Professional Documents
Culture Documents
Stamicarbon Project PDF
Uploaded by
Mir Hasib Ul LatifOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Stamicarbon Project PDF
Uploaded by
Mir Hasib Ul LatifCopyright:
Available Formats
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
1. INTRODUCTION
Urea (NH2CONH2) is produced at industrial scale by the reaction between ammonia and carbon
dioxide at high pressure (1330MPa) and high temperature (170200 0C) . There are different
types of processes to produce urea in the commercial units. