produced to counteract the change.
c Equilibrium shifts to left, where fewer molecules counteract the increase in
pressure.
d Equilibrium shifts to left as it is an endothermic reaction.
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
e No change in equilibrium position as the catalyst alters the rate of both forward
and reverse reactions.
2 a As [Fe
3+
] increases, equilibrium shifts to the left to counteract this change.
Increase in red as [FeSCN
2+
] increases.
b Removal of SCN
by precipitation shifts the equilibrium to the right. The resulting
solution becomes increasingly pale yellow.
Review exercise 15.3
1 a
] O [ ] SO [
] SO [
2
2
2
2
3
= K
b
5
2
4
3
6
2
4
] O [ ] NH [
] O H [ NO] [
= K
c K = [Pb
2+
] [I
]
2
d
] HF [
] F [ ] H [
+
= K
2 a [reactants] << [products]
b [reactants] >> [products]
c [reactants] > [products]
d [reactants] < [products]
3 a i Reaction shifts to right in order to minimise N
2
O
3
addition.
ii Stays the same.
b i Reaction shifts to left, where fewer molecules counteract the increase in
pressure.
ii Stays the same.
c i No change in concentrations of reactants or products. Forward and reverse
reactions affected equally.
ii Stays the same.
d i Shifts reaction to the right (endothermic reaction), to reduce temperature
applied.
ii Increases.
4 a 1 K Q = = 302 . 0
0049 . 0
0385 . 0
2
system is not at equilibrium
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
2 K Q = = = 213 . 0
0042 . 0
0299 . 0
2
system is at equilibrium
3 K Q = = 034 . 0
0031 . 0
0103 . 0
2
system is not at equilibrium
b i Q > K, so more reactants will be formed.
iii Q < K, so more products will be formed.
5 a Decrease
b Increase
Review exercise 15.4
1 a
2
2
2
] NOBr [
] Br [ ] NO [
= K
b K =
(2.0510
2
)
2
(5.24 10
3
)
(5.86 10
3
)
2
= 6.4110
2
2 a [H
2
] = 0.34075 mol L
1
[Br
2
] = 0.2200 mol L
1
H
2
(g) + Br
2
(g)
2HBr(g)
[initial] 0.34075 0.2200 0
[equilibrium] 0.1404
Thus 0.20035 mol L
1
of H
2
have reacted.
From equation, H
2
:Br
2
reacting is 1:1
Therefore at equilibrium, [Br
2
] = 0.2200 0.20035
and [HBr] = 2 0.20035
At equilibrium, [H
2
] = 0.1404 mol L
1
[Br
2
] = 0.01965 mol L
1
[HBr] = 0.4007 mol L
1
b K =
] Br [ ] H [
] HBr [
2 2
2
=
) 01965 . 0 ( ) 1404 . 0 (
) 4007 . 0 (
2
= 5.81981 10
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
3 a K =
] O H [
] H [ ] CO [
2
2
Carbon is a solid. Its concentration does not change and is excluded from the K
expression.
b K =
2
10 5
) 2 . 0 ( ) 2 . 0 (
= 0.8
Chapter 15 Application and investigation
1 Investigation
2 Investigation
3
4 Investigation
5 Investigation
6 Investigation
7 The rate of the reverse reaction becomes greater than that of the forward reaction, as the
equilibrium shifts to the left to decrease the effect of adding more NO
2
(Le Chateliers
principle). Thus, the concentration of N
2
O
4
increases until the rate of the forward
reaction equals the rate of the reverse reaction, and equilibrium is re-established.
8 a
2
2
2
] NOCl [
] Cl [ ] NO [
= K
b
] Cl [ ] CH [
] HCl [ ] Cl CH [
2 4
3
= K
c
] [O
] CO [
2
2
= K
d K = [Mg
2+
][OH
]
2
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
e
] NH [
] OH [ ] NH [
3
4
+
= K
f K = [CO
2
]
9 [Cl
2
] >> [Cl]
10 a K Q
A
= = = 050 . 0
02 . 0
) 04 . 0 ( ) 025 . 0 (
system is at equilibrium
K Q
B
= = 033 . 0
015 . 0
) 025 . 0 ( ) 020 . 0 (
system is not at equilibrium
b For system B, the reaction must move to the right in order to increase the
concentration of product.
11 a Equilibrium constant increases. Formation of products is endothermic.
b Reaction moves to the right (products) in order to counteract the addition of I
2
,
until equilibrium is re-established. The value of the equilibrium constant is
unchanged.
c No change, because there is an equal number of particles on both the reactant and
products sides. No change in the equilibrium constant.
12 K =
] Br [ ] NO [
] NOBr [
2
2
2
=
) 11 . 0 ( ) 30 . 0 (
) 046 . 0 (
2
2
K = 0.21
13 K =
2
2
2
2
] NO [
] O [ ] NO [
At equilibrium [NO] = 1.00 mol L
1
[O
2
] = 1.00 mol L
1
[NO
2
] = 0.464 mol L
1
K =
64 . 4
) 464 . 0 (
) 00 . 1 ( ) 00 . 1 (
2
2
=
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
14 K =
] S [ ] H [
] S H [
2
2
2
2
2
At equilibrium [H
2
S] = 0.725 mol L
1
[H
2
] = 0.208 mol L
1
[S
2
] = 1.125 10
6
mol L
1
K =
) 10 125 . 1 ( ) 208 . 0 (
) 725 . 0 (
6 2
2
K = 1.080 10
7
15 a i CO(g) + Cl
2
(g) COCl
2
(g)
[Initial] 0.2 0.5 0
[Equilibrium] 0.1
From equation CO : Cl
2
: COCl
2
1 : 1 : 1
[Cl
2
] at equilibrium = (0.5 0.1) = 0.4 mol L
1
[COCl
2
] at equilibrium = 0.1 mol L
1
ii K =
] Cl [ ] CO [
] COCl [
2
2
=
) 4 . 0 ( ) 1 . 0 (
1 . 0
= 2.5
b i decrease
ii decrease
16 a CO(g) + H
2
O(g) CO
2
(g) + H
2
(g)
[Initial] 0.100 0.100 0 0
[Equilibrium] 0.0665
At equilibrium [CO
2
] = [H
2
] = 0.0665 mol L
1
[CO] = [H
2
O] = (0.100 0.0665) = 0.0335 mol L
1
b K =
2
2
) 0335 . 0 (
) 0665 . 0 (
= 3.940
17 Initial concentration of NOCl = 1.666 mol L
1
28% dissociated = 0.4666 mol L
1
[NOCl] at equilibrium = 1.20 mol L
1
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
from equation, if 0.4666 mol L
1
of NOCl ionises, at equilibrium
[NO] = 0.4666 mol L
1
and [Cl
2
] = 0.2333 mol L
1
K =
2
2
2
] NOCl [
] Cl [ ] NO [
=
2
2
) 2 . 1 (
) 2333 . 0 ( ) 4666 . 0 (
= 0.0353
CHAPTER 16: THE INDUSTRIAL PRODUCTION OF SULFURIC ACID
Review exercise 16.1
1 Manufacture of superphosphate 72%
Manufacture of general chemicals 10%
Mining and metallurgy 6%
Manufacture of ammonium sulfate 6%
Other uses such as petroleum refining, explosives, detergents, batteries, dyes, pigments,
paint, paper, drugs 6%
2 a Ca
3
(PO
4
)
2
(s) + 2H
2
SO
4
(aq) + 5H
2
O(l) Ca(H
2
PO
4
)
2
.H
2
O(s) + 2CaSO
4
.2H
2
O(s)
b
Review exercise 16.2
1 Superheated steam pumped into underground deposits melts sulfur. Compressed air
pumped into deposits transports sulfur to surface. Sulfur has a relatively low melting
point which allows steam under pressure to melt it, and it is insoluble in water and has a
low density, which causes it to be easily extracted from water and in a pure form.
2 a 2SO
2
(g) + O
2
(g) 2SO
3
(g)
b High pressure as the products have fewer gas molecules than the reactants, and
low temperature because the reaction is exothermic.
c High temperature, high pressure, use of catalyst
d 600C, V
2
O
5
catalyst and atmospheric pressure. At room temperature a very high
yield could be achieved with a very slow reaction rate. At high temperature, the
yield is low but the reaction rate is high. The combination of moderately high
(600C) temperature and use of catalyst in the presence of excess O
2
gives a high
yield, so expensive, high-pressure equipment is not needed.
3 a S(s) + O
2
(g) SO
2
(g)
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
b 2SO
2
(g) + O
2
(g) 2SO
3
(g)
c SO
3
(g) + H
2
SO
4
(l) H
2
S
2
O
7
(l)
d H
2
S
2
O
7
(l) + H
2
O(l) 2H
2
SO
4
(l)
4 The exothermic reaction forms a fog of H
2
SO
4
droplets, which make working
conditions difficult.
5 Poisonous gas: pungent, can trigger asthma attacks. Very soluble in atmospheric water:
produces sulfurous acid rain.
Review exercise 16.3
1 a Zn(s) + H
2
SO
4
(aq) ZnSO
4
(aq) + H
2
(g)
b Cu(s) + 2H
2
SO
4
(aq) CuSO
4
(aq) + 2H
2
O(l) + SO
2
(g)
c
2 Safety goggles, gloves and protective clothing, as spitting may occur. Access to fume
cupboard for procedure and water supply. Add acid to the water slowly, with continuous
stirring, to minimise the potential for boiling from this exothermic reaction.
Chapter 16 Application and investigation
1
Properties Major uses
A strong acid that is non-volatile Manufacture of superphosphate and
ammonium sulfate (fertilisers)
Cleaning of coatings from iron or
steel before galvanising or
electroplating
Extracting titanium dioxide from
ilmenite
Strong affinity for water As a dehydrating agent in:
production of ethene from ethanol
esterification reactions
production of gases O
2
, N
2
, CO
2
Reacts with aromatic hydrocarbons to
form sulfonic acids
Manufacture of detergents
2 Low melting point, insoluble in water, low density.
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
3
Raw material Role
sulphur formation of sulfur dioxide
air/O
2
oxidation of sulfur to sulfur dioxide then to SO
3
V
2
O
5
catalyst for SO
2
SO
3
H
2
O H
2
S
2
O
7
(l) H
2
SO
4
(l)
4 a Sulfur burnt in air to form sulfur dioxide.
S(s) + O
2
(g) SO
2
(g)
b Sulfur dioxide is converted to sulfur trioxide over beds of V
2
O
5
catalyst in excess
O
2
.
c SO
3
(g) is absorbed in concentrated H
2
SO
4
to form oleum, H
2
S
2
O
7
.
SO
3
(g) + H
2
SO
4
(l) H
2
S
2
O
7
(l)
d Water is added to produce sulfuric acid.
H
2
S
2
O
7
(l) + H
2
O(l) 2H
2
SO
4
(l)
5 a Higher temperature increases the rate of reaction but decreases the yield, because
the reaction is exothermic. With a catalyst to increase the reaction rate, a lower
temperature enables an increased equilibrium yield of SO
3
.
b Reactions at high pressure require expensive high-pressure equipment. High
yields can still be obtained without high pressure with temperatures of 400
600C, V
2
O
5
catalyst and atmospheric pressure.
c SO
2
(g) is very soluble and could form H
2
SO
3
(aq) with moisture in the air.
6 The byproduct of SO
2
from sulfide ores can be used as the raw material for sulfuric acid
production.
7 Investigation
8 a As oxidising agent.
2KI(aq) + 2H
2
SO
4
(aq) I
2
(aq) + K
2
SO
4
(aq) + SO
2
(g) + 2H
2
O(l)
b As strong acid.
Zn(s) + H
2
SO
4
(aq) ZnSO
4
(aq) + H
2
(g)
c Concentrated acid is acting as a dehydrating agent, absorbing any H
2
O(g) mixed
in with N
2
(g).
N
2
.H
2
O(g) + H
2
SO
4
(l) N
2
(g) + H
2
SO
4
.H
2
O(aq)
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
9 Investigation
10 Main points for dilution procedure should include:
wearing goggles, gloves and protective clothing
use of fume cupboard, access to water and reasons
acid added to water
addition slow, with constant stirring
exothermic reaction, equations.
CHAPTER 17: THE INDUSTRIAL PRODUCTION OF SODIUM HYDROXIDE
Review exercise 17.1
1
Galvanic cell Electrolytic cell
Chemical energy is converted to electrical
energy (spontaneous reaction with a
positive voltage)
Electrical energy is converted to chemical
energy (reaction is not spontaneous and
energy must be supplied)
Anode (oxidation liberates electrons)
negative electrode
Cathode (reduction absorbs electrons)
positive electrode
Anode (oxidation liberates electrons)
positive electrode
Cathode (reduction absorbs electrons)
negative electrode
Electrons move from anode to metallic
conductor of external circuit to cathode
Electrons move from anode to voltage
source to cathode
Negative ions move from cathode to
anode
Negative ions move from cathode to anode
Positive ions move from anode to cathode Positive ions move from anode to cathode
2 a anode: 2H
2
O(l)
O
2
(g) + 4H
+
(aq) + 4e
cathode: 2Co
2+
(aq) + 4e
2Co(s)
overall: H
2
O(l) + 2Co
2+
(aq) O
2
(g) + 2Co(s) + 4H
+
(aq)
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
b
3 anode: 2H
2
O(l)
O
2
(g) + 4H
+
(aq) + 4e
cathode: 2Zn
2+
(aq) + 4e
2Zn(s)
overall: H
2
O(l) + 2Zn
2+
(aq) O
2
(g) + 2Zn(s) + 4H
+
(aq)
4 a anode: 2Cl
(l) Cl
2
(g) + 2e
cathode: Sr
2+
(l) + 2e
Sr(s)
b anode: Cu(s) Cu
2+
(aq) + 2e
cathode: Cu
2+
(aq) + 2e
Cu(s)
c anode: 4OH
(aq O
2
(g) + 2H
2
O(l) + 4e
cathode: 2H
2
O(l) + 2e
H
2
(g) + 2OH
(aq)
Review exercise 17.2
1 If aqueous solutions of active metals are electrolysed, less energy is needed to reduce
water rather than the metal. Hence molten compounds must be used in electrolysis.
2 Oxidation produces oxygen:
2H
2
O(l) O
2
(g) + 4H
+
(aq) + 4e
Reduction produces hydrogen:
2H
2
O(l) + 2e
H
2
(g) + 2OH
(aq)
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
3 a Water will be reduced before the sodium.
b At the cathode, possible reactions are:
Na
+
(aq) + e
Na(s) E = 2.71 V
2H
2
O(l) + 2e
H
2
(g) + 2OH
(aq) E = 0.41 V
Reduction of water has a more positive reduction potential, so hydrogen will be
evolved.
At the anode, possible reactions are:
2Cl
(aq) Cl
2
(g) + 2e
E = 1.36 V
2H
2
O(l) O
2
(g) + 4H
+
(aq) + 4e
E = 0.82 V
Since oxidation of water has a more positive E, oxygen will be produced.
4 a 2NaCl(aq) + 2H
2
O(l) 2NaOH(aq) + H
2
(g) + Cl
2
(g)
b 2Cl
(aq) + 2H
2
O(l) 2OH
+ H
2
(g) + Cl
2
(g)
5 Mercury process: Hg cathode absorbs Na to form an amalgam which is continually
removed. Chlorine produced at the inert anode is thus prevented from mixing with other
products.
Diaphragm process: An asbestos diaphragm lines the steel-mesh cathode to prevent Cl
2
mixing with other products.
Membrane process: Anode and cathode compartments are separated by a water-
impermeable membrane constructed from synthetic polymers such as Teflon.
Chapter 17 Application and investigation
1 Metal: metal structure with sea of electrons allows flow of electrons as current.
Molten ionic compound: ions are not confined to lattice, so the flow of ions conducts
current.
In aqueous solution of ionic compound, ions can move freely through the solution,
conducting current.
2 Cathode: 2K
+
(l) + 2e
2K(l)
Anode: 2OH
O
2
(g) + H
2
O(l) + 2e
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
3 a
b Anode: 2H
2
O(l) O
2
(g) + 4H
+
(aq) + 4e
2Cl
(aq) Cl
2
(g) + 2e
(with concentrated HgCl
2
)
Cathode: Hg
2+
(aq) + 2e
Hg(l)
4 a anode: 2I
(aq)
I
2
(aq) + 2e
colourless yellow/brown colour around anode
cathode: 2H
2
O(l) + 2e
H
2
(g) + 2OH
(aq)
colourless gas evolved
b anode: Cu(s)
Cu
2+
(aq) + 2e
anode erodes
cathode: Cu
2+
(aq) + 2e
Cu(s)
copper deposited on cathode
Equal loss and gain of Cu
2+
(aq) from solution maintains blue colour of solution.
c anode: 4OH
(aq)
O
2
(g) + 2H
2
O(l) + 4e
colourless gas evolved
cathode: 2H
2
O(l) + 2e
H
2
(g) + 2OH
colourless gas evolved
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
d anode: 2H
2
O(l)
O
2
(g) + 4H
+
(aq) + 4e
colourless gas evolved
cathode: Cu
2+
(aq) + 2e
Cu(s)
blue/green colour fades as pink/red copper deposited on cathode.
5 Electrolysis of molten sodium chloride involves:
anode oxidation: 2Cl
(l)
Cl
2
(g) + 2e
E = 1.36V
cathode reduction: Na
+
(l) + e
Na(s) E = 2.71 V
With aqueous solutions, reactions at the electrodes will favour those with the most
positive E value, so with NaCl(aq):
anode oxidation: 2H
2
O(l) O
2
(g) + 4H
+
(aq) + 4e
E = 0.82 V is favoured, unless a
more concentrated NaCl(aq) is used, and then Cl
2
may be evolved at the anode
2Cl
(aq) Cl
2
(g) + 2e
E = 1.36 V
cathode reduction: 2H
2
O(l) + 2e
H
2
(g) + 2OH
(aq) E = 0.41 V
6 a Water present in the aqueous solution would be reduced first at the cathode.
b Unless separated, the chlorine product at the anode and sodium at the cathode
would readily react to revert to sodium chloride.
c Carbon is an inert electrode and would not lose electrons in an oxidation reaction,
whereas iron would readily do so in preference to the chloride ions in solution.
7 Process Mercury Diaphragm Membrane
a Raw materials Concentrated
NaCl(aq)
Concentrated
NaCl(aq)
Concentrated
NaCl(aq)
b Nature of
electrodes
Cathode: flowing
Hg(l)
Anode: inert
(graphite)
Cathode: steel mesh
Anode: carbon
Cathode: iron mesh
Anode: titanium
c Cathode
reaction
Anode reaction
2Na
+
(aq) + 2e
+
Hg(l) 2Na(Hg)
2Cl
(aq) Cl
2
(g) +
2e
2H
2
O(l) + 2e
H
2
(g) + 2OH
(aq)
2Cl
(aq) Cl
2
(g) +
2e
2H
2
O(l) + 2e
H
2
(g) + 2OH
(aq)
2Cl
(aq) Cl
2
(g) +
2e
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
d Advantages High quality
products formed;
no chloride
contamination
No Hg
environmental
problems
No contamination of
NaOH product with
NaCl or formation of
NaOCl
Inert synthetic
diaphragm, only
allows Na
+
ions to
pass. Can withstand
long immersion in
OH
solutions.
Disadvantages Environmental issues
with Hg vaporisation,
leakage into
waterways.
NaOH is
contaminated with
NaCl(aq) and needs
to be separated for
commercial use.
Asbestos diaphragm
is carcinogenic
material.
CHAPTER 18: THE PRODUCTION OF SOAPS AND DETERGENTS
Review exercise 18.1
1 Soap is a sodium or potassium salt of a fatty acid.
2 a
b
c saponification
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Review exercise 18.2
1 a Polar solvents tend to dissolve polar solutes and non-polar solvents tend to
dissolve non-polar solutes.
b A solute will dissolve in a solvent only if the intermolecular forces within the
solute and those within the solvent are similar to those occurring between solute
and solvent molecules.
2 a CH
3
(CH
2
)
14
COO
Na
+
(s) CH
3
(CH
2
)
14
COO
(aq) + Na
+
(aq)
b CH
3
(CH
2
)
4
(CH
2
CH=CH)
2
(CH
2
)
6
COO
K
+
(s)
CH
3
(CH
2
)
4
(CH
2
CH=CH)
2
(CH)
6
COO
(aq) + K
+
(aq)
3 a
b
c The oil would disperse within the soap solution to form an emulsion.
4 a Hard water is water that contains significant concentrations of Ca
2+
, Mg
2+
, HCO
3
,
SO
4
2
, Cl
ions.
b The negative ion of soap combines with Ca
2+
, Mg
2+
to form an insoluble, greasy
precipitate known as scum.
c 2RCOO
Na
+
(s) + Ca
2+
(aq) Ca(RCOO)
2
(s) + 2Na
+
(aq)
d Hardness in water can be the result of the presence of Ca
2+
ions. These can be
removed by precipitation with CO
3
2
ions.
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Review exercise 18.3
1
Soap Detergent
Use Cleaning agent, surfactant Cleaning agent, surfactant
Nature Has a long hydrocarbon tail that
readily dissolves in oily or waxy
substances, and a polar or charged
particle head that easily dissolves
in water.
Has a long hydrocarbon tail that
readily dissolves in oily or waxy
substances, and a polar or charged
particle head that easily dissolves
in water.
Reaction
with Ca
2+
and Mg
2+
Precipitates No precipitate
Types One: soluble salts of fatty acids Three : anionic, cationic, non-ionic
2 a
b
3 The positive ends of cationic detergents are attracted to the surface of glass, leaving
their non-polar ends sticking out, forming a greasy layer.
Review exercise 18.4
1 Early detergents were non-biodegradable or degraded very slowly and caused excessive
frothing in waterways. These early detergents had branched hydrocarbon chains.
Current straight-chained hydrocarbon detergents have eliminated this problem.
Phosphate compounds included in anionic detergents enhance their cleaning power by
softening the water and increasing alkalinity. These phosphate compounds in waterways
can lead to algal blooms, which can cause depletion in water oxygen levels and kill
aquatic organisms. Measures to reduce the phosphate problem include the use of
substitutes such as sodium citrate, as well as greater use of non-ionic detergents.
2 The hydrocarbon chain is branched, and therefore the detergent is non-biodegradable.
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Chapter 18 Application and investigation
1 a
b It would be an oil as it contains only unsaturated fatty acids.
c
2 Investigation
3 a
b
4 a The hydrophobic end of a soap or detergent is the non-polar hydrocarbon end,
which will not dissolve in water. The hydrophilic end is the charged or polar end,
which will dissolve in water.
b The grease is surrounded by spherical aggregates of surfactant ions. The
hydrophobic tails attach to the grease, while the hydrophilic heads dissolve in the
water. The surfactant ion thus floats the grease spot from the cloth.
c Non-polar dry cleaning fluids would dissolve the non-polar grease spot (solute).
d Washing with detergent solution can remove both non-polar molecules and ionic
compounds from the fabric into an aqueous solution.
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Dry-cleaning fluid contains no water and dissolves non-polar molecules such as
grease into the fluid.
5 a Formation of scum
b 2RCOO
(aq) + Ca
2+
(aq) Ca(RCOO)
2
(s)
c Detergents do not form insoluble precipitates with Ca
2+
ions.
6 A scum ring indicates that the water is hard, that is, it contains Ca
2+
and/or Mg
2+
ions,
which form insoluble precipitates with soap. The bubble bath contained detergent,
which does not form precipitates with Ca
2+
or Mg
2+
ions.
7 a Praise French salad dressing: vegetable oil, vinegar and water are immiscible.
Emulsifier = soyabean lecithin
b Many cationic surfactants are effective biocidesthat is, they kill many
organisms, specifically bacteria, when used in mouth washes.
c Non-ionic detergents are used as substitutes for anionic detergents. Use of anionic
detergents that contain phosphates is kept at a minimum because of the resulting
environmental problem of eutrophication. Some non-ionic detergents also have
greater cleaning power than anionic ones.
8 The negative, anionic detergent ends are attracted to the positive surface of the plastic.
This leaves the long, non-polar hydrocarbon tails of the surfactant sticking out, forming
a thin, greasy film on the plastic.
9 Investigation
10 Investigation
11 Investigation
CHAPTER 19: THE INDUSTRIAL PRODUCTION OF SODIUM CARBONATE
Review exercise 19.1
1 Manufacture of glass, pharmaceuticals, soaps, detergents, dyes.
To neutralise excess acid from industrial chemical processes.
To remove ink from high-quality white waste paper in recycling process.
2
Formula Systematic name Common name
NaOH sodium hydroxide Caustic soda
Na
2
CO
3
sodium carbonate Soda ash
Na
2
CO
3
.10H
2
O sodium carbonate decahydrate Washing soda
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
3 2HCl(aq) + Na
2
CO
3
(aq) 2NaCl(aq) + H
2
O(l) + CO
2
(g)
Review exercise 19.2
1 CaCO
3
is insoluble. (Reverse process occurs if product solutions are mixed).
2 a CaCO
3
(s)
heat
CaO(s) + CO
2
(g)
b NH
3
(aq) + CO
2
(aq) + NaCl(aq) + H
2
O(l) NaHCO
3
(aq) + NH
4
Cl(aq)
c Na
+
(aq) + HCO
3
(aq) NaHCO
3
(s)
d 2NaHCO
3
(s) Na
2
CO
3
(s) + CO
2
(g) + H
2
O(l)
e CaO(s) + H
2
O(l) Ca(OH)
2
(s)
f Ca(OH)
2
(s) + 2NH
4
Cl(aq) 2NH
3
(g) + CaCl
2
(aq) + 2H
2
O(l)
3 a CO
2
from 2a is used in 2b above, regenerated in 2d above for use in 2b again.
b NH
3
is used in 2b above, regenerated in 2f above for use in 2b again
Review exercise 19.3
1 Thermal pollution.
Excess quantities of CaCl
2
are produced and cannot be used in other applications.
CaCl
2
returned to the environment adversely affects aquatic ecosystems.
Disposal of excess Ca(OH)
2
which must be neutralised and stored before release into
the environment.
2 Large quantities of heat from the process are absorbed by water from rivers or lakes.
This resultant hot water, unless cooled before being returned to the environment, will
lower O
2
water concentrations and harm or destroy aquatic organismsthermal
pollution. To avoid thermal pollution, expensive heat diffusers are needed or alternative
ways of dissipating heat must be devised.
3 Ca(OH)
2
(s) + 2HCl(aq) CaCl
2
(aq) + 2H
2
O(l)
250 kg Ca(OH)
2
= 250 10
3
g
=
mol
1 . 74
10 250
3
= 3373.82 mol
(Molar mass of Ca(OH)
2
is 74.10 g mol
1
)
From equation, nHCl for neutralisation is 2nCa(OH)
2
6747.64 mol HCl needed
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
c =
V
n
V = HCl L 6 . 1124 L
6
64 . 6747
=
4 Availability and cost of land, raw materials, transport, energy and water supply.
Supply of labour, accessibility to markets, social and safety issues as well as those of
waste disposal and the environment.
Solvay process plant: waste disposal plus thermal pollution, environmental issues and
cost of raw materials.
Chapter 19 Application and investigation
1 a Raw materials: limestone CaCO
3
(s), water, brine NaCl(aq), ammonia NH
3
(g).
b End products: i CaCl
2
(aq) and ii Na
2
CO
3
(s).
i In the recovery process to obtain NH
3
(g), which is recycled, the by-product
CaCl
2
(aq) is formed:
Ca(OH)
2
(s) + 2NH
4
Cl(aq) 2NH
3
(g) + CaCl
2
(aq) + 2H
2
O(l)
ii Na
2
CO
3
(s) is formed by heating NaHCO
3
(s):
2NaHCO
3
(s) Na
2
CO
3
(s) + CO
2
(g) + H
2
O(l)
c Na
2
CO
3
(s) is produced by heating NaHCO
3
(s) (equation b ii)
NaHCO
3
(s) is obtained from bubbling CO
2
(g) through a concentrated brine
solution which has absorbed NH
3
(g):
CO
2
(g) + NaCl(aq) + NH
3
(g) + H
2
O(l) NaHCO
3
(aq) + NH
4
Cl(aq)
d Recycling of CO
2
(g) and NH
3
(g) make the process efficient. CO
2
(g) is first
generated by heating limestone:
CaCO
3
(s)
heat
CaO(s) + CO
2
(g)
and it is then used to make NaHCO
3
(aq) (equation c ii).
CO
2
(g) is regenerated in the heating of NaHCO
3
(s) (equation b ii).
NH
3
(g) is used to form NaHCO
3
(aq) (equation c ii) and is regenerated in the
reaction with Ca(OH)
2
(s) and NH
4
Cl(aq) (equation b i)
e Main environmental problems associated with the Solvay process are:
i thermal pollution: can be minimised by use of heat diffusers to cool the
heated water before it is returned to the environment. Heated water
discharged into ocean where significant dilution occurs may avoid critical
thermal pollution.
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
ii disposal of excess by-product CaCl
2
: excess CaCl
2
may be used up in other
processes. If not, rapid release into environment/waterways would cause
ecosystem damage. Discharge of diluted CaCl
2
into the ocean could also be
less harmful, given the high [Ca
2+
] in the sea.
Other environmental problems could include leaks of NH
3
gas and problems
associated with the mining and extraction of ores for limestone and sodium
chloride.
2 a CaCO
3
(s)
heat
CaO(s) + CO
2
(g)
b CaO(s) + H
2
O(l) Ca(OH)
2
)(s)
c 2NH
4
Cl(aq) + Ca(OH)
2
(s) 2NH
3
(g) + CaCl
2
(aq) + 2H
2
O(l)
3 From: NH
3
(aq) + CO
2
(aq) + NaCl(aq) + H
2
O(l) NaHCO
3
(aq) + NH
4
Cl(aq)
1 mol NaCl produces 1 mol NaHCO
3
Molar mass of NaCl = 58.44 g mol
1
Molar mass of NaHCO
3
= 84.01 g mol
1
1000 kg NaCl =
NaCl mol
44 . 58
10 1000
3
= 1.711 10
4
mol NaCl
= mol NaHCO
3
produced
From: 2NaHCO
3
(s) Na
2
CO
3
(s) + CO
2
(g) + H
2
O(l)
2 mol NaHCO
3
produces 1 mol Na
2
CO
3
thus 1.711 10
4
mol NaHCO
3
produces 0.5 (1.711 10
4
) mol Na
2
CO
3
Mass of Na
2
CO
3
produced = moles molar mass
= (0.5 1.711 10
4
)(84.01) g
= 718 706 g = 718.7 kg
4 From overall chemical reactions in the Solvay process, the production mole ratio of
Na
2
CO
3
(s) : CaCl
2
(aq) is 1 : 1.
n(Na
2
CO
3
) in 1000 kg =
mass molar
mass
=
3 2
3
CO Na mol
99 . 105
10 1000
= 9434.85 mol Na
2
CO
3
= n(CaCl
2
)
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
mass CaCl
2
= n M = (9434.85 110.98) g
= 1047 kg
5 NH
3
(aq) + CO
2
(aq) + NaCl(aq) + H
2
O(l) NaHCO
3
(aq) + NH
4
Cl(aq)
100.00 L of 6.00 mol L
1
NaCl(aq)
c(NaCl) =
V
n
n(NaCl) = (6.00 100.0) mol = 600.0 mol NaCl
nNH
3
: nNaCl is 1 : 1
n(NH
3
) = 600 mol
At 0C and 101.3 kPa 1mole = 22.41 L
volume of NH
3
= (n 22.41) L = 13 446 L of NH
3
(g)
6 2NaHCO
3
(s) Na
2
CO
3
(s) + CO
2
(g) + H
2
O(l)
1000 kg NaHCO
3
=
M
m
=
01 . 84
10 1000
3
mol NaHCO
3
= 11903.34 mol
n(Na
2
CO
3
) produced = 0.5n(NaHCO
3
)
mass Na
2
CO
3
produced = n(Na
2
CO
3
) M
= (5951.67 105.99) g
= 631 kg of Na
2
CO
3
produced
7 Investigation
Module 4
REVIEW
1 Natural material = rubber
a Original raw rubber from the bark of rubber trees was vulcanised to increase its
elasticity, hardness and resistance to chemical attack. As the automotive industry
increased, the demand for rubber used in tyres escalated. The source of natural
rubber was restricted during World War II, which promoted the need to develop
synthetic rubber.
b Styrenebutadiene. Also vulcanised. Raw materials for production are plentiful as
they are by-products of petroleum refining.
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
2 a 2SO
2
(g) + O
2
(g) 2SO
3
(g)
b high pressure, low temperature
c Conditions used: moderately high temperatures 400600C, V
2
O
5
catalyst in
excess O
2
and atmospheric pressure.
d Since the reaction is exothermic, the yield would be better at low temperatures.
However, at low temperatures the reaction rate is very slow. High pressure
equipment is very expensive. It has been found that an excellent yield is achieved
at atmospheric pressures if V
2
O
5
catalyst is used.
e K =
] O [ ] SO [
] SO [
2
2
2
2
3
f Q =
2
2
2
10 42 . 1
) 450 . 0 ( ) 250 . 0 (
) 00 . 2 (
=
This is less than the K value of 6.50 10
2
, so the system is not at equilibrium. The
system must shift to increase products, [SO
3
], in order to reach equilibrium.
3 a i Superheated steam pumped into underground deposits melts sulfur.
Compressed air pumped into deposits transports sulfur to surface.
ii Low melting point, insoluble in water, low density.
iii Care must be taken to prevent possible oxidation or reduction of sulfur at
the Earths surface. Low concentrations of SO
2
and H
2
S are serious
pollutants. Impurities dissolved in superheated water may also cause
environmental damage on discharge to surrounds, plus thermal pollution.
Possibility of earth subsidences over mining area where the underground
caverns left after extracting sulfur are difficult to back-fill.
b i High temperature, large surface area of S to increase reaction rate.
ii A mixture of SO
2
and excess O
2
is passed over several layers of V
2
O
5
catalyst in pellet form. Temperature is at 600C, then cooled slightly
(400C) between each pass over catalyst layer.
iii SO
3
(g) + H
2
SO
4
(l) H
2
S
2
O
7
(l)
H
2
S
2
O
7
(l) + H
2
O(l) 2H
2
SO
4
(l)
c Manufacture of fertilisers such as superphosphate and ammonium sulfate.
Cleaning iron before galvanising or electroplating.
d i Cu(s) + 2H
2
SO
4
(aq) CuSO
4
(aq) + 2H
2
O(l) + SO
2
(g)
ii C
2
H
5
OH(l)
H
2
SO
4
(l )
C
2
H
4
(g) + H
2
O(l)
iii Ba(NO
3
)
2
(aq) + H
2
SO
4
(aq) BaSO
4
(s) + 2HNO
3
(aq)
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
4 a 2Na(Hg) + 2H
2
O(l) 2NaOH(aq) + H
2
(g) + 2Hg(l)
b 2Cl
(aq) Cl
2
(g) + 2e
c Overall equation for mercury cell is
2NaCl(aq) + 2H
2
O(l) 2NaOH(aq) + Cl
2
(g) + H
2
(g)
1 mole NaCl produces 1 mole NaOH
1.00 10
3
kg of NaCl =
M
m
mol of NaCl
n(NaCl) =
44 . 58
10 10 1
3 3
mol NaCl
= 1.711 10
4
mol NaCl
= 1.711 10
4
mol NaOH produced
mass (NaOH) = n molar mass
= (1.711 10
4
) (40.00)
= 6.84 10
5
g
= 6.84 10
2
kg
d i Main hazard is Hg pollution. Hg can be lost from the process in vapour form
or in contaminated brine. The Hg in brine released to the environment
(usually the ocean) is taken up by organisms and passed along the food
chain, becoming more concentrated at each stage. Mercury can damage the
human nervous system/brain.
ii Using the membrane process, a concentrated NaCl solution is electrolysed
to produce NaOH and H
2
at the cathode and Cl
2
at the anode. The anode and
cathode compartments are separated by a water-impermeable membrane
made of synthetic polymers. This membrane only allows Na
+
ions to pass
through it and so prevents contamination of the NaOH with NaCl and the
formation of other products such as NaOCl.
e Uses of NaOH:
manufacture of soaps and detergents
manufacture of rayon and plastics
extraction of alumina from bauxite.
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
5 a
b
The hydrophobic tail of soap attaches to oily particles and the other charged end
dissolves in water. Soap floats off the oil particles from the plate.
c In hard water, the action of soap is much less effective because it reacts with Ca
2+
and Mg
2+
ions to form insoluble precipitates (scum).
d Early detergents were anionic branched-chain alkylbenzene sulfonates. They were
not easily biodegradable and caused foam build-up in waterways. Phosphates
were added to detergents to enhance their cleaning power by softening the water
and increasing alkalinity. In waterways these phosphates caused algal blooms and
eutrophication.
6 a Manufacture of glass, pharmaceuticals, soaps, detergents, dyes.
To neutralise excess acid from industrial chemical processes.
To remove ink from high-quality white waste paper in recycling process.
b X = brine (NaCl(aq))
Y = limestone (Na
2
CO
3
)
c CaCO
3
(s) + 2NaCl(aq) Na
2
CO
3
(s) + CaCl
2
(aq)
d The statement involves the conversion of two cheap abundant raw materials into
two useful products infers a simple reaction, whereas five chemical reactions take
place in the process.
NH
3
(g) is not mentioned, although it is essential in the process. Recycling of NH
3
reduces the cost, but it is an expensive raw material need for the initial process.
There is a possibility of pollution with any NH
3
(g) loss to the atmosphere.
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
The other recycled substance, CO
2
, is used continuously and still needs additional
CO
2
supplied from heating limestone.
Geographic location dominates access to raw materials which, even if present in
abundance, may still have to be mined, treated for undesirable side products, and
purified.
Calcium chloride may not be such a useful product. Often its production far
exceeds its demand. Although the total outcome of the process produces thermal
pollution, considerable energy input is still necessary to make the whole process
workpumping, bubbling, heating limestone, NaHCO
3
and so on.
e Thermal pollution; disposal of excess Ca(OH)
2
which must be neutralised and
stored before release into the environment.
f Availability and cost of land, raw materials, transport, energy and water supply
need to be determined; supply of labour, accessibility to markets, social and safety
issues as well as those of waste disposal and the environment..
7 a
b anode: 2Cl
(aq) Cl
2
(g) + 2e
oxidation
cathode: 2H
2
O(l) + 2e
H
2
(g) + 2OH
(aq) reduction
8 Production of SO
3
(g) during the industrial manufacture of H
2
SO
4
(aq):
a In a closed system a reaction that is in dynamic equilibrium is one in which the
rate of the forward reaction equals the rate of the reverse reaction. The
macroscopic (observable) properties of a dynamic system in equilibrium are
constant, but both forward and reverse reactions are occurring at equal rates at the
molecular level.
b The production of SO
3
(g) is an exothermic reaction:
2SO
2
(g) + O
2
(g) 2SO
3
(g) + energy
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Lowering the temperature would cause the system to shift to the right-hand side of
the equation (products) in order to counteract the drop in temperature, since the
formation of products releases energy. However, the drop in temperature would
also lead to a very low reaction rate. Thus a compromise must be reached between
yield and rate. Similarly, an increase in pressure would cause the reaction to move
to the products side in order to minimise the increase in pressure. Increasing
pressure would increase both reaction rate and yield, but the necessary equipment
is very expensive to use on an industrial scale.
c Industrially, a very high yield is obtained by using moderately high temperatures
and atmospheric pressure together with a very effective catalyst, V
2
O
5
, in the
presence of excess O
2
.
9 a K =
] O [ ] HCl [
] Cl [ ] O H [
2
4
2
2
2
2
b 4HCl + O
2
2H
2
O + 2Cl
2
[initial] 2 0.5 0 0
Let x mol L
1
of O
2
react in order to achieve equilibrium.
Then 4x mol L
1
of HCl will react with x mol L
1
O
2
and 2x mol L
1
are formed of
H
2
O and Cl
2
.
Then at equilibrium: [HCl] = (2 4x) mol L
1
[O
2
] = (0.5 x) mol L
1
[H
2
O] = 2x mol L
1
[Cl
2
] = 2x mol L
1
K =
) 5 . 0 ( ) 4 2 (
) ( ) (
4
2 2
x x
2x 2x
x, and thus equilibrium concentrations, can only be expressed as a function of K,
which is unknown. Insufficient information.
c Since the reaction is exothermic, if temperature is decreased, the system will shift
to the product side of the equation in order to counteract the change in
temperature until equilibrium is re-established. Thus the K value would increase.
d If pressure is decreased, the system moves to the side of the equation which has
more gas particles in order to counteract the loss of pressure imposed on it.
The reactants side has 5 mol of reactants compared to 4 mol of products. So the
system shifts to the left and the K value decreases.
e High pressure, low temperature. Low temperature would decrease the reaction
rate so that it became an unviable commercial proposition, so probably a catalyst
to increase reaction rate would have to be introduced. Alternatively, one of the
Solutions Manual: Module 4
Industrial chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
products could be removed from the system on formation, thus producing a small
but constant flow of products.
10 a Extraction of a metal from its sulfide ores; and extracted from natural gas and
petroleum crudes in which it is a pollutant.
b S(s) + O
2
(g) SO
2
(g)
c SO
3
(g) + H
2
O(l) H
2
SO
4
(aq)
d SO
3
(g) is absorbed in concentrated H
2
SO
4
to form oleum, H
2
S
2
O
7
SO
3
(g) + H
2
SO
4
(l) H
2
S
2
O
7
(l)
Water is added to produce sulfuric acid
H
2
S
2
O
7
(l) + H
2
O(l) 2H
2
SO
4
(l)
e The exothermic reaction forms a fog of H
2
SO
4
droplets, which make working
conditions difficult.
Solutions Manual: Module 5
Shipwrecks, corrosion and conservation
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
CHAPTER 20: THE OCEAN ENVIRONMENT
Review exercise 20.1
1 The bonding and intermolecular forces within the solute.
The forces between the solute and water.
The temperature of the water.
2 High intermolecular forces between gas molecules and water molecules, resulting in
ionisation reactions.
Review exercise 20.2
1 As temperature increases, solubility of gas decreases.
As pressure of the gas above water increases, solubility of gas increases.
2 CO
2
(aq) CO
2
(g) so when the container is opened, the pressure of CO
2
(g) above the
liquid rapidly decreases. The equation shifts to the right-hand side in order to counteract
the fall in pressure by producing more CO
2
(g) molecules from the solution (Le
Chateliers principle).
3 [O
2
] is greatest near the surface due to atmospheric O
2
and O
2
produced by
photosynthetic aquatic organisms. [O
2
] decreases as depth of ocean increases due to use
of O
2
in respiration of organisms and their decomposition. At great depths, [O
2
] may
increase due to presence of dense, cold water containing high O
2
levelsglobal
conveyor belt. [CO
2
] increases with ocean depth. At the surface, CO
2
is used for
photosynthesis. With increasing depth and less light penetration, less photosynthesis
occurs and more CO
2
is produced due to respiration and decomposition of organisms.
Review exercise 20.3
1 The leaching effects of rain water passing through terrestrial environments and
hydrothermal vents in mid-ocean ridges.
2 Aquatic electric current transfer requires mobile ions and sea water has these.
3 No outlet to large sea mass. High evaporation, low precipitation due to regional weather
interferes with water cycle.
4 Salts of chromium, copper, gold, manganese and nickel are generally insoluble.
Superheated water from volcanic mid-ocean ridges readily dissolves these minerals,
which become dispersed and form underwater mineral deposits.
Chapter 20 Application and investigation
1 KClsoluble. Ions surrounded by polar water molecules.
C
6
H
12
O
6
soluble. Polar molecule forms hydrogen bonds with water molecules.
Solutions Manual: Module 5
Shipwrecks, corrosion and conservation
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
CO
2
slightly soluble. Reacts with water to form carbonic acid which then partially
ionises.
C
2
H
6
insoluble. Non-polar solute in polar solvent. Will not react with water.
2 NH
3
, SO
2
, O
2
decreasing order of solubility. Decreasing order of reaction with water.
NH
3
readily reacts with water forming ammonium and hydroxide ions. SO
2
reacts with
water, forming H
2
SO
3
which partially ionises. O
2
molecules have only dispersion forces
with water molecules.
3 a Weak dispersion forces between water and O
2
decrease as temperature or average
kinetic energy of particles increases. Hence, as temperature increases, solubility of
O
2
decreases. Water which has been boiled is depleted of dissolved O
2
. After
cooling, a goldfish would not survive in this water.
b Pressure increases the number of gas molecules per unit volume. This causes an
increase in the rate at which the gas enters the solution and so the concentration of
dissolved gas increases. Carbonated drinks are bottled at high pressures of carbon
dioxide to ensure high concentration of dissolved CO
2
in the liquid.
4 Investigation
5 Investigation
6 a Weigh a sample of sea water in a sealed container (W
1
).
b Remove top from container and warm.
c To ensure all the gas has escaped from the seawater, replace top securely, shake
conatiner and contents. When liquid has settled, remove top. If any further gas
appears to be released, repeat b.
d Replace top, re-weigh bottle (W
2
).
e Mass of dissolved gases = (W
1
W
2
) g.
7 Investigation
8 Investigation
9 Investigation
CHAPTER 21: OXIDATIONREDUCTION REACTIONS
Review exercise 21.1
1 Oxidation is loss of electrons. Reduction is gain of electrons.
For electron transfer to occur, the two processes must occur simultaneously, e.g. zinc in
a solution of copper(II) sulfate will lose electrons (oxidation) to copper(II) ions, which
are reduced.
Zn(s) Zn
2+
(aq) + 2e
Solutions Manual: Module 5
Shipwrecks, corrosion and conservation
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Cu
2+
(aq) + 2e
Cu(s)
Overall: Zn(s) + Cu
2+
(aq) Zn
2+
(aq) + Cu(s)
2
Fe(s) Fe
2+
(aq) + 2e
E = 0.44 V
2Ag
+
(aq) + 2e
2Ag(s) E = 0.80 V
Fe(s) + 2Ag
+
(aq) Fe
2+
(aq) + 2Ag(s) E =1.24 V
3 Cu(s) |Cu
2+
(aq)| |Ag
+
(aq)| Ag(s)
4 Galvanic: Chemical energy is converted to electrical energy. The reaction is
spontaneous with a positive voltage.
Electrolytic: Electrical energy is converted to chemical energy. The reaction is not
spontaneous and energy must be supplied.
Review exercise 21.2
1 a Galvani: The muscle contracted due to the passage of an electric current.
The muscle had generated the electricity.
Volta: The different metals in a solution generated the electricity, not the muscle.
b Volta made the first galvanic cell to produce an electric current.
2 Na, K, Mg and Ca are found in nature in compounds which contain their stable ions.
Electrolytic techniques enabled electrons to be added to these metal ions and convert
them to elements.
Solutions Manual: Module 5
Shipwrecks, corrosion and conservation
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Review exercise 21.3
1 To obtain a reactive metal by electrolysis, the metal ion must be reduced at the cathode.
If any water is present, it will be reduced preferentially at the cathode, since the energy
required to do so is less than that needed to reduce the metal ion. Thus the metal ion
must be in a liquid, not aqueous, state.
2 Oxidation: oxygen 2H
2
O(l)
O
2
(g) + 4H
+
(aq) + 4e
Reduction: hydrogen 2H
2
O(l) + 2e
H
2
(g) + 2OH
(aq)
3 Possible cathode reactions:
Cu
2+
(aq) + 2e
Cu(s) E = 0.34 V
2H
2
O(l) + 2e
H
2
(g) + 2OH
(aq) E = 0.83 V
Deposition of Cu(s) is favoured as it has a more positive E value. This remains
unaltered as [CuCl
2
] increases.
Possible anode reactions:
2Cl
(aq)
Cl
2
(g) + 2e
E = 1.36 V
2H
2
O(l)
O
2
(g) + 4H
+
(aq) + 4e
E = 1.23 V
Under standard conditions, the oxidation of water is favoured as it has a more positive
E value. However, as [Cl
] increases, the discharge of chlorine gas becomes more
favourable.
4 a Cathode: silver 2Ag
+
(l) + 2e
2Ag(l)
Anode: iodine 2I
(l) I
2
(l) + 2e
b Cathode: silver 2Ag
+
(aq) + 2e
2Ag(s)
Anode: oxygen 2H
2
O(l) O
2
(g) + 4H
+
(aq) + 2e
c Cathode: hydrogen 2H
2
O(l) + 2e
H
2
(g) + 2OH
(aq)
Anode: oxygen 2H
2
O(l) O
2
(g) + 4H
+
(aq) + 2e
Chapter 21 Application and investigation
1 a Fe(s) + Cu
2+
(aq) Fe
2+
(aq) + Cu(s)
brown
b Copper ions are reduced to copper atoms by the addition of electrons. These
electrons come from iron atoms which are oxidised. The transfer of electrons from
one species to another constitutes a redox reaction involving simultaneous gain
and loss of electrons.
Solutions Manual: Module 5
Shipwrecks, corrosion and conservation
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
2 a, b
3 Investigation
4 Investigation
5 Electrons are gained in reduction and lost in oxidation. The number of moles of
electrons lost in oxidation equals the number of electrons gained in reduction. The
charge on ions is equal in magnitude to exactly one, two, three or four times that of one
electron. This corresponds to the transfer of electrons during the formation of ions.
By using the formula Q = It and
4
10 65 . 9
) e (
Q
n , it is possible to work out the
number of moles of electrons which flow through a circuit when a given current flows
for a fixed time.
Volumes and masses of products can be calculated, knowing the numbers of electrons
involved at each electrode half equation.
6 CrO
4
2
(aq) + 8H
+
(aq) + 6e
Cr(s) + 4H
2
O(l)
Solutions Manual: Module 5
Shipwrecks, corrosion and conservation
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
7
8 Silver anode would dissolve as the silver metal is oxidised.
Ag(s) Ag
+
(aq) + e
E = 0.80 V
which has a higher positive value of E and therefore occurs in preference to
2H
2
O(l) O
2
(g) + 4H
+
(aq) + 4e
E = 1.23 V
9 In electrolysis, the most positive E values of possible half reactions at the electrodes
will nominate which reaction occurs, e.g.
reduction at cathode: Na
+
(l) + e
Na(s) E = 2.71 V
or 2H
2
O(l) + 2e
H
2
(g) + 2OH
(aq) E = 0.83 V
Reduction of water is preferred.
Other factors affecting products at anode and cathode:
i nature of electrodesrefer to text page 378
ii molten or aqueous electrolyterefer to text page 377
iii concentration of electrolyterefer to text page 378
CHAPTER 22: CORROSION
Review exercise 22.1
1 Corrosion involves the oxidation of a metal to form positively charged ions by the loss
of electrons. The electrons are generally transferred to atmospheric O
2
or other
substances acting as oxidising agents.
2 A very thin coating of metal oxide on the surface of the metal can form a protective
layer, preventing any further reaction between the metal and the oxidising agent.
Solutions Manual: Module 5
Shipwrecks, corrosion and conservation
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
3 Chromium, zinc, aluminium or manganese.
Review exercise 22.2
1 Ready availability. Economical extraction and refining methods.
2 Iron is an active metal susceptible to corrosion. Moist, salty sea conditions accelerate
the rusting process. Rusting requires moisture and oxygenconditions to which ships
are always exposed.
3 An iron bar on the ground can trap moisture between the ground and the bottom layer.
The underside becomes an anodic site where the iron oxidises. The surfaces of the iron
bar exposed to atmospheric oxygen become cathodic sites of reduction. The moisture
between the ground and the iron bar would contain certain dissolved salts which act as
an electrolyte and would accelerate the rusting process.
Iron nails stored in a sealed bottle would have atmospheric oxygen surrounding them
but only water vapour in the air, so the rate of corrosion is much less.
4 a The differential aeration principle applies. Between the tightly woven central
strands, water may be trapped and oxygen is excluded. These surfaces become
anodic sites. The external strands are exposed to air and become cathodic sites.
b Central strands, anodic sites:
Fe(s) Fe
2+
(aq) + 2e
Outer strands, cathodic sites:
O
2
(g) + 2H
2
O(l) + 4e
4OH
(aq)
5 Copper is a less reactive metal than iron, and galvanic action occurs. The iron becomes
anodic and corrodes:
Fe(s) Fe
2+
(aq) + 2e
The copper becomes cathodic where oxygen from the air is reduced:
O
2
(g) + 2H
2
O(l) + 4e
4OH
(aq)
Review exercise 22.3
1 The magnesium is a sacrificial anode. It corrodes preferentially to the steel tank.
2 Pipelines may be painted, put at a negative potential at intervals, or have attached
sacrificial anode blocks at intervals. They may also be mounted above ground to reduce
corrosion by the differential aeration principle.
3 The standard reduction potential:
Cu
2+
(aq) + 2e
Cu(s) E = 0.34 V
This is more likely to occur than the oxidation of copper atoms or the Cu
+
ion:
Solutions Manual: Module 5
Shipwrecks, corrosion and conservation
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Cu(s) Cu
2+
(aq) + 2e
E = 0.34 V
Cu
+
(aq) Cu
2+
(aq) + e
E = 0.16 V
The higher E value of the reduction half equation for Cu
2+
ions protects the metal from
corrosion.
Chapter 22 Application and investigation
1 In coastal areas such as Kiama and Byron Bay, sea spray produces the necessary
conditions for rustingwater, oxygen and electrolytic salts. These conditions promote
accelerated rusting of steel structures compared to inland areas such as Dubbo where
there is less water in the atmosphere and negligible electrolytic salts present in this
atmospheric water.
2 Aluminium is a passivating metal which forms a very thin, but strongly adhering, layer
of aluminium oxide on its surface. This oxide layer protects the metal from further
oxidation and is not soluble in water.
3 Investigation
4 a i Yes. No reaction between the nickel and iron(III) salt would occur.
ii No. The nickel can react with the iron(III) salt:
Ni(s) + 2Fe
3+
(aq) Ni
2+
(aq) + 2Fe
2+
(aq) E(cell) = +1.03 V
b No. The steel tank would corrode:
Fe(s) + Cu
2+
(aq) Fe
2+
(aq) + Cu(s) E(cell) = +0.78 V
5 Magnesium is a more active metal than iron. It would form its own protective oxide
coating and protect the underlying steel. It would also act as a sacrificial anode if the
magnesium layer were cracked. However, magnesium is readily attacked by mild acids.
Gold, copper and nickel are less reactive metals than iron. Copper and nickel will form
their own protective oxide coating and gold will remain untarnished. All will protect the
iron. However, any tiny gaps in the surface layer will lead to galvanic action in which
the iron is preferentially oxidised. In addition, these are expensive metals.
6 Tin is a less reactive metal than iron. Once the tinplate of a can is broken, the iron will
preferentially corrode by galvanic action.
Galvanised iron has a protective zinc coating. If the protective coating is broken, the
zinc will preferentially corrode before iron because zinc is a more active metal than
iron.
7 Aluminium forms a tenacious, impervious oxide layer on its surface.
Incorporating it with zinc in Zincalume
will enhance the protective oxide layer that
zinc would form by itself.
Solutions Manual: Module 5
Shipwrecks, corrosion and conservation
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
8 Just below the surface the steel becomes the anodic site for oxidation. The sea water,
rich in electrolytes, transfers the electrons to the steel surface exposed to atmospheric
oxygen above the water, which then acts as the cathodic site.
Iron just under water dissolves away, while rust forming on the cathodic area flakes
away faster due to the action of the sea.
9 a Magnesium bar: Mg(s) Mg
2+
(aq) + 2e
Steel pipe: 2H
2
O(l) + 2e
H
2
(g) + 2OH
(aq)
b Electrons flow from the magnesium bar to the pipe.
10 Investigation
CHAPTER 23: CAN CHEMISTRY SAVE THE TITANIC?
Review exercise 23.1
1 Sunlight would not affect the temperature of water in deep, dark depths. There are fewer
significant convection currents that would mix water from different depths.
2
Variable Effect
Temperature Increase temperature, increase reaction rate of corrosion.
Light Increase light, increase photosynthetic aquatic organisms and their
consumers, which could increase corrosion below water line.
Dissolved O
2
Corrosion is greatest where [O
2
] is greatest. O
2
is reduced at the
cathode, as Fe is oxidised at anode site.
Dissolved CO
2
As [CO
2
] increases, acidity increases as carbonic acid provides H
+
ions to promote reduction of O
2
and so increase corrosion rate.
Salinity As salinity increases, the number of ions increases. The electrolyte
becomes more effective and so accelerates corrosion.
Review exercise 23.2
1 The lower temperatures would decrease the rate of the corrosion reaction.
2 Anaerobic bacteria are prevalent on ships since the hull provides a more ready supply of
iron than sea water. The bacteria reduce sulfates to hydrogen sulfide, which in turn
increases the acidity of the sea water. This promotes the reduction of O
2
as well as
aiding in dissolving rust. Hydrogen sulfide also reacts with other metals to form
insoluble metal sulfides.
Solutions Manual: Module 5
Shipwrecks, corrosion and conservation
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
3 Less active metals in sea water close to the steel hull accelerate the corrosion of iron.
An electrochemical cell is set up with, for example, the less active copper acting as a
cathode to the iron anode of the hull.
Review exercise 23.3
1 In oak artefacts, the cellular structure of the wood is destroyed by crystallising sulfate
and chloride ions. Salt crystals in fine pores or cracks can distort the shape of an
artefact, break it up or react chemically with it.
2 Oxidation of any exposed silver would take place, removing more silver from the coin.
3 Preservative of wood and it restores supple structure to leather.
Chapter 23 Application and investigation
1
Factor a
Surface
sea water
b
Deep
sea water
Difference from a b in rate of Fe
rusting
Temperature higher low Rate decreases with decrease in
temperature.
Light intensity high low Damaging sea life decreases with decrease
in photosynthetic organisms together with
associated consumers.
Dissolved O
2
high low Oxidation of Fe decreases with reduction of
oxygen levels.
Dissolved CO
2
low higher As pH decreases with dissolving CO
2
, the
oxidation of Fe would be promoted.
Salinity high higher Improved electrolytic action with more ions
present would increase corrosion rate.
2 a In deep waters it was expected that the lack of damaging sea life, salinity levels,
freezing temperatures and low oxygen content would leave the Titanic well
preserved.
b Galvanic action was set up between the steel and the presence on board of a
number of other less active metals. Differential aeration caused by sediment or
calcareous deposits from marine organisms resulted in high levels of localised
corrosion. Oxygen levels were higher than anticipated. This increased the
corrosion rate.
Solutions Manual: Module 5
Shipwrecks, corrosion and conservation
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Slight lowering of pH levels due to the presence of anaerobic bacteria accelerated
the reduction of O
2
and hence the oxidation of iron. Hydrogen ions may aid in the
dissolving of rust.
3 a SO
4
2
(aq) + 10H
+
(aq) + 8e
H
2
S(aq) + 4H
2
O(l)
b 2Ag(s) + H
2
S(aq) Ag
2
S(s) + H
2
(g)
4 a In oak artefacts, the cellular structure of the wood is destroyed by crystallising
sulfate and chloride ions. Salt crystals in fine pores or cracks can distort the shape
of an artefact, break it up or react chemically with it.
b Treatment with weak acids such methanoic acid or ammonium citrate. Mechanical
cleaning.
c Chloride and sulfate ions are allowed to diffuse out of the wood by replacing the
ionic solution with polyethylene glycol as a preservative.
In leather, the salts are leached out by placing it in distilled water and then in a
30% PEG solution for approximately 2 weeks. The leather absorbs PEG to keep it
supple even after drying.
5 a The formation of silver sulfide can be reversed by making the silver coin the
cathode in an electrolyte cell:
cathode: Ag
2
S(s) + 2e
2Ag(s) + S
2
(aq)
also 2H
2
O(l) + 2e
H
2
(g) + 2OH
(aq)
The resulting H
2
(g) helps to loosen any corroded material on the surface of the
coin.
b Oxides and chlorides of iron may be reduced to form elementary iron if the
affected object is placed in an alkaline solution and made into the cathode in an
electrolysis reaction.
Fe
2
O
3
(s) + 3H
2
(g) 2Fe(s) + 3H
2
O(l)
FeCl
2
(s) + H
2
(g) Fe(s) + 2HCl(g)
68 Investigation
Module 5
REVIEW
1 Galvanic action was set up between the steel and the presence on board of a number of
other less active metals. Differential aeration caused by sediment or calcareous deposits
from marine organisms resulted in high levels of localised corrosion. Oxygen levels
were higher than anticipated. This increased the corrosion rate.
Solutions Manual: Module 5
Shipwrecks, corrosion and conservation
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Slight lowering of pH levels due to the presence of anaerobic bacteria accelerated the
reduction of O
2
and hence the oxidation of iron. Hydrogen ions may aid in the
dissolving of rust.
2 a Paint prevents oxygen and water coming into contact with the iron.
b Cathodic protection of steeluse of a sacrificial anode such as Mg or Zn which
oxidises preferentially to the iron, preventing the formation of Fe
2+
ions. A
sacrificial anode is attached to the hull at regular intervals and must be covered by
sea water so that ions can migrate between anode and cathode.
c Iron alloyed with elements such as chromium, carbon, molybdenum and
manganese form steels which are resistant to corrosion. Pure stainless steel
effectively resists rust but is prohibitively expensive. Hulls of new ships now have
surface stainless steel alloys which protect the bulk of the steel underneath from
corrosion.
d Corrosion of steel requires the presence of O
2
, water and electrolytes in the water.
The ocean environment has an abundance of sea water and sea spray containing
many ions. O
2
is in the air and is dissolved in upper layers of the ocean. Unless
steel is protected from the water and oxygen, the corrosion rate is very high.
3 Iron corrodes to form Fe
3+
ions:
4Fe(s) + 3O
2
(g) + 2H
2
O(l) 2Fe
2
O
3
.H
2
O(s)
The resulting oxide is soft and porous, allowing O
2
and H
2
O to pass through it to
another Fe layer, which corrodes again.
Aluminium oxidises to form a very thin coat of aluminium oxide, which strongly
adheres to the metal layer, is insoluble in water and thus forms a protective barrier
against more oxidation occurring.
4Al(s) + 3O
2
(g) 2Al
2
O
3
(s)
4 a For a reaction to occur spontaneously, the sum of the two half reactions in the
redox reaction must have a positive E value.
Anode: Fe(s) Fe
2+
(aq) + 2e
E = 0.44 V
Cathode: O
2
(g) + 2H
2
O(l) + 4e
4OH
(aq) E = 0.40 V
The sum of these two reactions is E = +0.84 V
Hence, providing an electrolyte is present to allow migration of ions between
electrodes, the reaction is spontaneous.
b Acidic conditions could change the cathode reaction to:
O
2
(g) + 4H
+
(aq) + 4e
2H
2
O(l) E = 0.82 V
which would increase the overall value of the redox reaction and hence accelerate
the rusting process.
Solutions Manual: Module 5
Shipwrecks, corrosion and conservation
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
5 Soft drink is carbonated:
CO
2
(aq) CO
2
(g)
Releasing the pressure above the drink when the can is opened causes more CO
2
(g) to
come out of solution and the solution to go flat. As temperature increases, the reaction
rate would increase as well.
As temperature increases outside the refrigerator, the solubility of CO
2
in solution
decreases and more CO
2
is released to the environment.
6 a Ca
2+
ions are present in sea water. Decomposing organisms which act on paper
and leather produce CO
2
(g) which in turn reacts with water to form HCO
3
(aq).
CO
2
(g) + H
2
O(l) H
2
CO
3
(aq)
H
2
CO
3
(aq) H
+
(aq) + HCO
3
(aq)
Thus the two ions Ca
2+
(aq) and HCO
3
(aq) can react to form calcium carbonate.
b Careful mechanical cleaning and chemical cleaning with relatively weak acids
such as methanoic acid or ammonium citrate.
7 a When twisted, the surface oxide layer will develop fine cracks and expose the
metal. The orderly crystal structure of iron is distorted, making it easier for
individual Fe atoms to break away from the crystal as Fe
2+
ions from anodic
oxidation.
b Greater moisture in the air which contains sea salts acts as an effective electrolyte,
which accelerates corrosion.
c Zinc is a passivating metal which forms a coating to protect steel nails. If the
coating is damaged, exposing the steel beneath, zinc will act as a sacrificial anode
since it is oxidised more readily than iron.
A tin coating also excludes oxygen and water from the steel nail surface, but if the
steel is exposed, the iron has a lower ionisation energy than tin and will oxidise
first.
8 Faraday established the basic laws of electrolysis that govern the quantitative
relationships in electrolytic processes.
Faradays 1st lawrefer to text page 375
Faradays 2nd lawrefer to text page 375
9 a It is a sacrificial anode.
b Zn(s) Zn
2+
(aq) + 2e
E = 0.76 V
As the zinc is oxidised, it forms Zn
2+
(aq), so must be replaced regularly.
c The iron in ocean oil and gas rigs can corrode or oxidise according to the half-
reaction
Solutions Manual: Module 5
Shipwrecks, corrosion and conservation
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Fe(s) Fe
2+
(aq) + 2e
E = 0.44 V
Any metal which has a more positive E value than Fe(s) for the oxidation half-
reaction will oxidise first and so can act as a sacrificial anode, provided it does not
react with water itself.
Instead of Zn, you could use Cr, Mn, Al, Mg.
10 a In a galvanic cell, a spontaneous chemical reaction takes place in which stored
chemical energy is converted to electrical energy.
b
c anode: Zn(s) Zn
2+
(aq) + 2e
cathode: Ag
+
(aq) + e
Ag(s)
For every mole of Zn (65.39 g) lost at the anode, 2 mol of Ag (2 107.9 g) are
deposited at the cathode.
Mass Zn lost : mass Ag deposited
65.39 g : 215.8 g
1 : 3.3
11 a Copper is resistant to corrosion and has a higher ionisation energy value than Mg.
This enables Mg to act as a sacrificial anode.
The oxidation half reaction for Cu(s) has E = 0.34 V
Fe(s) has E = +0.44 V
Mg(s) has E = +2.37 V
The highest E value will occur first.
Solutions Manual: Module 5
Shipwrecks, corrosion and conservation
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
b From 11a, magnesium will have the greatest tendency to oxidise. As well as
reducing O
2
, the electrons from magnesium can reduce any Fe
2+
ions that are
formed and so inhibit corrosion.
c Underground pipes are exposed to O
2
and particularly water containing dissolved
salts. The presence of an efficient electrolyte accelerates corrosion.
12 a Chemical weathering from terrestrial environments.
Hydrothermal vents in mid-ocean ridges.
b Cl
, SO
4
2
c Desalination: Leaching takes time. Chloride ions are difficult to remove and
further electrolysis is required. Materials often have to be treated with
preservatives.
Electrolysis: An example is the reduction of Ag
2
S on silver objects with an inert
anode in an alkaline solution. It is very effective as this method allows restoration
of Ag markings on objects. Hydrogen gas produced by water reduction at the
cathode on the surface of the object can help to loosen any flakes of corroded
material.
13 Galvani joined two different metallic wires together and placed the unjoined ends into a
freshly extracted frog muscle. The electric current that was generated contracted the
muscle. Galvani thought the muscle had generated electricity, not the wires in the
solution. Galvani is credited with the first generation of an electric current.
Alessandro Volta showed that Galvanis frog muscle contraction was the consequence
of metallic electricity resulting from contact between the two different metals when he
made the first battery with two different metals coming into moist contact with each
other. He went on to develop the Voltaic Pile, the first direct current battery.
Davy developed improved versions of Voltas pile, which he recognised as producing a
source of electrons from a chemical reaction. Electric current could be used in
electrolysis reactions to decompose water and prepare samples of the very reactive
elements of potassium and sodium from their molten salts.
Thus, Galvani unknowingly introduced the notion of electricity as being associated with
differences between two different metals, Volta knowingly developed this idea and
generated electricity in a prototype battery with the idea that something was able to flow
between the metals, and Davy extended the concepts to chemical compounds.
14 a zinc, magnesium
b Zn(s) Zn
2+
(aq) + 2e
E = +0.76 V
Fe
2+
(aq) + 2e
Fe(s) E = 0.44 V
Solutions Manual: Module 5
Shipwrecks, corrosion and conservation
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
The E value for the overall reaction is + 0.32 V, which indicates that the reaction
can occur spontaneously. Electrons are lost at the zinc anode (oxidation) and
transferred to the iron cathode.
c Cathode. Reduction of any Fe
2+
(aq) occurs through the electrons being lost by the
sacrificial anode.
15 a 2H
2
O(l) + 2e
H
2
(g) + 2OH
(aq) E = 0.83 V
b 1 mole of electrons has a charge of 9.649 10
4
coulombs. For one mole of H
2
(g)
to be produced,
2 (9.649 10
4
) C are needed.
For 7 10
8
mol of H
2
gas,
2 (9.649 10
4
) (7 10
8
) C are needed = 1.351 10
14
C
c 7.0 10
8
mol of H
2
gas is approximately 1.71 10
10
L at 25C and 101.3 kPa.
Vast quantities of H
2
bubbling to the surface would displace the air above the H
2
source, leading to a lack of O
2
for the crew.
H
2
gas is highly flammable. With air it forms an explosive mixture.
16 a Reduced as silver ions gain electrons:
Ag
+
(aq) + e
Ag(s)
b Aluminium metal acts as the anode in the galvanic cell that is set up, donating
electrons to the silver ions.
Anodic oxidation: Al(s) Al
3+
(aq) + 3e
c An abrasive cleaner removes the silver content in the tarnish layer on the metal
surface. Reduction of silver ions restores the silver to the object.
17 Crystallisation of Cl
and SO
4
2
salts can cause considerable damage to the structure of
materials such as wood and leather.
Non-metal porous artefacts must be leached of salts, and protected by preservatives and
fungicides before they are brought to the surface and dried.
Artefacts must also be cleaned of calcium carbonate encrustations and sediments.
18 Shipwreck A: Much greater corrosion rate of hull if it is made of iron. Plentiful
supply of O
2
. Does not dry out. Temperature high, good for reaction
rate due to location. Hull in greater light, more organisms to aid in
breakdown of materials. Because of shallow waters, artefacts of
different metals have probably been removed.
Shipwreck B: Limited supply of sea O
2
. Cold water. Lower number of destructive
sea organisms. Anaerobic bacteria play larger role in corrosion.
Interacting different metal artefacts could accelerate corrosion.
Solutions Manual: Module 5
Shipwrecks, corrosion and conservation
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
19 a Low O
2
content of water; very low temperatures; lack of damaging sea life.
b Higher O
2
content in the deep water currents than expected, accelerated rate of
corrosion of steel structure as oxygen reduced cathodically.
Presence of different metal artefacts set up galvanic corrosion in localised areas of
shipwreck.
20 a In an electrolytic cell, electrical energy is converted to chemical energy. The
reaction is not spontaneous and energy must be supplied.
b
c Cu
2+
(aq) + 2e
Cu(s)
d Concentration of ions in electrolyte.
Quantity of electrical charge passing through the circuit.
Solutions Manual: Module 6
Forensic chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
CHAPTER 24: THE ROLE OF FORENSIC CHEMISTRY
Review exercise 24.1
1 Theft, fraud, counterfeiting, drink driving, arson
2 Forensic chemists may test samples associated with a crime and be asked to testify in
court. They do not solve a crime individually; rather, they provide specific pieces of
evidence used in constructing a case.
3 All scientific tests, including chemical ones, have errors associated with them. It is
possible (although sometimes highly unlikely) to get a false positive (or false negative)
result. A forensic chemist has an ethical responsibility to explain how likely or unlikely
this is to happen for a particular test. This can make it difficult to answer questions in
court that require a yes or no response, such as Are you 100% sure that the sample
you tested came from the suspect? The answer might be no even when the test is
positive and 99.9% accurate! One way to solve this ethical dilemma would be for the
forensic chemist to try to give a more complex answer that conveys the accuracy of the
test and meets the requirements of the court. A forensic chemist must be impartial. This
can raise ethical difficulties if the forensic chemist is employed by the prosecution or
the defence. Ideally, this should not be the case.
Review exercise 24.2
1 The possibility of sample contamination can be used to discredit chemical forensic
evidence. For example, this happened in the 1997 trial of O.J. Simpson, in which DNA
evidence to be used in the case against Simpson was successfully discredited. Forensic
laboratories need to ensure that such contamination cannot occur.
2 A DNA sample can be contaminated by a flaking skin cell from another person. An
unsealed or unsecured sample of blood or urine from an athlete could be contaminated
by the addition of a banned substance.
3 Chain of custody refers to the full tracking of samples from athlete to final result. It
includes observation of athletes while samples are being provided, labelling and
packing of samples in the athletes presence and transportation of samples under
security.
4 Napoleon died in 1821 after a long period of ill health. Doctors performing the autopsy
concluded that a perforated, cancerous stomach ulcer was the cause of death. In the
1950s forensic scientists found that samples of Napoleons hair contained very high
levels of arsenic and suggested that Napoleon had been poisoned with arsenic oxide or a
similar compound. By the 1950s it was known that arsenic bonds strongly to sulfur
atoms, including those found in the hair protein keratin. It remains bound to keratin as
hair grows, thus providing evidence of arsenic intake. This knowledge was unavailable
in 1821.
Solutions Manual: Module 6
Forensic chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Another forensic chemist showed that the wallpaper in Napoleons residence was a
source of arsenic that could have adversely affected Napoleons health. Wallpaper made
in the 1800s commonly used a green pigment called Scheeles green, containing
copper(II) arsenite. This chemical breaks down, releasing a vapour containing arsenic
when the wallpaper became damp or mouldy. The scientist suggested that Napoleon
probably died of stomach cancer as proposed in the original diagnosis but that arsenic in
the wallpaper caused a decline in his health. The chemical contents of the green pigment
and factors relating to its breakdown were unknown in 1821.
All of the evidence relating to Napoleons ill health and/or death due to arsenic
poisoning was gathered by analysing trace quantities of arsenic from hair and wallpaper
samples. Such analyses require sophisticated extraction methods and the use of
instrumentation such as spectrometers, as well as knowledge unavailable at the time of
Napoleons death.
Chapter 24 Application and investigation
1 Investigation
2 Investigation
3 Forensic pharmacists study medicinal drugs and forensic toxicologists study poisons.
4 Investigation
5 Analytical chemists seek to identify or work out the concentrations of chemicals often
in trace quantities. Sophisticated separation and analytical techniques have been
developed by analytical chemists to aid this process. Such techniques are invaluable in
forensic chemistry where samples to be used as evidence only contain trace quantities of
the chemicals to be identified and/or matched with the victim, suspect or scene of the
crime.
6 If samples may have been contaminated, the evidence associated with them can be
discredited. This was illustrated in the 1997 trial of O.J. Simpson in the US, in which
Simpsons lawyers used the possibility of sample mishandling or tampering to discredit
DNA evidence presented against Simpson. A chain of custody in sample testing helps to
overcome this problem. In this process (which is often used with athletes), all samples
are tracked throughout the testing process from suspect to final result.
7 Duplicate samples are collected so that a second independent test can be performed if
the first test identifies the presence of a banned substance. Athletes personally select and
seal their sample containers to prevent the possibility of someone else tampering with
samples. Athletes have the right to a second analysis if a positive test result is obtained
in case the test result is a false positive, i.e. a positive result has appeared for some
reason other than the presence of a banned substance in the athletes blood or urine.
8 Investigation
Solutions Manual: Module 6
Forensic chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
CHAPTER 25: ANALYSIS OF MATERIAL
Review exercise 25.1
1 Compounds can be classified according to (i) their original source; (ii) their solubility in
water; (iii) the types of particles found in the substance.
2 Millions of compounds exist on Earth. The process of identifying a compound utilises
the classification process. For example, if a compound undergoes combustion,
producing carbon dioxide or carbon monoxide and/or soot under reduced oxygen
conditions, then it is organic. Organic compounds can be further classified into groups
including hydrocarbons, alkanols and alkanoic acids. Having established that a
compound is organic, further tests can be performed to determine to which group of
organic compounds it belongs. This process can be continued until the compound is
specifically identified. Forensic chemists need to be able to classify compounds because
the process of classifying a compound using chemical testing enables identification of
the compound.
3 Soil particle size, microorganism content and chemical composition (such as
percentages of quartz and calcium carbonate) can be used to provide specific location
details. The presence of foraminifers indicates that a soil is of marine origin and may
sometimes provide specific location details due to the wide variety of foraminifers
geographically and over geological time.
Glass fragments can be used to match cars with particular car accidents and criminals
with crime scenes. This is possible because glasses vary in composition and physical
properties. Density and refractive index measurements are used to identify glass
samples.
Metals and metallic ions occur in soils, paints, glass and metal tools associated with
crimes. Metal ion compositions can be used to match a sample with its source.
Techniques utilised include mass spectra, atomic absorption spectra and scanning
tunnelling microscopy. It is possible to use fingerprinting to determine the sources of
gold samples because two different samples mined from the same source should have
the same distribution of impurities.
4 a The forensic chemist would be involved in the detection and identification of
substances such as accelerants as well as substances that might be matched with
the suspect, such as trace fibres, paint and glass.
b Matching paint fragments from victims clothing with the car, matching blood
from the victim with residues found on the car, matching the rubber of the tyres,
and oil from the car with samples taken from the scene of the accident.
5 Gold contains trace quantities of specific impurities relating to the mineralisation event
in which it was formed. Forensic chemists use mass spectrometry to match gold
associated with crimes with samples mined in the same location. Both samples should
Solutions Manual: Module 6
Forensic chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
have the same distribution of impurities (fingerprint) if they come from the same
location.
6 The fibres do not have the same composition. In a gas chromatograph, different
components are identified by different peak locations and the area under each peak
provides a measure of mole per cent concentration. The two samples have some
significantly sized peaks in quite different locations. For example, sample 1 has a large
peak at 35 minutes but sample 2 has no peak in this position. Sample 2 has a moderately
sized peak at 21 minutes but sample 1 only has a small peak in this position. Sample 1
has a large peak at 13 minutes but sample 2 has no peak in this position. This implies
that sample 1 has some components in significant proportions which are not in sample 2
and vice versa.
Review exercise 25.2
1 Decolourisation of bromine water. Ethene reacts with Br
2
(aq), forming
1,2-dibromoethane. The red-brown colour of the Br
2
disappears. Ethane does not react
with Br
2
(aq).
2 Primary alkanols are oxidised most readily by acidified potassium permanganate.
Secondary alkanols are oxidised more slowly. Tertiary alkanols are resistant to
oxidation by acidified permanganate because a large amount of energy is necessary to
break a CC bond in order to form an oxidised product.
3 a Orange to green as the chromium is reduced from an oxidation state of +7 in the
dichromate ion to an oxidation state of +3
3CH
3
CH
2
OH(aq) + Cr
2
O
7
2
(aq) + 8H
+
(aq)
3CH
3
CHO(aq) + 2Cr
3+
(aq) + 7H
2
O(l)
(orange) (green)
b The driver would blow into the breathalyser bag. If there was a high concentration
of alcohol in the drivers blood, there would also be considerable alcohol in his or
her breath. This alcohol (ethanol) would dissolve in the dichromate solution,
changing it from orange to green.
Review exercise 25.3
1 a
Carbohydrate class Carbohydrate Origins in nature
Monosaccharide glucose fruits, honeys
Monosaccharide fructose flowers, fruits, honeys
Disaccharide sucrose cane sugar, sugar beet
Disaccharide lactose milk
Disaccharide maltose grainshydrolysis of starch
Solutions Manual: Module 6
Forensic chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Polysaccharide starch plantsenergy source
Polysaccharide glycogen animalsenergy source
Polysaccharide cellulose plantsstructural material
b cellulose
2 In the case of maltose, which is a reducing sugar, a red precipitate of copper(I) oxide
should be formed. Benedicts solution is an alkaline solution containing copper(II) ions.
The copper(II) ions will be reduced, forming red copper(I) oxide. The carbonyl group in
the maltose will be oxidised to a carboxylic acid group. Sucrose is not a reducing sugar
because the normally reactive carbonyl group within the glucose and fructose units
making up sucrose is involved in the glycosidic bond linking the two molecules.
Sucrose therefore cannot be readily oxidised, so no reaction should take place.
3 Starch, glycogen and cellulose are all polymers consisting of linked glucose units. They
have the general formula (C
6
H
10
O
5
)
n
. Starch and glycogen consist of long chains of
glucose units but the chains are longer and more highly branched in glycogen than in
starch. Cellulose consists of long chains of glucose units but the glucose molecules are
oriented differently to give a more linear molecule. This permits the polymer molecules
to pack more closely together in bundles linked together by hydrogen bonds. (Both
starch and glycogen are made up of -glucose monomers; cellulose is made up of -
glucose monomers with every second glucose monomer inverted.)
4 Substances containing starch change colour to blue-black in the presence of an
iodine/potassium iodide solution.
Starch + iodine solution
starchiodine complex
(brown) (blue-black)
This test gives a negative result with cellulose, due to the strong hydrogen bonds
between cellulose polymer chains that prevent an interaction with iodine.
5 a The two identifying functional groups of amino acids are the amine (NH
2
) and
carboxylic acid (COOH) groups.
b Amino acids can link together by undergoing condensation reactions to form
peptide bonds. This process can be repeated many times to form polypeptide
chains or proteins which consist of a series of amino acids linked through peptide
bonds.
Chapter 25 Application and investigation
1 Inorganic evidence: soils, glass and paint fragments, metals.
Organic evidence: carbohydrates, lipids, proteins, nucleic acids.
Solutions Manual: Module 6
Forensic chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
2 a Add silver nitrate solution. The formation of a white precipitate that is insoluble in
nitric acid and the absence of a precipitate when barium chloride is added to a
fresh sample of water indicates the presence of chloride ions.
b Calcium carbonate should react with hydrochloric acid, forming carbon dioxide
gas. Carbon dioxide turns a drop of barium hydroxide in an eyedropper milky.
Calcium sulfate should not react with hydrochloric acid.
3 a 2-hexene decolourises bromine solution (i.e. the red-brown bromine solution
changes to colourless). Hexane does not react with bromine solution.
b 1-pentanol is oxidised readily by acidified permanganate solution, i.e. the purple
colour of the permanganate ion changes to colourless. 2-methyl-2-propanol resists
oxidation by acidified permanganate.
c Glucose, a reducing sugar, can be oxidised by Benedicts solution, forming a red
precipitate of copper(I) oxide. Sucrose cannot be oxidised by Benedicts solution.
d Starch turns blue-black in the presence of an iodine/potassium iodide solution,
i.e. the solution changes from brown (the colour of iodine) to blue-black. Glucose
does not react with iodine in this way.
4 a CH
3
CH=CHCH
2
CH
3
(l) + Br
2
(aq) CH
3
CHBrCHBrCH
2
CH
3
(l)
b 3CH
3
CHOHCH
2
CH
3
(l) + Cr
2
O
7
2
(aq) + 8H
+
(aq) 3CH
3
COCH
2
CH
3
(l) +
2Cr
3+
(aq) + 7H
2
O(l)
c 2Cu
2+
(aq) + 2H
2
O(l) + C
6
H
12
O
6
(aq) Cu
2
O(s) + 4H
+
(aq) + C
5
H
9
O
5
COOH(aq)
5 The refractive index of glass is a measure of the change in direction of light as it enters
or leaves glass. Glass will be invisible if it is placed in a medium with the same
refractive index. This makes it possible to quickly determine whether a sample consists
of a particular type of glass and therefore to ascertain its possible uses.
6 a Test for the presence and concentration of ethanol (an alkanol) in the drivers
blood; mass spectrometry.
b Identify the accelerant (a fuel) and try to determine its source; mass spectrometry.
c Fingerprint test the gold, comparing it against gold mined at the minesite.
Compare the mass spectra of the two samples.
d Match the paint sample with the suspects car using gas chromatography.
7
Type of compound Elements present Monomer units
Carbohydrate carbon, hydrogen, oxygen C
6
H
10
O
5
(for polysaccharides)
Lipids carbon, hydrogen, oxygen
Proteins carbon, hydrogen, amino acids
Solutions Manual: Module 6
Forensic chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
oxygen, nitrogen
Nucleic acids carbon, hydrogen,
oxygen, nitrogen
nucleotides
8 a Starch, glycogen and cellulose are all polymers consisting of linked glucose units.
They have the general formula (C
6
H
10
O
5
)
n
. Starch and glycogen consist of long
chains of glucose units but the chains are longer and more highly branched in
glycogen than in starch. Cellulose consists of long chains of glucose units but the
glucose molecules are oriented differently to give a more linear molecule. This
permits the polymer molecules to pack more closely together in bundles linked
together by hydrogen bonds.
b Cellulose is insoluble in water because the hydrogen bonds that could be formed
between water molecules and cellulose are not strong enough to disrupt the
regular arrangement of hydrogen bonds among the closely arranged polymer
molecules.
9 Reducing sugars contain a reactive carbonyl (C=O) group and are therefore easily
oxidised by weak oxidising agents such as copper(II) or silver cations. Two examples of
reducing sugars are glucose and lactose.
10 Investigation
11 a Investigation
b Proteins consist of amino acids linked together by peptide bonds. The 20 amino
acids found in the human body can link together in a myriad of different
combinations, giving rise to the vast number of proteins found in humans.
12 Investigation
13 DNA is characteristic of a particular organism and is independent of the organisms age
and the type of tissue sampled. This means that it can be used to identify a species or an
individual member of a species. Comparisons can be made between different types of
biological samples because every cell contains DNA. DNA can remain intact for
thousands of years, making it possible to preserve evidence. DNA analyses have high
analytical sensitivity. Only tiny samples (about 100 molecules) are needed for an
analysis.
CHAPTER 26: TOOLS OF A FORENSIC CHEMIST
Review exercise 26.1
1 Chromatography involves the separation of a mixture (often in solution) by passing it
over an inert substance like alumina, silica or special paper. The mixture is called the
mobile phase and the inert substance is called the stationary phase. The components of
the mixture are separated because they are adsorbed onto the surface of the stationary
phase with different strengths depending on their particular properties.
Solutions Manual: Module 6
Forensic chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
2 Gas chromatography: used to measure the blood alcohol (ethanol) level of drivers.
Paper chromatography: used to provide evidence about inks and pigments used in
forgeries.
Ion exchange chromatography: used to separate different proteins from each other.
Column chromatography: used for the separation of phenols.
3 Some peaks in the athletes sample coincide with peaks in the standard. It is likely that
the weight lifter was using steroids.
4 Stepwise elution involves passing solutions containing different salt concentrations
and/or different pHs through the column. Proteins with the lowest affinities are eluted
through the column first, followed by those with higher affinities.
5 a Electrophoresis involves separation of a mixture using the property of different
rates of ion migration towards electrodes. For proteins, the rate of migration varies
with molecular mass and total charge of the molecules. The charge on proteins
changes with the pH of the solution. At higher pH values, the charge on proteins
is more negative, owing to the neutralisation of any acidic side chains. After
separation, the proteins are stained and identified. A reference sample is often
used to aid in identification.
b The proteins should be negatively charged because of the neutralisation of any
acidic side chains by the hydroxide ions present in an alkaline solution of pH 9.
Review exercise 26.2
1
Relative mass Fragment
27
29 CH
3
CH
2
41
57 CH
3
CH
2
CH
2
CH
2
and CH
3
CH
2
CO
72
85 COCH
2
CH
2
CH
2
CH
3
114 molecular ion
2 a infra-red, ultraviolet, visible, gamma
b An atom is in its ground state when it has the lowest possible energy, i.e. when the
electron in a hydrogen atom is in the first energy level, the hydrogen atom is in its
ground state.
Solutions Manual: Module 6
Forensic chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
c An excited state is produced by supplying the atom with sufficient energy for
one or more electrons to move to a higher energy level than it would normally be
in if it were in the ground state.
3 Strontium produces a scarlet colour in a flame test and barium a yellow-green colour.
These colours are characteristic of the predominant wavelength of light emitted when
electrons in each type of atom jump from higher energy levels to lower energy levels.
Different atoms have different electron arrangements and energy level differences and
hence emit different colours.
4 a The emission spectrum of an element is the characteristic pattern of wavelengths
emitted by atoms of that element when they are in an excited state.
b The emission spectrum of an element consists of a series of lines because
electrons in the atoms of the element cannot have an infinite number of possible
energy values greater than zero. They can only have energy characteristic of the
energy level they occupy. When electrons are transferred from a higher energy
level to a lower one, radiation of characteristic energy (equivalent to the
difference between the energy levels) is emitted. This radiation has a
characteristic wavelength and colour and gives rise to a particular line in the
emission spectrum of that element.
c Each element has a signature line emission spectrum because it has a unique
pattern of energy level differences between the shells (and subshells) that
electrons can occupy in excited and ground states. This means that the emission
spectrum of an element can be used to identify the element. For example, the
elements in stars can be identified using this technique.
5 Forensic chemistry often involves the analysis of minute samples. For example, one
drop of blood may be the only organic material available for analysis. Ideally, the
sample should be preserved throughout the investigation, i.e. the techniques used should
be non-destructive. Atomic force spectroscopy and scanning-tunnelling microscopy are
two less destructive techniques.
Review exercise 26.3
1 The protein cytochrome c is found is almost all living things. Different species have
slightly different sequences of amino acids in their cytochrome c. The degree of
similarity of the amino acid sequences among different species is an indicator of the
degree of genetic similarity among these species.
2 a A DNA fingerprint consists of single strands of DNA, which have been treated
with DNA probes to become visible. A DNA fingerprint is unique to a particular
individual and can be compared with DNA from suspects and victims to locate
matches.
b DNA is extracted from a sample, then cut into fragments using a restriction
enzyme. DNA fragments are then separated into bands according to size using
Solutions Manual: Module 6
Forensic chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
electrophoresis. DNA probes are bound to the bands. The DNA band pattern is
then transferred to a nylon membrane, which is developed using X-ray film or
other photographic methods.
c DNA fingerprinting is a tremendously powerful tool for forensic chemists because
a DNA fingerprint is unique to an individual.
d If the sample is very small, e.g. a drop of blood, the DNA is amplified by a
polymerase chain reaction (PCR). In this process, the DNA is gently heated to
separate the two strands of the double helix. An enzyme is added which causes
each strand to produce its complementary other half. This process can be
repeated several times until sufficient DNA is available for further analysis.
3 If F and M are both the parents of the child, then each band in the childs DNA
fingerprint should correspond with a band from at least one parent. The childs DNA
fingerprint has some bands corresponding with those of the mother and others that do
not correspond with either parent. This suggests that M is the childs mother but F is not
the childs father.
4 a Advantages: Many criminals reoffend. A DNA database would make it easier
to identify and convict criminals and reduce the chances of
convicting someone who is innocent.
Disadvantages: It would interfere with the freedom of criminals because they
would be forced to contribute to the database.
b Advantages: It would be possible to conduct extensive medical research
using the material in the database together with other
information gathered from medical records (if this was
permitted).
Disadvantages: Once information is readily available in a DNA database, it has
the potential to be misused. For example, many people are
concerned that insurance companies and some employers might
use such information in making decisions about who to insure,
employ, promote etc.
Chapter 26 Application and investigation
1 Many samples used in chemical analysis are very small quantities of mixtures.
Chromatography is particularly useful for separating such samples into the elements and
compounds that make them up.
2 Ion exchange chromatography is used to separate different proteins. A solution
containing the protein mixture is passed through an ion-exchange column. Different
proteins bind to the ion-exchanger with different affinities. Proteins with low affinities
for the ion exchanger move quickly down the column. Those with higher affinities
move more slowly. The affinity of proteins for the ion-exchanger depends on the nature
of their amino acid side chains and on the pH.
Solutions Manual: Module 6
Forensic chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
3 In electrophoresis, the ions in a mixture migrate to either the positive or the negative
electrode at characteristic rates. For proteins, the rates of migration depend on molecular
mass and total charge of the molecules. Different animal species including different
species of fish have characteristic protein profiles. This means that electrophoresis can
be used to distinguish between them.
4 Flame tests: sodium chloride gives a bright yellow colour (characteristic of sodium)
when a solution is sprayed into a flame. Potassium chloride solution gives a lilac colour
characteristic of potassium.
5 The presence of characteristic absorption and emission spectra for particular elements
suggests that electrons in an atom have only certain allowed energy levels. The energy
of the radiation emitted in an emission spectrum or absorbed in an absorption spectrum
represents the energy difference between the level the electron occupied and the level to
which it transferred.
6 Ultraviolet radiation damages the hereditary material in the skin cells (DNA).
7 Destructive analysis occurs when a sample is destroyed during the analysis. This is a
problem in forensic chemistry, because often only very small quantities of samples are
available to be used as evidence.
8 Peaks in the athletes sample match the standard peaks for amphetamine and ephedrine.
9 a The mass spectrum has peaks corresponding to the ions of copper. The height of
each peak indicates the relative abundance of each ion.
b In this case,
63
Cu has an abundance of approximately 70% and
65
Cu has an
abundance of approximately 30%. These relative abundances would give rise to
an average atomic mass of 63 0.7 + 65 0.3 = 63.6, which is very close to the
atomic mass of copper (63.55).
10 Blood, skin, hair and semen.
11 Investigation
12 Investigation
13 a The molecular ion peak corresponds to the relative mass of the molecular ion. The
fragmentation pattern provides information about molecular structure, i.e. the
relative mass of the groups present in the molecule. This information can be used
to determine some of the groups present in the molecule.
b i Top spectrum = 2-methylhexanal
Bottom spectrum = benzaldehyde
ii Top spectrum: 29 = CHO, 43 = CH
3
CH
2
CH
2
,
100 = CH
3
CH
2
CH
2
CH(CH
3
)CHO
+
.
Bottom spectrum: 29 = CHO, 77 = C
6
H
5
, 106 = C
6
H
5
CHO
+
Solutions Manual: Module 6
Forensic chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
14 a The two semen DNA fingerprints (left and centre) match exactly. If one was taken
from a suspect and the other from the victim then it is highly likely that this
suspect was responsible for the crime.
b The childs entire DNA fingerprint features match with either the mother or
possible father. It is highly likely that the possible father is the father of the child.
Module 6
REVIEW
1 a Glucose is a reducing sugar and will give a brick-red precipitate when warmed
with Benedicts solution. The two remaining solutions will not. If acidified
potassium permanganate is added to the remaining two compounds, glycerol will
be oxidised (i.e. the purple colour of the permanganate ion will change to
colourless due to the formation of Mn
2+
). Ethanoic acid will not react with
acidified potassium permanganate, i.e. the solution will remain purple.
b Forensic chemists could distinguish between the three substances using a mass
spectrometer. Each would give a different relative mass characteristic of the
molecular ion and a different pattern of fragments characteristic of the groups in
each compound.
2 a 1-propanol can be oxidised by acidified potassium permanganate more rapidly
than 2-propanol can. In each case, the rate of reaction can be estimated from the
rate of disappearance of the purple colour of permanganate ion from the solution.
b These two alcohols could be distinguished used mass spectroscopy. The spectrum
of 1-propanol should have a peak at 43 corresponding to the fragment
CH
3
CH
2
CH
2
. This peak should be absent from the spectrum of 2-propanol.
3 a Pure: metal (elements pure)
Impure: concrete, cloth, metals (alloys impure), paint and glass. Any of these
samples could also contain organic material such as drops of blood or other
fragments of skin.
b Chromatography: this separation process is based on the differing adsorbances of
the pure substances in the mixture for the material in the column.
c Scanning-tunnelling microscope: this type of microscope scans the surface of
substances using the current flowing between the surface and the probe to map the
sample surface.
4 Electrophoresis could be used to identify the types of vegetables in the vegetable debris.
Electrophoresis uses the rate of migration of ions to identify the biological molecules in
a mixture. Each species of vegetable would have a characteristic protein profile that
could be matched with a reference sample. DNA fingerprinting could be used to identify
Solutions Manual: Module 6
Forensic chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
the species of rodent and even matched with hairs from the actual rodent or rodents. In
this process, DNA is extracted from a sample, cut into fragments using a restriction
enzyme and the fragments are separated into bands using electrophoresis. A radioactive
probe is then used to bind the bands onto a membrane and the bands are detected using
X-ray film. The developed film can be matched with reference samples.
5 a Starch turns blue-black in the presence of an iodine/potassium iodide solution, i.e.
the solution changes from brown (the colour of iodine) to blue-black. Glucose
does not react with iodine in this way.
b Glucose, a reducing sugar, can be oxidised by Benedicts solution, forming a red
precipitate of copper(I) oxide. Sucrose cannot be oxidised by Benedicts solution.
6
Polysaccharide Monomer
units
Shape of polymer Function
Starch glucose branched energy storage
in plants
Glycogen glucose highly branched energy storage
in animals
Cellulose glucose straightevery second
monomer is inverted
structural, in
plants
7 a amino acids
b
c The two compounds have different molecular masses, so their ions migrate
towards the electrodes at different speeds when a voltage is applied.
d At high pHs the acidic side chains on these molecules will be neutralised, forming
negatively charged ions, which will be attracted to the positive electrode.
8 If samples become contaminated with other DNA-containing material, the results of the
analysis will be invalid. Some of the bands on the DNA fingerprint may be caused by
the DNA of the contaminating substance rather than by the DNA being analysed.
Sources of potential contamination include drops of blood, flakes of skin and hair.
9 a The emission spectrum of an element is the characteristic pattern of wavelengths
emitted by atoms of that element when they are in an excited state.
Solutions Manual: Module 6
Forensic chemistry
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
b Each element has an individual emission spectrum because it has a unique pattern
of energy level differences between the shells (and subshells) that electrons can
occupy in excited and ground states. This means that the emission spectrum of
an element can be used to identify the element.
c The flame colours are the colours produced by combining the brightest lines in the
emission spectrum of the metal ion of the salt.
10 a Non-destructive analysis includes analytical methods in which the sample is not
destroyed during the process of the analysis.
b Often only minute quantities of samples are available for analysis, e.g. one drop of
blood. It is desirable to carry out as many tests as possible on these samples and to
preserve them in case further testing is required.
c Atomic force microscopy: in this process, a microscope scans a very sharp tip
with a flexible lever over the surface of the substance. The tip moves up and down
as the forces between it and the sample surface vary. A computer uses the
variation in the forces to produce a topographic image of the surface of the
sample.
Solutions Manual: Module 7
The biochemistry of movement
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
CHAPTER 27: THE FOODS WE EAT
Review exercise 27.1
1 carbon, hydrogen and oxygen
2 Glucose is a six-membered ring compound with the formula C
6
H
12
O
6
. It is the
compound from which all livings derive chemical energy during respiration. It is also a
building block for many other carbohydrate structures. Glycogen is a condensation
polymer of glucose found in animals as a storage compound for glucose.
3 Glycogen is a polysaccharide made up of individual glucose monosaccharides. When
two of these monosaccharides link up, a molecule of water is eliminated. This is a
condensation reaction.
Review exercise 27.2
1 One of the major functions of fats in the diet of humans is to provide energy.
2 a Palmitic acid is saturated. The ester is a fat.
Solutions Manual: Module 7
The biochemistry of movement
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
b Two saturated fatty acids and one unsaturated. The ester is a fat.
c Both are unsaturated fatty acids. The ester is an oil.
glycerol + 1 oleic acid + 2 linoleic acid 1-oleoyl-2-linoleoyl-3-linoleoyl-
glycerol
3 a As the number of carbon atoms increases, their melting points rise.
b As the number of carbon atoms increases, the molar mass increases and the
intermolecular attractions increase. This will elevate the melting points.
Review exercise 27.3
1 Fibrous proteins tend to be long, tough molecules that provide structure for many body
components including bone, hair, skin, tendon and cartilage.
Globular proteins tend to be compact, roughly spherical molecules that can move freely
in blood and other tissues. They transport materials, fight invading organisms and
catalyse many biochemical reactions.
2 a Amino acids contain an amine group (NH
2
) and an alkanoic (carboxylic) acid
group (COOH).
b Essential amino acids, of which there are eight (nine for children), must be taken
in with the diet as they cannot be sythesised in the body.
c When amino acids link, between the carboxyl group of one molecule and the
amine group of the other molecule, a peptide bond is formed; water is eliminated
during this condensation reaction.
Solutions Manual: Module 7
The biochemistry of movement
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
3 When biochemists describe protein structure they do so using the following sequence:
The primary structure of proteins refers to the sequence of amino acids contained
within the molecule.
The secondary structure refers to the spatial arrangement of the polypeptide chain.
The tertiary structure refers to the overall way the protein folds.
The quaternary structure refers to the way several polypeptide chains form an
aggregated whole.
4 If the complex three-dimensional shape of a protein is destroyed by such agents as
heating, a change in pH or reaction with other chemicals, it no longer functions as it
should and is described as having been denatured. Denaturing destroys the secondary,
tertiary and any quaternary structure of the protein.
Review exercise 27.4
1 Enzymes:
are specific to particular reactions
provide a pathway of lower activation energy
speed up biochemical reactions
remain unchanged at the completion of the chemical reaction
do not alter the equilibrium of the reaction
function within restricted pH and temperature ranges.
2 Chymotrypsin is one of the pancreatic enzymes that breaks down protein into
polypeptides. Catalase breaks down toxic hydrogen peroxide into oxygen and water.
3 a In order to work, enzymes must come in contact with the material they react with.
This material is the substrate.
b Where substrate and enzyme meet is known as the active site.
c If an enzyme is to function it must fit the substrate sitehence the specificity of
that enzyme.
d Some enzymes have cofactors that are required for them to work effectively.
Chapter 27 Application and investigation
1 Glucose, fructose and galactose all share the same molecular formula but each has a
different structural formula and therefore each will behave chemically differently.
Glucose and galactose are 6-membered rings but fructose is a 5-membered ring.
Glucose and galactose differ only in the orientation of the OH group on one of the
carbon atoms.
Solutions Manual: Module 7
The biochemistry of movement
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
2 Sucrose is a disaccharide made up of the monosaccharides glucose and fructose. When
two monosaccharide molecules combine, a condensation reaction occurs and a water
molecule is produced as a by-product. When disaccharides are broken down into their
two separate monosaccharides, a water molecule is required. This reaction is the reverse
of a condensation reaction and is known as hydrolysis. When sucrose is hydrolysed,
glucose and fructose are produced.
3 Plants store surplus glucose as starch within the cells. Animals use another form of
polymerised glucose known as glycogen to store their glucose. Glycogen is more highly
branched than starch. The glucose molecules are connected through different carbon
atoms in the two different compounds.
4 Approximately 1499 (243 000/162.08) glucose units make up this starch molecule.
Each time a glucose unit is added to the starch molecule in the condensation reaction,
one molecule of water is eliminated. This means that the repeating unit is C
6
H
10
O
5
.
5 a The structural formula of glycerol is:
The structural formula of stearic acid is:
The structural formula of glyceryl tristearate is:
b Glycerol: The glycerol molecule has three hydroxy groups which are hydrophilic.
The remaining three-carbon hydrocarbon chain is hydrophobic.
Stearic acid: The carboxyl group forms hydrogen bonds and is hydrophilic. The
17-carbon chain is non-polar and hydrophobic.
Glycerol tristearate has three carboxyl groups which will form hydrogen bonds.
This end of the molecule is hydrophilic. However, the three 17-carbon atom
hydrocarbon chains are hydrophobic.
Solutions Manual: Module 7
The biochemistry of movement
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
c Glycerol is soluble in water. It has a large hydrophilic and a small hydrophobic
component.
Stearic acid has a small hydrophilic component but a large hydrophobic
component and will be insoluble in water.
Glycerol tristearate has a small hydrophilic component but a very large
hydrophobic component and will be even less soluble in water.
6 Saturated fatty acids belong to a homologous series with the general formula
C
n
H
2n+1
COOH or CH
3
(CH
2
)
n
COOH. They contain no double bonds. Stearic acid is an
example.
Monounsaturated fatty acids contain one C=C double bond in the hydrocarbon chain.
Oleic acid is an example.
Polyunsaturated fatty acids contain two or more C=C bonds in the hydrocarbon chain.
Linoleic acid is an example.
7 Investigation
8 a CH
3
(CH
2
)
4
(CH=CHCH
2
)
2
(CH
2
)
6
COOH
b Linoleic acid has two double bonds. Each molecule of linoleic acid requires four
hydrogen atoms for saturation. One mole of linoleic acid reacts with 2 moles of
H
2
.
9 Take as a typical fat, say, glycerol monostearate C
21
H
42
O
4
. Compare this with a
carbohydrate with the same number of carbon atoms, C
21
H
42
O
21
.
Fat Carbohydrate
Molar mass 358.336 630.336
% Carbon 70.3 40.0
% Hydrogen 11.8 6.7
% Oxygen 17.9 53.3
The molecular formula of fat shows it to have much less oxygen per mole than
carbohydrate.
The percentage composition shows fats to be higher in carbon and hydrogen and lower
in oxygen. The combustion of fats and carbohydrates yields carbon dioxide and water in
both cases. Molecules with more oxygen/less hydrogen release less energy on
combustion than molecules with less oxygen. This makes sense, as combustion is
oxidation and a molecule with oxygen in it is already partly oxidised. Thus, fats release
more energy upon metabolism than carbohydrates because they contain more hydrogen
and less oxygen per carbon than carbohydrate.
Solutions Manual: Module 7
The biochemistry of movement
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
10 Weight lifters and body builders are attracted to protein supplements because the nature
of their sport requires the development and maintenance of larger-than-usual muscles. A
pure supply of protein therefore is seen as a quick route to the deposition of bulk tissue.
11 a When two amino acids link together, a condensation reaction occurs, with the
elimination of a water molecule. This link is between the anion (COO
) of one
amino acid and the cation (NH
3
+
) of another. This link is known as a peptide
bond.
b Glycine plus alanine gives the dipeptide Gly-Ala or Ala-Gly.
i
ii
c 6 (= 3 2 1)
d 6.7 10
11
(= 20 19 18 17 16 15 14 13 12 11)
12 a Fibrous proteins are long, tough molecules that provide structure for many body
components including bone, hair, skin, tendon and cartilage.
Globular proteins are more compact, roughly spherical molecules that perform a
vast array of functions including the transport of oxygen, fighting disease as
antibodies, and in catalysing biochemical reactions.
b When biochemists describe protein structure, they do so using the following
sequence: The primary structure of proteins refers to the sequence of amino acids
contained within the molecule. The secondary structure refers to the spatial
arrangement of the polypeptide chain. The tertiary structure refers to the overall
way the protein folds. The quaternary structure refers to the way several
polypeptide chains form an aggregated whole.
c The secondary structure is held in place by hydrogen bonding, while the tertiary
and quaternary structures are held in place by electrostatic forces, hydrogen
bonding, hydrophobic forces and covalent disulphide bonds. Of course, the
primary structure is held in place by peptide bonds.
d Disruption of the shape of the protein molecule renders it biologically inactive.
This is known as denaturation. It involves disrupting the forces and bonds which
determine secondary, tertiary and, if relevant, quaternary structure of the protein,
while leaving the chain of amino acids intact. Extremes of temperature, pH and a
variety of chemicals can denature proteins.
Solutions Manual: Module 7
The biochemistry of movement
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
13 Investigation
14 Investigation
15 Blood contains protein. Warm water is not recommended for the removal of blood
stains from garments because the proteins may denature and unravel and then coagulate
into solid lumps, making the stain harder to remove.
16 a Enzymes are responsible for catalysing almost all of the chemical reactions that
occur in our bodies. Clearly, without enzymes, living organisms including
ourselves could not survive.
b Because of the need for a precise fit between the enzyme and substrate
molecules, the mechanisms of enzyme-catalysed reactions are often discussed in
terms of a lock and key mechanism. If the active site of the enzyme has a
specific shape (similar to a lock) then only the right-shaped substrate (key) will fit
that site.
17 Investigation
18 a i Each hydroxyl group on the glycerol bonds with the corresponding hydroxyl
of the stearic acid. The linkage is an ester linkage.
ii The hydrogen of the hydroxyl group of the first molecule reacts with the
OH of the hydroxyl group of the second molecule. The linkage is a
glycosidic linkage.
Solutions Manual: Module 7
The biochemistry of movement
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
iii Amino acids link together with dipeptide bonds from the amino group of
one and the hydroxyl group of the other.
b i Esterificationcondensation reaction
ii Condensation polymerisation
iii Condensation polymerisation
c Proteins and polysaccharides are biopolymers.
CHAPTER 28: CELLULAR RESPIRATION
Review exercise 28.1
1 ATP is adenosine triphosphate.
ADP is adenosine diphosphate.
2 When glucose in the cell is oxidised to water and carbon dioxide, this energy is stored in
the form of ATP. This ATP can be used to provide energy for various endothermic
biochemical reactions.
Review exercise 28.2
1 a The reactants of glycolysis are glucose, nicotinamide adenine dinucleotide
(NAD
+
), ADP and free phosphate. The products of glycolysis are pyruvate ion,
reduced NAD
+
(NADH), ATP, water and hydrogen ions.
b 38 molecules of ATP
c The lactate ion
d Glycolysis, a non-aerobic process, occurs within the cytoplasm of the cell and is
the preliminary stage to aerobic respiration in humans and most other species.
2 pyruvate ion + coenzyme A + nicotinamide adenine dinucleotide acetyl-coenzyme A
+ carbon dioxide + reduced NAD
+
3 a Carbon dioxide and coenzyme A
b For each molecule of acetyl-CoA that is oxidised, one molecule of ATP, three
molecules of NADH and one molecule of FADH
2
are produced.
Solutions Manual: Module 7
The biochemistry of movement
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
c The TCA cycle occurs within the mitochondrial matrix.
4 a The oxidation of NADH and FADH
2
is coupled with the phosphorylation of ADP
to produce ATP. This oxidative phosphorylation is a multi-stage pathway, where a
total of 10 redox reactions occur.
b 30 molecules of ATP are generated from the oxidation of one pyruvate ion.
c O
2
+ 4e
+ 4H
+
2H
2
O
5
One molecule of CO
2
is produced during the conversion of glucose to pyruvate and two
molecules are produced in the TCA cycle.
Chapter 28 Application and investigation
1 a Cellular respiration involves the conversion of glucose and oxygen to carbon
dioxide, water and energy. The energy evolved is stored in the form of ATP and
enables the cell to perform other biochemical reactions. The reactants are glucose
and oxygen. The products are water, carbon dioxide and energy.
b Combustion of carbon compounds involves the oxidation of the compound with
oxygen to produce carbon dioxide, water and energy. In cellular respiration the
energy produced is stored as ATP and only a small amount of heat energy is
evolved. Simple combustion requires an initial and considerable amount of heat
before it occurs and releases its energy as heat. Such heat would destroy living
tissue. Cellular respiration occurs as a many-stepped pathway requiring enzymes
to proceed. Simple combustion is straightforward and requires no enzymes.
2 C
6
H
12
O
6
+ 2NAD
+
+ 2ADP + 2P
i
2CH
3
COCOO
+ 2NADH + 2ATP + H
2
O + 4H
+
Solutions Manual: Module 7
The biochemistry of movement
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
3 Cellular respiration in the absence of oxygen is known as anaerobic respiration. The
products of anaerobic respiration are the pyruvate ion and water. In plant and fungi
tissue, anaerobic respiration produces ethanol and carbon dioxide. In animal tissue,
anaerobic respiration produces lactic acid.
4 pyruvate ion + coenzyme A + nicotinamide adenine dinucleotide acetyl-coenzyme A
+ carbon dioxide + reduced NAD
+
5 The following equation shows the conversion of acetyl-CoA to its final product, carbon
dioxide. Note the conversion of ADP to ATP, the reduction of NAD
+
to NADH and the
reduction of FAD to FADH
2
. Restored coenzyme A becomes a product.
acetyl-CoA + ADP + 3NAD
+
+ FAD + P
i
2CO
2
+ ATP + 3NADH + FADH
2
+ CoA
6
OOC-CH=CH-COO
7 Investigation
8 a NADH NAD and ADP + P
i
ATP
b FADH
2
FAD and ADP + P
i
ATP
9 Investigation
10 Investigation
11 The radioactive carbon-14 would emerge in carbon dioxide at steps 3 and 4 of the TCA
cycle.
12 i (a) glycolysis; (b) tricarboxylic acid cycle or Krebs cycle; (c) respiratory chain
ii X is 2pyruvate; Y 2CO
2
; Z ATP
13 Investigation
14 Investigation
15 This amount of heat energy in one burst would cause excess heat within the cells and
destroy living tissue.
CHAPTER 29: MUSCLE ACTION AND EXERCISE
Review exercise 29.1
1 Skeletal muscle: voluntary movement of muscles.
Cardiac muscle: involuntary muscles of the heart.
Smooth muscle: involuntary muscles of the digestive and circulatory systems.
2 Myosin, actin, tropomysin and troponin.
Solutions Manual: Module 7
The biochemistry of movement
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
3 Thick filaments are made up of the protein myosin. This molecule is unusual in having
at one end a fibrous structure and at the other, a globular end. Several hundred of these
myosin molecules make up a filament.
Thin filaments are made up from the protein actin, which has a fibrous structure. Two
other proteins, tropomysin and troponin, combine with actin to form thin filaments.
Tropomysin and troponin together prevent actin and myosin interacting when the
muscles are in a relaxed state.
Both thin and thick filaments are found in skeletal (striated) muscles.
Review exercise 29.2
1 Exothermic. When ATP is hydrolysed to ADP and phospate ion, energy in the form of
heat is given out.
2 During muscle contraction, an interaction between the proteins tropomysin and troponin
and calcium ions occurs. A site on the actin becomes available, allowing the myosin
head to bond. This process allows the myosin to catalyse the hydrolysis of ATP. The
energy released allows the head of the myosin molecule to bend, putting it into a higher
energy state. Sufficient energy of the myosin allows it to bind with another myosin
molecule further along the filament, which liberates phosphate and ADP.
Review exercise 29.3
1
Type 1 cells Type 2 cells
Slow twitching (contraction) Fast twitching (contraction)
Rich supply of blood cells Reduced supply of blood vessels
Adequate oxygen supply Limited amount of oxygen available
Many mitochondria Fewer mitochondria
ATP is derived from the process of oxidative
phosphorylation (aerobic respiration)
Use muscle-stored glycogen as initial fuel
(anaerobic respiration)
Contain fewer contractile filaments than do
type 2 cells
Can use glucose, fatty acids and amino acids
as fuels
2 Anaerobic glycolysis; the pyruvate produced is reduced to make lactate. This lactate is
harmful to the muscle cells and if it accumulates will lead to cramp. It occurs in skeletal
muscle cells. (The blood takes the lactate away from the muscles to the liver where it is
oxidised back into pyruvate when oxygen is again available.)
Solutions Manual: Module 7
The biochemistry of movement
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Review exercise 29.4
1 Fats, carbohydrates, proteins.
2
Aerobic respiration Anaerobic respiration
Energy is derived from carbohydrates, fats
and proteins.
It is carbohydrate dependent, in particular,
glucose.
Initially it involves glycolysis, which leads
into the tricarboxylic acid cycle, part of
which involves oxidative phosphorylation.
Stored glycogen (muscles and liver) is
converted to glucose.
Acetyl CoA provides the initial fuel for the
TCA cycle.
The breakdown of glycogen followed by
glycolysis ensures a rapid and immediate
supply of ATP, which can be supplied in a
short period of time but only in short bursts.
3 Athletes who compete in short but rapid events such as the 100 m sprint or the 100 m
freestyle benefit from the anaerobic respiration of glycolysis because it provides high
levels of ATP at a rapid rate.
4 The metabolism of both glucose and fatty acids requires the presence of the carrier
molecule, coenzyme A, but there is a limited amount of this within the cell at any time.
If high levels of fatty acids enter the cell, the CoA is consumed, leaving insufficient
quantities for glucose metabolism.
5 In long-distance running, most of the energy required is derived from the metabolism of
carbohydrates, fats and proteins by aerobic respiration. At rest, approximately one-third
of the energy required comes from carbohydrate metabolism; the remaining two-thirds
comes from the metabolism of fat. Once into the run, carbohydrates become the primary
fuel. The muscles require more oxygen for aerobic respiration as the demand for oxygen
rises. During this period, anaerobic respiration supplies the extra ATP needed. With
oxygen levels restored, metabolism is fuelled by fats.
Chapter 29 Application and investigation
1 It is composed of fibre-like cells and has a banded appearance.
Solutions Manual: Module 7
The biochemistry of movement
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
2 a Thick filaments are made up of the protein myosin. This molecule is unusual in
having at one end a fibrous structure and at the other, a globular end. Several
hundred of these myosin molecules make up a filament.
Thin filaments are made up from the protein actin, which has a fibrous structure.
Two other proteins, tropomysin and troponin, combine with actin to form thin
filaments.
b Thick filaments appear as dark bands in striated muscle. They alternate with thin
filaments, which appear light in colour.
3 Skeletal muscle consists of thin actin filaments which overlap thick myosin filaments.
During muscle contraction, these filaments slide over each other but do not individually
alter in length. Calcium ions are liberated in the region of the sarcomeres, interacting
with the proteins tropomysin and troponin. A site on the actin becomes available,
allowing the myosin head to bond. This process allows the myosin to catalyse the
hydrolysis of ATP. The energy released allows the head of the myosin molecule to
bend, putting it into a higher energy state. Sufficient energy of the myosin allows it to
bind with another myosin molecule further along the filament, which liberates
phosphate and ADP.
4 a Slow twitch muscles contract slowly and contain mostly type 1 muscle cells. They
are richly supplied with blood vessels, making them suited to aerobic respiration
because oxygen is well supplied. They have abundant supplies of mitochondria
and ATP is generated from them through the process of oxidative
phosphorylation. Slow twitch muscles are able to provide the long-distance runner
or the migratory bird with a steady and sufficient supply of energy from fuel
reserves of fats, proteins and carbohydrates.
Fast twitch muscles contract relatively quickly and contain mostly type 2 muscle
cells; they contain fewer mitochondria and are poorly supplied with blood vessels
compared with slow twitch muscle. Respiration is mostly by anaerobic means.
Glucose available in the blood and glycogen stores provide the primary fuel. Fast
twitch cells supply small amounts of high energy, making them suitable for high-
intensity athletic events such as sprints.
b Cheetahs hunt their food by making short but rapid attacks on their prey. Their
skeletal muscles would be mostly made up of the type 2 fast twitch muscles cells.
Elephants, being herbivores, do not ambush their prey, but they do need muscles
that will sustain their large bulk. Elephants have muscles made up primarily of
type 1 slow twitch muscles.
5 Investigation
Solutions Manual: Module 7
The biochemistry of movement
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
6
7 Investigation
8 The fuels of food compounds include glucose, amino acids, fatty acids, lactate and
ketone bodies. All of them can be converted into acetyl-CoA by a variety of
biochemical pathways. Acetyl-CoA is the starting compound for the TCA cycle. It is the
common intermediate product formed by the breakdown of the major primary fuels.
During the TCA cycle, the acetyl group of acetyl-CoA is oxidised to give carbon
dioxide and energy, which can be used to generate ATP. ATP generated can thus be
used as a raw fuel in other biochemical processes in the body.
9 Anaerobic (without air) respiration or glycolysis is the preliminary stage to aerobic
(with air) respiration in many living organisms. Anaerobic respiration does not use
oxygen as a reactant. It produces carbon dioxide and alcohol and relatively small
amounts of ATP. Yeasts used in fermentation employ this kind of metabolism. Aerobic
respiration uses copious amounts of oxygen in the cellular metabolism of food. It
produces energy in the form of ATP, carbon dioxide and water as products. Muscles in
higher organisms obtain most of their energy (ATP) from aerobic respiration.
Solutions Manual: Module 7
The biochemistry of movement
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
10 For much of their time on the competition circuit, basketball players and triathletes rely
on sudden bursts of muscular activity. Fast twitch muscles that provide these sudden
exertions need to be supplied with a steady supply of glucose. A consequence of this
activity is an accumulation of acid in the muscles and a drop in pH. This leads to fatigue
and cramp. Such athletes train to utilise as much oxygen as possible and eat
carbohydrates (the major source of glucose) prior to such events.
1113 Investigation
Module 7
REVIEW
1 a Glucose
b The pyruvate ion, ATP, water, hydrogen ions and reduced NAD.
c The pyruvate ion becomes acetyl-CoA, which enters the TCA cycle to be finally
oxidised into water and carbon dioxide.
2 a X is water and Y is carbon dioxide.
b There are some 140 intermediate compounds before the waste products are
produced.
3 a (i) amino acid, (ii) fatty acid, (iii) carbohydrate
b glycosidic linkage forms:
4 a carbohydrates
Solutions Manual: Module 7
The biochemistry of movement
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
b
5 a peptide bond
b
6 a The sudden release of the energy derived from the breakdown of glucose to water
and carbon dioxide would generate too much heat at one time; the energy could
not all be converted to useful ATP molecules.
b Glycolysis, tricarboxylic acid cycle, oxidative phosphorylation. TCA cycle and
oxidative phosphorylation release most energy.
7 Food is essential for the healthy functioning of the human body. A balance of
carbohydrates, fats, proteins, vitamins, minerals and water is required. As a fuel, food
supplies the necessary energy for body functions. Food provides the raw materials from
which the body is made and repairs damaged tissue. Some essential nutrients play a role
in the biochemical processes that take place within the body.
Carbohydrates provide the bulk of energy and should generally make up about 60% of
the total calorie intake. Most elite athletes rely on sudden bursts of muscular activity, so
they must be supplied with a steady supply of glucose. The major source is
carbohydrates. Fats serve as energy sources, particularly long-term reserves. Proteins
provide structure for many body parts including bone, hair, skin, tendon and cartilage.
Solutions Manual: Module 7
The biochemistry of movement
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Others aid in the transport of oxygen to the tissues, fight disease or catalyse biochemical
reactions.
8 The primary structure of proteins refers to the sequence of amino acids contained within
the molecule which are linked by covalent peptide bonds. The secondary structure refers
to the spatial arrangement of the polypeptide chain (made up from amino acids). It is
held in place by H-bonding between the CO and nearby NH groups. The tertiary
structure refers to the overall way the protein folds and it is held in place by bonds and
forces from the side chains of the amino acids. These include electrostatic forces caused
by ionic interactions between amino acids, hydrogen bonds, hydrophobic forces derived
from the side chains of amino acids and some cross-linking of parts of proteins caused
by disulphide bonds. Any quaternary structure is determined by the same type of bonds
and forces that determine tertiary structure.
9 a Enzymes function as catalysts for many biochemical reactions. Enzymes are
specific to particular biochemical reactions speeding up reaction rates. They are
more specific than most chemical reactions, and act upon specific substrates.
b Enzymes are proteins that catalyse biochemical reactions.
c The enzyme is A. An enzyme remains unchanged at the end of the reaction.
10 The two functional groups are amine (NH
2
) and carboxylic acid (COOH).
11 a Glucose
b Glycogen (from storage) and glucose (in blood).
c Glycolysis is the series of biochemical reactions in which glucose is broken down
to pyruvate with the release of usable energy in the form of ATP. The net energy
yield is two ATP molecules per glucose molecule.
d A side-effect is the production of lactate and hydrogen ions. The hydrogen ion
surplus lowers the pH in the muscle, causing cramps and fatigue.
12 a Glycerol and alkanoic or fatty acids.
b
Solutions Manual: Module 7
The biochemistry of movement
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
c Ester; the ester linkage occurs between the glycerol and each of the fatty acids.
d Fuel
13 a Glycogen
b There are two types of muscle cells:
Type one cells contain slow twitch fibres and have a rich blood supply and many
mitochondria.
Type two cells contain fast twitch fibres, have fewer mitochondria and a reduced
supply of blood.
c Skeletal muscle is striated in appearance. It is composed of fibre-like cells
containing many nuclei. The banded appearance is due to the presence of
alternating bands of thick and thin filaments. Thin filaments appear lighter in
colour than the thick filaments.
14 a
Monosaccharides Disaccharides Polysaccharides
glucose sucrose starch
fructose lactose glycogen
b Glucose
c At rest, about one-third of the bodys energy comes from the metabolism of
carbohydrates, with about two-thirds being supplied by the metabolism of fats.
As exercise gets under way, the total energy consumption increases and the fuel
balance changes, with the metabolism of carbohydrates becoming dominant.
Athletes should consume more carbohydrates in the 24 hours before competing.
15 a The enzyme fumarase acts on the compound fumarate. Also citrate synthase
(acetyl-CoA), aconitase (citrate), isocitrate dehydrogenase (isocitrate), malate
dehydrogenase (malate), succinate dehydrogenase (succinate), succinyl-CoA
synthetase (succinyl-CoA), -ketoglutarate dehydrogenase (-ketoglutarate).
b The coenzymes NADH/NAD
+
and FADH
2
/FAD. Reversible reactions between
the two forms facilitate the flow of electrons through the cycle.
c ATP is adenosine triphosphate. It is an energy-rich compound used to drive
many biochemical reactions. In the TCA cycle, ATP is generated as a product and
stores potential chemical energy.
Solutions Manual: Module 8
The chemistry of art
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
CHAPTER 30: PIGMENTS IN PAINTING
Review exercise 30.1
1 Early artists relied upon the pigments available to them from the Earth. The only
technique available for producing them at the time was grinding of local materials.
These materials were red, brown and yellow ochres (containing iron), white clays, chalk
and gypsum and black charcoal, graphite and manganese dioxide.
2 All the ochres contain the metal iron, either as anhydrous or hydrated iron(III) oxide or
as limonite, FeO(OH).
3 All paints are made up of a pigment (the colouring matter) and a medium (the liquid
that carries the pigment).
4 The pigment needs to be insoluble so that as the colourless medium dries, the colour
remains.
Review exercise 30.2
1 Egyptian ores:
Ore used as pigment Main metal in ore Colour
Malachite Copper Green
Azurite Copper Blue
Cinnabar Mercury Red
Orpiment Arsenic Yellow
Realgar Arsenic Orange-scarlet
Egyptian blue Copper Blue
Stibnite Antimony Black
2 A pigment is a coloured insoluble solid suspended in a medium; the pigment remains
after the medium dries, e.g. malachite, orpiment, realgar. A dye is a soluble colouring
agent that colours the medium, usually water, e.g. Madder lake, indigo.
3 Lakes are produced from dyes. The soluble colouring agent in the dye is fixed onto an
insoluble white powder, such as chalk or gypsum, which can then be extracted and
dried.
4 CuCO
3
.Cu(OH)
2
(g) 2CuO(s) + CO
2
(g) + H
2
O(g)
Review exercise 30.3
1 Some cosmetics, such as cinnabar, white lead, red lead, stibnite and manganese dioxide
as kohl, contain heavy metals that act as cumulative poisons in the body. Romans would
Solutions Manual: Module 8
The chemistry of art
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
not have been aware of the connection between the long-term use of these cosmetics
and any illnesses they may have developed. Orpiment, containing arsenic, may have
produced more immediate effects but the Romans may have seen no obvious
connection.
2 mercury + sulfur
mercury(II) sulfide (vermilion)
Hg(s) + S(s)
HgS(s)
Review exercise 30.4
1 When hydrated iron oxides such as siennas and umbers are heated, the water of
crystallisation is driven off, so the oxides become anhydrous. This changes the colour of
the compounds.
2 Current price of gold (September 2005) is AUS$577 an ounce.
One ducat weighs 0.12 of an ounce, so eight ducats will be worth 8 0.12 $577 =
$553.92 .
Review exercise 30.5
1 Chromite, FeO.Cr
2
O
3
, was discovered in commercial quantities in America in 1820.
Chromite is the source of the pigments chrome yellow, chrome red and chrome green.
These pigments give colours of great depth and covering ability.
2 Chrome yellow is manufactured by the precipitation of lead(II) chromate from a
solution of a lead salt, such as lead(II) nitrate, and chromate compound, such as sodium
chromate.
lead(II) nitrate + sodium chromate
lead(II) chromate + sodium nitrate
Pb(NO
3
)
2
(aq) + Na
2
CrO
4
(aq)
PbCrO
4
(s) + 2NaNO
3
(aq)
3 cadmium(II)
chloride
+ hydrogen
sulphide
cadmium
sulphide
+ hydrochloric
acid
CdCl
2
(aq) + H
2
S(aq)
CdS(s) + 2HCl(aq)
4 titanium(IV) chloride + oxygen
titanium dioxide + chlorine
TiCl
4
(l) + O
2
(g)
TiO
2
(s) + 2Cl
2
(g)
Review exercise 30.6
1 Most paintings consist of the following layers: a support (such as wood panel or
canvas), a ground (such as gesso), paint and then varnish.
Solutions Manual: Module 8
The chemistry of art
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
2 Gesso was a common ground for paintings. It was usually a mixture of chalk or gypsum
with animal glue. It was applied as a warm, thick liquid and allowed to set. It dried to a
creamy colour and was then scraped and rubbed smooth.
3 Varnishing a painting protects the paint layers, enhances clarity and depth of saturation.
Some varnishes were chosen for the golden glow they gave to paintings.
4 Colour can be produced in glass by:
grinding metal oxide pigments to a fine powder and adding them to the glass mixture
before melting
flashing, which involves the coating of clear (white) glass with a thin layer of
coloured glass at the molten stage
staining, which involves painting silver nitrate onto the glass and then firing it in
the kiln
mixing pigments (from powdered copper or iron oxides or ground enamel pigments)
with a medium such as wine or urine, then painting onto the glass and firing it again.
Review exercise 30.7
1 Tempera is used as a paint medium made from egg yolk mixed with water. It was mixed
with pigments and used immediately.
2
Pigment Metal component
Red ochre Iron
Minium (red lead) Lead
Ultramarine Sodium, aluminium
Crimson lake (cochineal) (none)
White lead Lead
Lead tin yellow Lead, tin
Vermilion Mercury
Green earth Iron
Gilding Gold
Review exercise 30.8
1 a The common elements of pigments are metals, especially transition metals.
Solutions Manual: Module 8
The chemistry of art
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
b Most metals are found in the d-block; and the heavier p-block metals
Chapter 30 Application and investigation
16 Investigation
7 Early Aboriginal people, with their limited technology, derived their pigments directly
from their immediate environment or from occasional trade with other groups.
Therefore their pigment palette was limited to easily extracted earth products and some
plant and animal products.
8 Early Aboriginal people attached pigments, such as red ochre, to surfaces such as rock
faces, using media such as saliva, water and birds eggs to carry the pigment and adhere
to the surface. Early Egyptians used saliva to carry and adhere kohl to their eyes.
CHAPTER 31: THE STRUCTURE OF THE ATOM AND COLOURS
Review exercise 31.1
1 Many metallic elements emit light of a distinctive colour when their compounds are
heated in a flame. If it is known that the sample of compound tested is pure and a
particular colour is given out in a flame test, then a comparison can be made with
known flame colours and a possible identification of the metal made.
2 If a flame test is made of both potassium and sodium chlorides, the sodium salt will give
a yellow flame and the potassium salt will give a violet flame. (The chloride does not
give a colour.)
Review exercise 31.2
1 Electromagnetic radiation includes gamma rays, X-rays, ultraviolet light, visible light,
infra-red radiation, microwaves, TV and radio waves. As the wavelength gets shorter
(from radio waves to gamma rays), the electromagnetic radiation becomes more
energetic and penetrating.
Solutions Manual: Module 8
The chemistry of art
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
2 A line emission spectrum is the spectrum of visible light that results from energetically
excited atoms; it is seen as a series of discrete lines of colour separated by blank areas.
A line absorption spectrum results when light is passed through a substance in the
vapour phase and the resulting spectrum observed; it is seen as a pattern of dark lines
across a continuous spectrum. For a given element, its line emission spectrum is the
reverse of its line absorption spectrum.
3 a i Sample A is pure and is cadmium (Cd).
ii Sample B is impure and contains sodium (Na) and lithium (Li).
b Sample A contains seven spectral lines and they correspond exactly to the seven
spectral lines of cadmium below 600 nm; they do not match any other elements.
Sample B contains the three lithium lines that can be found between 300 and
600 nm and the two visible sodium lines; these lines do not exactly match any
other elements.
Review exercise 31.3
1 Niels Bohr (18851962) was studying the line spectrum of hydrogen and proposed that
in a hydrogen atom the electron moves around the nucleus without radiating energy.
Only orbits of certain energy levels are allowed, and electrons can jump from one
orbital to another by absorbing or emitting energy of a particular quantised amount. The
allowed orbits are designated a principal quantum number, n, with values of n = 1,
n = 2, etc.
2 An atom is in its ground state when its electrons are in their lowest possible energy
levels. An atom is in an excited state when any of its electrons are in higher energy
levels than their ground state. An atom can become excited when its electrons absorb
photons of energy and can move to a higher energy level.
3 Bohr postulated that every line in the emission spectrum of hydrogen represents a
transition of electrons from one energy level to a lower one. When an electron jumps
from one energy level to a lower energy level, a photon of radiation is emitted. For
some of these emissions, the energy released is in the visible spectrum range and so can
be seen as the line spectrum.
Review exercise 31.4
1 Bohrs model of the atom successfully explained the line spectrum of hydrogen and
introduced the idea of quantised energy states for electrons in atoms. This feature is
incorporated into our current model of the atom.
2 Bohrs model was not as successful at explaining the atomic spectra of atoms other than
hydrogen. Nor could it explain the different intensities of spectral lines, the fact that
some spectral lines actually consist of a number of very fine close lines, or the splitting
of such spectral lines in magnetic fields.
Solutions Manual: Module 8
The chemistry of art
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
3 The double yellow lines that appear in the sodium line spectrum at 589 and 590 nm are
the result of emission from excited electrons as they fall back from two different p sub-
shell orbitals to the s sub-shell in the n = 3 energy level.
Review exercise 31.5
1 Both absorption and emission spectra are used to identify different components of
pigments, media and varnishes by comparing the spectrum produced to known spectra.
This allows conservators to decide on the products and techniques they will use to
restore or conserve different paintings.
2 A reflectance spectrum is produced by shining white light on the surface of a painting
and analysing the reflected light with a spectrophotometer. The reflectance spectrum
obtained can then be compared to known spectra gathered using the same technique for
identification of the pigments used in the painting.
3 Laser is an acronym for light amplification by stimulated emission of radiation. Laser
microspectral analysis can be used to identify pigments by either destructively
vaporising a small sample and subjecting the vapour to analysis or by non-destructively
reflecting the laser light from the surface of the pigment to be analysed. Either way, the
resulting spectrum is compared to known spectra gathered using the same technique and
a match and identification made.
4
Radiation Zinc oxide pigment Copper-containing pigments
Infra-red Change from white to lemon-
yellow, change back again when
IR light removed
Permanent chemical change to
black copper(II) oxide
Ultraviolet Fluoresces a pale yellow Malachite fluoresces a dirty
mauve
5 Laser microspectral analysis is a useful analytical tool for pigment identification in the
art world. As the equipment becomes more portable and less expensive, it can be used
by more conservators to aid in the conservation and restoration of paintings, and by
those needing to establish the autheniticity of some paintings.
Chapter 32 Application and investigation
1 Investigation
2 In a flame test, potassium ions give a violet colour and strontium gives a red colour.
You could demonstrate that it is the metal ion that produces the colour by using
potassium chloride and strontium chloride for testing, or both potassium nitrate and
strontium nitrate. In each case, the same flame colours of violet and red will result.
3 Laser light with a wavelength of 780 nm will appear in the red light region of the
electromagnetic spectrum.
Solutions Manual: Module 8
The chemistry of art
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
4 Different elements can produce different colours when, in the vapour state, they are
subjected to electric discharge. Neon gas, with its distinctive red colour, was one of the
first elements used in this way for lighting. Since then other elements have been used to
produce lights of different colours.
5 The complex will appear green. The complex absorbs most light from the blue (400 nm)
and red (650750 nm) regions of the visible light spectrum and so will transmit mostly
in the green region.
68 Investigation
9 He
+
has two protons in its nucleus, unlike hydrogen, which has only one proton. This
increased nuclear attraction changes the energy levels of the helium, so the He
+
ion
gives spectral lines with higher energy/frequency.
10 760 ppm
CHAPTER 32: ELECTRONS IN THE ATOM
Review exercise 32.1
1 Bohrs model of the hydrogen atom postulated allowed energy levels for electrons. The
quantum mechanical model suggests that the electrons should be viewed as waves and
that the orbits do not represent the paths of the electrons but rather the amplitudes of the
electron waves at different points in space. It is possible to determine only the
probability of locating an electron at a certain point, and thus we speak of the electrons
existing in an electron cloud.
2 An orbit is a region in space with a fixed radius in which Bohr postulated that an
electron could be found. An orbital is a region of space around a nucleus through which
an electron with a given energy may move.
3 a Shell n = 2: two sub-shells, an s and a p; one orbital in the s sub-shell and three
orbitals in the p sub-shell; maximum of eight electrons can be accommodated in
this shell.
b Shell n = 4: four sub-shells, an s, a p, a d and an f; one orbital in the s sub-shell,
three orbitals in the p orbital, five orbitals in the d sub-shell and seven orbitals in
the f sub-shell; a maximum of 32 electrons can be accommodated in this shell.
4 a 16
b 3
c 0
d 1
e 5
f 7
Solutions Manual: Module 8
The chemistry of art
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Review exercise 32.2
1 a helium
b beryllium
c nitrogen
d sodium
e sulfur
2 a 1s
2
2s
1
b 1s
2
2s
2
2p
6
3s
2
3p
4
c 1s
2
2s
2
2p
4
d 1s
2
2s
2
2p
6
3s
2
3p
5
e 1s
2
2s
2
2p
1
f 1s
2
2s
2
2p
6
3s
2
3p
6
4s
2
3 a Ground state atoms: i, iii and iv. Excited atoms: ii and v.
b i oxygen, 8; ii neon, 10; iii aluminium, 13; iv calcium, 20; v zinc, 30
4 a
b
c
d
e
Review exercise 32.3
1 a s
2
b p
4
2 fluorine: 1s
2
2s
2
2p
5
3 a i Group 2 ii 3 iii 12 iv Mg
b i Group 17 ii 3 iii 17 iv Cl
c i Group 15 ii 4 iii 33 iv As
Solutions Manual: Module 8
The chemistry of art
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Review exercise 32.4
1 The negative electrons are held in place by their electrostatic force of attraction for the
positively charged nucleus. It takes energy to overcome this force.
2 a B(g)
B
+
(g) + e
H
b F(g)
F
+
(g) + e
H
c K(g)
K
+
(g) + e
H
Review exercise 32.5
1 The first ionisation energy for the successive elements boron, carbon and nitrogen
increases. As you move across the period, the outer electrons (which are in the same
shell) are held by progressively increasing nuclear charge and so it takes progressively
more energy to remove them from this electrostatic hold.
2 The first ionisation energy for the elements Mg, Ca and Sr decreases. As you move
down the group to which they all belong, the outer electrons are held in progressively
further out energy levels. They are increasingly further away from the electrostatic
attraction of the nucleus and are increasingly shielded from this same pulling attraction,
so it takes increasingly less energy to remove an outer shell electron.
3 Carbon will have a higher first ionisation energy than boron, as its outer electrons are
held in place by a higher nuclear charge. Aluminium will have a lower first ionisation
energy than boron and carbon, as its outer electrons are further away from the pull of
the nucleus and shielded by an extra shell. Carbon will have the largest first ionisation
energy (B, I
1
807 kJ mol
1
; C, 1093 kJ mol
1
; Al, 584 kJ mol
1
).
Review exercise 32.6
1 a B(g)
B
+
(g) + e
B
+
(g)
B
2+
(g) + e
B
2+
(g)
B
3+
(g) + e
B
3+
(g)
B
4+
(g) + e
b Removing the first three electrons from boron removes the three that occupy the
outer n = 2 shell. The fourth electron to be removed occupies the n = 1 energy
level, which is closer to the positive nucleus and so this requires much more
energy.
c Three valence electrons.
2 The first three electrons removed from aluminium occupy the n = 3 energy level. The
fourth and fifth electrons occupy the n = 2 energy level, which is closer to the nucleus
and so require much more energy to remove than those in the further out n = 3 shell.
Solutions Manual: Module 8
The chemistry of art
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Review exercise 32.7
1 Electronegativity is a measure of the ability of an atom in a molecule to attract electrons
to itself in a chemical bond. Atoms with strong electron pulling power in covalent
bonds are said to be highly electronegative. Electronegativity can be used to determine
the polarity of a covalent bond: the greater the difference in electronegativity, the more
polar the bond.
2 The most electronegative element, fluorine, has been arbitrarily assigned a value of 4.0
and all other values are relative to this. The least electronegative element is caesium,
with a value of 0.7. The values for all other elements lie between these two extremes.
3 As you move from left to right across a period, electronegativity steadily increases. This
reflects the increasing nuclear charge across the period.
Chapter 32 Application and investigation
1 a 2
b 6
c 2
d 10
e 14
2 a Period 3, Group 1, sodium
b Period 2, Group 16, oxygen
c Period 3, Group 14, silicon
d Period 3, Group 18, argon
3 Ionisation energy is influenced by position across a period (group numberincreasing
number of protons), position down a group (period numberdistance from nucleus and
number of shells shielding outer electrons).
4 a Ne: 1s
2
2s
2
2p
6
; F: 1s
2
2s
2
2p
5
; C: 1s
2
2s
2
2p
2
; O: 1s
2
2s
2
2p
4
; Cl: 1s
2
2s
2
2p
6
3s
2
3p
5
; He: 1s
2
b Ne: p block; F: p block; C: p block; O: p block; Cl: p block; He: s block
c Ne: period 2, group 18; F: period 2, group 17; O: period 2, group16; Cl: period 3,
group 17; He: period 1, group 18; C: period 2, group 14
d Number of valence electrons: Ne: 0; F: 7; C: 4, O: 6; Cl: 7; He: 0
e Increasing first ionisation energies: C< Cl < O < F < Ne < He.
5 The first ionisation energy of sodium is less than that of magnesium because the
effective nuclear charge of sodium is 11
+
and that of magnesium is 12
+
, yet their
valence electrons are in the same energy level (at the same distance). Thus sodiums
Solutions Manual: Module 8
The chemistry of art
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
electrons are not as strongly held as magnesiums. The second ionisation energy of
sodium is higher than that of magnesium because the second electron to be removed
from sodium must come from a closer energy level to the nucleus than that of
magnesiums.
6 a 1s
2
2s
2
2p
6
3s
2
3p
3
b 1s
2
2s
2
2p
6
3s
2
3p
6
7 Investigation
8 Electronegativity values are determined by giving a high value to the known most
electronegative element, fluorine, and then comparing the other elements to this.
Investigation for the other scales possible.
9 Investigation, however detail provided in Chapter 33.
CHAPTER 33: THE TRANSITION METALS
Review exercise 33.1
1 The transition metals have electron configurations that include the gradual filling of the
d-block orbitals. The other elements in the main groups of the periodic table have their
d-block orbitals either completely empty or completely filled.
2 a V: 1s
2
2s
2
2p
6
3s
2
3p
6
3d
3
4s
2
b Ni: 1s
2
2s
2
2p
6
3s
2
3p
6
3d
8
4s
2
c Cr: 1s
2
2s
2
2p
6
3s
2
3p
6
3d
5
4s
1
d Pd: 1s
2
2s
2
2p
6
3s
2
3p
6
3d
10
4s
2
4p
6
4d
8
5s
2
3 The d-block elements have similar properties because their electron configurations
(which give rise to their properties) have their outer shells as s
1
or s
2
. The p-block
elements have greater differences in their outer shells, ranging from p
1
to p
6
, and hence
a greater difference in their properties.
Review exercise 33.2
1 Chromium, [Ar]4s
1
3d
5
, exhibits several oxidation states in its compounds as a result of
being able to lose or bond its 3d electrons as well as its 4s electron. Aluminium, [Ne]3s
2
3p
1
, has only the three valence electrons, which are all lost or shared during bonding,
resulting in only the +3 oxidation state.
2 a The transition metals commonly have s
2
electrons as their outer electrons, making
+2 a common oxidation state for them.
b Transition metals can also lose or combine their many d orbital electrons, which
can give rise to many oxidation states.
3 The oxidation state of chromium shifts from 0 to +2:
Solutions Manual: Module 8
The chemistry of art
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Cr(s) + 2H
+
(aq) Cr
2+
(aq) + H
2
(g)
4 Vanadium, [Ar]3d
3
4s
2
and 23 protons, forms VCl
2
by releasing its two 4s
2
electrons.
Scandium, [Ar]3d
1
4s
2
and 21 protons, does not readily form ScCl
2
; its 3d and 4s
electrons are not as strongly held by the attraction from the nucleus, so it more readily
releases these three electrons rather than just two of them.
Review exercise 33.3
1 a Ni
2+
: [Ar]3d
8
; Cu
2+
: [Ar]3d
9
; Zn
2+
: [Ar]3d
10
b Both Ni
2+
and Cu
2+
will form coloured compounds as they both have unfilled
d-orbitals which can be occupied by excited electrons as they absorb light. Zn
2+
is
colourless because the d-orbitals are completely filled and no electrons can be
promoted, so no light is absorbed.
c Because Cu
2+
ions are typically blue they must be absorbing light of the
complementary colours, that is, in the red-orange range.
2 Sc
3+
: [Ar] and Ti
4+
: [Ar] do not have coloured compounds because they have no
electrons occupying d-orbitals that can be promoted to higher energy level orbitals.
However, Ti
3+
: [Ar]3d
1
and V
3+
: [Ar]3d
2
both have partially filled d-orbitals with
electrons that can be promoted into higher energy d-orbitals when they absorb photons
of light.
Review exercise 33.4
1 a Cr
3+
acts as a Lewis acid (electron pair acceptor) and the water molecules act as
Lewis bases.
b Monodentate
2 The ethylenediamine (en) has two sites with lone pairs of electrons (see Figure 33.9 in
text), which it is able to donate and hence act as a Lewis base. Three en molecules
donate both their lone pairs to Ni
2+
, which thus acts as a Lewis acid and forms
[Ni(en)
3
]
2+
.
3 a [Ir(NH
3
)
3
Cl
3
]
b [Cr(H
2
O)
2
(C
2
O
4
)
2
]
c [Co(CN)
6
]
3
Review exercise 33.5
1 The colour depends on the type of ligand present; the water molecule produces the
violet colour and the cyanide ion produces the yellow colour.
2 Cu
2+
: [Ar]3d
9
and Zn
2+
: [Ar]3d
10
; the copper ion will produce coloured complexes
because it has an unfilled d-orbital which can be occupied by electrons as they absorb
Solutions Manual: Module 8
The chemistry of art
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
light. However, the zinc ion will produce colourless complexes, as there are no unfilled
d-orbitals.
3 a i The metal ion is Ni
2+
and its oxidation state is +2.
ii Each water molecule is bonded to the metal ion through one of the lone
pairs of electrons on the oxygen atom.
b i The metal ion is Ni
2+
and its oxidation state is +2.
ii Each ammonia molecule is bonded to the metal ion through the lone pair of
electrons on the nitrogen atom.
c i The metal ion is Ni
2+
and its oxidation state is +2.
ii Each en molecule is bonded to the metal ion through the lone pair of
electrons on each on the two nitrogen atoms.
4 Because it is impossible to actually see the bonding arrangement with complexes
containing chelated ligands, such as iron(III)hexahydrate, we use models such as
diagrams, modelling kits and computer images to show what we think the arrangement
is like. We can illustrate the way we believe the six water molecules are arranged
around the iron(III) ion and can use models to represent the coordinate covalent bonding
in the Lewis acidbase interaction. However, we cannot show how this happens, the
positions and movements of electrons, the energy changes involved or the rate at which
this happens. Overall, modelling is the most useful method we have at the moment to
depict our understanding.
Chapter 33 Application and investigation
1 Investigation
2 a MnO
4
(aq) (purple) + 8H
+
(aq) + 5e
Mn
2+
(aq) (colourless) + 4H
2
O(l)
b 2CrO
4
2
(aq) (yellow) + 2H
+
(aq) Cr
2
O
7
2
(aq) (orange) + H
2
O(l)
c Fe
3+
(aq) (brown) + (SCN)
(aq) [Fe(SCN)]
2+
(aq) (deep red)
d 3Cu(s) (colourless)+ 2NO
3
(aq) + 8H
+
(aq) 3Cu
2+
(aq) (blue) + 2NO(g) + 4H
2
O(l)
e Cr
2
O
7
2
(aq) (orange) + 2OH
(aq) 2CrO
4
2
(aq) (yellow) + H
2
O(l)
3 +2
46 Investigation
Module 8
REVIEW
1 a Red ochre, Fe
2
O
3
Solutions Manual: Module 8
The chemistry of art
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
b The colour of red ochre comes from the presence of the Fe
3+
ion. Iron, [Ar]3d
6
4s
2
, is a transition metal with unfilled d-orbitals. The electrons in the 3d subshell
are able to absorb quanta of energy and move to the available unfilled orbitals.
The energy absorbed is from the green range of the visible spectrum and so the
compound appears as the reflected complementary colour red. The Australian
Aboriginal people commonly applied their pigments to rock surfaces by mixing
them with saliva, fats, waxes, water or blood, and allowing these media to dry.
2 a Colour can be added to the bulk of a material such as glass by finely powdering
metal oxide pigments and adding them to the glass mixture before melting the
mixture. Other methods, such as staining and flashing, involve coating the
previously prepared glass and then refiring it.
b Cobalt oxide, manganese oxide, iron oxide, copper(II) oxide and silver nitrate can
be used to add colour to glass.
3 In the mediaeval painting of about 1365, St John the Baptist with St John the Evangelist
(?) and St James, by Nardo di Cione, the picture is on wood and the ground is gesso.
This gesso is a mixture of gypsum, CaSO
4
.2H
2
O, and animal glue. The figures were
drawn onto the ground and then painted using an egg tempera medium. The paints
involved in this painting include the following:
Pigment Chemical composition Method of preparation
Red ochre Fe
2
O
3
Grinding of the natural ore.
Minium (red lead) PbO
2
.2PbO Oxidation of molten lead.
Ultramarine 3Na
2
O.3Al
2
O
3
.6SiO
2
.2Na
2
S Grinding, kneading in lye
solution with a special dough.
Insoluble colour in suspension
is then allowed to settle, is
washed and dried.
Crimson lake
(cochineal)
C
11
H
12
O
17
Colour from solution is fixed
onto an insoluble white
powder such as gypsum,
which is then extracted and
dried.
White lead 2PbCO
3
.Pb(OH)
2
Stack processlead in clay
pots with vinegar and manure
and left to ferment.
Lead tin yellow PbSnO
4
Roasting of lead and tin
mixture.
Solutions Manual: Module 8
The chemistry of art
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
Vermilion HgS Heating mercury and sulfur
together and HgS condenses
from the vapours.
Green earth Various Fe, Mg, Al and K
compounds
Grinding of the natural ores.
Gilding Au Found in native condition.
This painting was not varnished; the egg tempera of the paint layer provided a self-
protective finish.
4 a An emission spectrum is produced when the visible light emitted from excited
atoms of an element is passed through a narrow slit and then a prism. An
absorption spectrum is the result of light first passing through a substance in the
vapour phase and then producing a pattern of dark lines across a continuous
spectrum. A reflectance spectrum results when white light is shone onto a surface
and the reflected light is analysed.
b A reflectance spectrum can be obtained from a painting by shining white light on
to the surface of the chosen pigment and analysing the resulting light reflected
from the surface of the pigment. The reflectance spectrum can then be compared
to those of known compounds and an identification made of the pigment. A 5%
tint of cobalt blue shows over 80% reflectance of light with a wavelength of 480
490 nm (blue at the green end) and 90% reflectance of light with a wavelength of
700 nm (deep red). A 5% tint of Prussian blue has over 60% reflectance of light in
the 440490 nm range (violet blue).
Laser microspectral analysis can use laser reflectance to identify pigments very
accurately.
5 The Bohr model of the atom described the orbit of electrons around the atom as being at
fixed radii and energy; the electrons could absorb energy in fixed quanta and move to a
higher energy level and then emit these same quanta of energy when they fell back to a
lower energy level. This model was very useful in explaining the line spectrum of
hydrogen, and it supplied the notion of quanta of energy able to be absorbed and
emitted, but it was not as useful for explaining the atomic spectra of atoms other than
hydrogen. Nor could it explain the different intensities of spectral lines, the fact that
some spectral lines actually consist of a number of very fine close lines, or the splitting
of such spectral lines in magnetic fields.
6 A piece of platinum wire was thoroughly cleaned by immersion in hydrochloric acid
and heating in a non-luminous Bunsen burner flame. Then the wire was dipped in a
small sample of sodium chloride and placed in the non-luminous Bunsen flame and the
colour of the flame observed. Sodium ions produced a strong yellow flame. This colour
is produced because the electrons of the sodium absorb quanta of energy and move to
Solutions Manual: Module 8
The chemistry of art
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
higher energy levels. This absorbed energy is emitted as the excited electrons jump back
to their ground states. The emitted energy is the yellow range of visible light.
7 a
b The 27 electrons of the cobalt atom fill in the shell order 1s, 2s, 2p, 3s, 3p, 4s,
then 3d. In filling the orbitals, two restrictions apply: the Pauli exclusion principle
states that an orbital can only hold a maximum of two electrons (and that these
must have opposite spins); and Hunds rule states that if two or more orbitals are
available (as in the 3d orbitals for cobalt), then one electron goes into each orbital
until all the orbitals are half-full, and then a second electron can go into the
orbitals.
8 The transition metals have valence electrons in both the 3d and 4s sub-shells. Because
there is very little difference in energy between these sub-shells and their orbitals, the
transition metals can lose these electrons with almost equal ease, resulting in a number
of oxidation states for most of these metals. The unfilled orbitals in the sub-shells of
some of the transition metals also have good electron acceptor properties in forming
coordination compounds and so make good oxidising agents because they are
themselves so easily reduced. Metals from the s and p blocks do not have the same
d-orbital configurations and so have little or no variation in their oxidation states.
9 Colour in aqueous ions of transition metals results from the ability of these metals to
absorb quanta of energy and promote electrons to unfilled d-orbitals. The absorbed
energy is in the range of visible light and the compounds appear the complementary
colour of the energy absorbed: the [Cr(H
2
O)
6
]
3+
ions appear violet because they have
absorbed energy in the yellow-green range and reflect or transmit in the violet range.
Aqueous ions of metals other than transition metals do not have unfilled d-orbitals and
so have no opportunity to produce colours.
10 a The ligand EDTA forms a coordinate covalent bond with cadmium ions in
complex formation.
b The ligand EDTA has six sets of lone pair electrons through which it bonds to the
Cd
2+
ion. As the EDTA is donating the pairs of electrons, it is acting as a Lewis
base and the metal ion is acting as a Lewis acid. Hence, the sequestering of
cadmium into an EDTA complex is a Lewis acidLewis base interaction.
11 A strong oxidising agent is itself readily reduced. Potassium permanganate solution,
KMnO
4
(aq), is a deep purple colour as a consequence of the +7 oxidation state of
manganese in the MnO
4
(aq) ion. If this ion is reduced, its colour should quickly
change.
Steps:
1 Make up a 0.1 M solution of acidified (with sulfuric acid) potassium
permanganate, KMnO
4
(aq).
Solutions Manual: Module 8
The chemistry of art
Pearson Education Australia (a division of Pearson Australia Group Pty Ltd) 2006.
This page from Chemistry Contexts 2 Teachers Resource second edition may be reproduced for classroom use.
2 Add it dropwise to a solution of violet vanadium(II) ions formed from oxidising
dioxovanadium(V) ions using granulated or powdered zinc metal added to
acidified (with about 9 M sulfuric acid) 0.1M ammonium metavanadate solution.
3 If you dont agitate the mixture too much you will see the colours as the
vanadium(II) is oxidised progressively to the vanadium(III) ion, to the
oxovanadium(IV) ion, to the dioxovanadium(V) ion. The colour goes from violet
to green to blue to yellow and the purple permanganate decolourises.
4 As the vanadate ion (converted to dioxovanadium(V) ion by the sulfuric acid) is
also a strong oxidising agent, permanganate must be a very strong oxidising agent.
The net ionic equation for the permanganate reduction is:
MnO
4
(aq) (purple) + 8H
+
(aq) + 5e
Mn
2+
(aq) + 4H
2
O(l) (colourless)
The oxidation state of the Mn
2+
ion is +2.
The ionic equations for the vanadium(II) oxidation are:
V
2+
(aq) V
3+
(aq) + e
V
3+
(aq) + H
2
O(l) VO
2+
(aq) + 2H
+
(aq) + e
VO
2+
(aq) + H
2
O(l) VO
2
+
(aq) + 2H
+
(aq) + e
Because acidification of the metavanadate is with concentrated acid, safety goggles,
gloves and protective clothing must be worn. Also, the metavanadate is toxic and a
possible mutagen; gloves, goggles and protective clothing must be worn.
12 A ligand is a species (acting as a Lewis base) with a lone pair of electrons able to be
donated to a metal cation (acting as a Lewis acid) with orbitals available to receive
them. Thus, a coordinate covalent bond forms in this Lewis acidbase interaction as
both electrons in the bond originate from the one atom. Chelates are ligands able to
form at least two coordinate bonds to the one cation.
For example, ethylene diamine is a ligand that has two nitrogen atoms that can each
bond to a cation such as nickel(II).
You might also like
- Chemistry Contexts PreliminaryDocument377 pagesChemistry Contexts PreliminaryEshayz111100% (3)
- Biology: in FocusDocument14 pagesBiology: in FocusJoshua John0% (1)
- HSC Biology NotesDocument31 pagesHSC Biology NotesSilvia100% (1)
- Avax PDFDocument401 pagesAvax PDFSancocho LibrosNo ratings yet
- Solutions Manual PDFDocument113 pagesSolutions Manual PDFfdsgNo ratings yet
- Chemistry Formula SheetDocument193 pagesChemistry Formula SheetGadde Gopala KrishnaNo ratings yet
- Chapter 2 VCE Chemistry AnswersDocument50 pagesChapter 2 VCE Chemistry AnswersNicholas EkkelNo ratings yet
- Full PDFDocument445 pagesFull PDFعلي مؤيد مطشر صدامNo ratings yet
- Ebin - Pub Introduction To Modern Inorganic Chemistry 2nbsped 9843000870Document840 pagesEbin - Pub Introduction To Modern Inorganic Chemistry 2nbsped 9843000870Sumiya Aziz100% (2)
- A Local EcosystemDocument44 pagesA Local EcosystemHad MoeNo ratings yet
- Jacaranda HSC Chemistry Chapter 15Document42 pagesJacaranda HSC Chemistry Chapter 15Fúul 'O' Reagrett100% (1)
- Chemistry Unit 3 Student GuideDocument28 pagesChemistry Unit 3 Student GuideApollo WongNo ratings yet
- Matter and OthersDocument54 pagesMatter and OthersAnonymous p1txoupQGvNo ratings yet
- 10th Biology English PDFDocument242 pages10th Biology English PDFImam GajulaNo ratings yet
- Basic Chemistry 1Document246 pagesBasic Chemistry 1FalcoOon100% (1)
- 0 - NSW Surfing Physics Modules 5and6 Sample PDFDocument19 pages0 - NSW Surfing Physics Modules 5and6 Sample PDFAmelia Rahmawati0% (1)
- Investigating Science: PearsonDocument29 pagesInvestigating Science: PearsonnatsdorfNo ratings yet
- NSM Enhanced 7 CBDocument530 pagesNSM Enhanced 7 CBRenee Ng0% (8)
- Nutrient and Food TestsDocument2 pagesNutrient and Food Testsapi-222503660No ratings yet
- Frank Jenkins, Hans Van Kessel, Dick Tompkins, Oliver Lantz, Lucille Davies, Patricia Thomas - Nelson Chemistry 11 - Nelson (2002) PDFDocument699 pagesFrank Jenkins, Hans Van Kessel, Dick Tompkins, Oliver Lantz, Lucille Davies, Patricia Thomas - Nelson Chemistry 11 - Nelson (2002) PDFRuby Angel Mann100% (2)
- PDFDocument226 pagesPDFAa50% (2)
- Jacaranda Chemistry Units 3 - 4Document450 pagesJacaranda Chemistry Units 3 - 4josh soon100% (1)
- New School PhysicsDocument541 pagesNew School PhysicsjaphethatongaNo ratings yet
- Chap01 Jacaranda Chemistry PrelimDocument38 pagesChap01 Jacaranda Chemistry PrelimPatrick ChenNo ratings yet
- Essentials Pearson 2016Document253 pagesEssentials Pearson 2016Devansh SharmaNo ratings yet
- SM 11 Chemistry Eng 201617Document185 pagesSM 11 Chemistry Eng 201617Anonymous 9uu04el100% (1)
- Biology in Focus Prelim Sample ChapterDocument37 pagesBiology in Focus Prelim Sample ChapterLuke Vincent100% (1)
- Biology Dot Point SummaryDocument91 pagesBiology Dot Point Summaryjulz230100% (4)
- Economics Year 12 PDFDocument136 pagesEconomics Year 12 PDFNavinesh Nand100% (1)
- 93, 94Document282 pages93, 94Sulfa MubarikaNo ratings yet
- Year 11 Chemistry Study NotesDocument39 pagesYear 11 Chemistry Study NotesRaghav Jaitely100% (1)
- HSC Preliminary Chemistry NotesDocument32 pagesHSC Preliminary Chemistry NotesHarvey Luo100% (1)
- Aqa Science Igcse Chemistry SowDocument103 pagesAqa Science Igcse Chemistry SowAnthonyNo ratings yet
- First 5 Chapters Chemistry XiiDocument201 pagesFirst 5 Chapters Chemistry XiiIkram ali khan100% (2)
- Chemistry Module IV Organic Chemistry I PDFDocument323 pagesChemistry Module IV Organic Chemistry I PDFdebasij83% (6)
- Chemistry Grade 7 PDFDocument140 pagesChemistry Grade 7 PDFBD TUBENo ratings yet
- PracticalsDocument158 pagesPracticalsBirajuNo ratings yet
- Calculations in Chemistry PDFDocument869 pagesCalculations in Chemistry PDFMandel Mach80% (5)
- Yr 11 Chemistry Module 4 Revision TestDocument3 pagesYr 11 Chemistry Module 4 Revision TestRicha NgNo ratings yet
- Vceasy Visual Chemistry Student Booklet v1 33Document68 pagesVceasy Visual Chemistry Student Booklet v1 33James Wong100% (1)
- c3 Revision WorksheetsDocument5 pagesc3 Revision Worksheetsapi-236179294No ratings yet
- Properties and Structure of MatterDocument20 pagesProperties and Structure of MatterBella PurserNo ratings yet
- Pearson Chemistry Chapter 2 SlidesDocument54 pagesPearson Chemistry Chapter 2 SlidesRocket FireNo ratings yet
- PUC Chemistry Question BankDocument214 pagesPUC Chemistry Question BankSathish Kumar100% (1)
- Full HSC BioDocument37 pagesFull HSC BioAnna Gotham LiNo ratings yet
- Matriculation Chemistry (Hydrocarbon) Part 2 AlkaneDocument30 pagesMatriculation Chemistry (Hydrocarbon) Part 2 AlkaneridwanNo ratings yet
- Year 11 Chemistry WorkbookDocument147 pagesYear 11 Chemistry WorkbookVikas86% (7)
- The EASY Guide To Ace General Chemistry I and II - General Chemistry Study Guide, General Chemistry ReviewDocument202 pagesThe EASY Guide To Ace General Chemistry I and II - General Chemistry Study Guide, General Chemistry Reviewaaaaa100% (2)
- NSW Biology 01Document75 pagesNSW Biology 01maria paulNo ratings yet
- Production of MaterialsDocument27 pagesProduction of MaterialsdasdaNo ratings yet
- 25 Polylactic Acid PDFDocument8 pages25 Polylactic Acid PDFFrench Sarah NeraNo ratings yet
- 2011 Chemistry NotesDocument70 pages2011 Chemistry NotesAbdullionNo ratings yet
- HSC Production of Materials. BestnotesDocument24 pagesHSC Production of Materials. Bestnotes16choi.michaelNo ratings yet
- Production of MaterialsDocument12 pagesProduction of MaterialsBen ReynoldsNo ratings yet
- 2008 Chemistry NotesDocument20 pages2008 Chemistry NotesJohndoeNo ratings yet
- HSC Chemistry Production of MaterialsDocument75 pagesHSC Chemistry Production of MaterialsRichardZhangNo ratings yet
- Preliminary Chemistry Notes NSWDocument56 pagesPreliminary Chemistry Notes NSWfocuc980% (1)
- Production Of: Mate RialsDocument40 pagesProduction Of: Mate RialsWulan SariNo ratings yet
- 1 s2.0 S0032386119303970 MainDocument10 pages1 s2.0 S0032386119303970 Mainali kasiriNo ratings yet
- Introduction to Renewable Biomaterials: First Principles and ConceptsFrom EverandIntroduction to Renewable Biomaterials: First Principles and ConceptsAli S. AyoubNo ratings yet
- Autumn Lecture 1 (Isotopes Mass Spec)Document32 pagesAutumn Lecture 1 (Isotopes Mass Spec)Eugenia MigranovaNo ratings yet
- Aakash Foundations - Online Test on PMT Pattern for Class-X with Hints & SolutionsDocument15 pagesAakash Foundations - Online Test on PMT Pattern for Class-X with Hints & Solutionssohan12345No ratings yet
- Class 9 Med Exam 2019Document10 pagesClass 9 Med Exam 2019Khalid HassanNo ratings yet
- Atomic Math ChallengeDocument1 pageAtomic Math Challengeapi-378350740No ratings yet
- O Levels Cehimstry-Atomic Structure - Chemical BondingDocument16 pagesO Levels Cehimstry-Atomic Structure - Chemical Bondingjave_yeongNo ratings yet
- 11 Stem - Khayecee Gail Aya Ay Mayor Gen Chem 1 Module 2Document16 pages11 Stem - Khayecee Gail Aya Ay Mayor Gen Chem 1 Module 2Khayecee Gail Aya-ay MayorNo ratings yet
- Folio Chemistry Form 4 Chapter 2Document34 pagesFolio Chemistry Form 4 Chapter 2Nurul Syafiqa MahadhirNo ratings yet
- Everyday ScienceDocument117 pagesEveryday ScienceFawad Hayat MohmandNo ratings yet
- C.S.E. (MAIN) PHYSICS PAPER - I EXAM SOLUTIONSDocument9 pagesC.S.E. (MAIN) PHYSICS PAPER - I EXAM SOLUTIONSRATNADEEP BANERJEENo ratings yet
- Download ebook The Linemans And Cablemans Handbook Pdf full chapter pdfDocument67 pagesDownload ebook The Linemans And Cablemans Handbook Pdf full chapter pdfsummer.stephens690100% (18)
- Radiation Physics Lecture Notes 2018Document75 pagesRadiation Physics Lecture Notes 2018Niyas UmmerNo ratings yet
- The Torsion Field and the Aura ExplainedDocument48 pagesThe Torsion Field and the Aura ExplainedCambiador de Mundo100% (9)
- Cosmic Abundance of Element NotesDocument42 pagesCosmic Abundance of Element NotesSyed Aquib ShamshadNo ratings yet
- Properties of Matter and SoundDocument40 pagesProperties of Matter and SoundrrtilakNo ratings yet
- Nuclear Gravitation Field TheoryDocument62 pagesNuclear Gravitation Field TheoryKen WrightNo ratings yet
- Technical Data On Nucleonic Gauges: IAEA-TECDOC-1459Document118 pagesTechnical Data On Nucleonic Gauges: IAEA-TECDOC-1459Ashish FatingNo ratings yet
- Curriculum Map G8 ScienceDocument7 pagesCurriculum Map G8 ScienceReynald AntasoNo ratings yet
- Formation of Light and Heavy Elements: Prepared By: Jerome A. Bigael, Leyte Progressive High SchoolDocument17 pagesFormation of Light and Heavy Elements: Prepared By: Jerome A. Bigael, Leyte Progressive High Schoolshermaine geniston100% (1)
- Activity Sheet-09 (Symbols and Atomic Numbers of The 1st 20 Elements)Document2 pagesActivity Sheet-09 (Symbols and Atomic Numbers of The 1st 20 Elements)Nkemzi Elias NzetengenleNo ratings yet
- 2020-APR - GWG - Unit-IG2 - Element - 5 - v03 - SDocument48 pages2020-APR - GWG - Unit-IG2 - Element - 5 - v03 - SJakeer hussain ShaikNo ratings yet
- JC1 Atomic Structure NotesDocument35 pagesJC1 Atomic Structure NotesLeng RyanNo ratings yet
- Chem Lec ReviewerDocument16 pagesChem Lec ReviewerSophia EspirituNo ratings yet
- Nuclear Physics-Critical Thinking SkillsDocument36 pagesNuclear Physics-Critical Thinking SkillsNitin SharmaNo ratings yet
- Dwnload Full Inquiry Into Life 14th Edition Mader Test Bank PDFDocument35 pagesDwnload Full Inquiry Into Life 14th Edition Mader Test Bank PDFpommettepresume401u100% (9)
- Physical Design and Technology Development of CSNS Target StationDocument280 pagesPhysical Design and Technology Development of CSNS Target StationsamginNo ratings yet
- Tesla Coils Plans PDFDocument153 pagesTesla Coils Plans PDFroseli100% (3)
- Medical Physics Syllabus Notes - Daniel WilsonDocument14 pagesMedical Physics Syllabus Notes - Daniel WilsonPosclutoNo ratings yet
- CBSE NCERT Solutions For Class 12 Physics Chapter 13: Back of Chapter QuestionsDocument29 pagesCBSE NCERT Solutions For Class 12 Physics Chapter 13: Back of Chapter QuestionsBikash DeyNo ratings yet
- N He O H: Chapter 10: Nuclear EnergyDocument7 pagesN He O H: Chapter 10: Nuclear EnergyShwetaNo ratings yet
- Marius Arghirescu - The Cold Genesis of Matter and Fields (2015)Document222 pagesMarius Arghirescu - The Cold Genesis of Matter and Fields (2015)Arghirescu MariusNo ratings yet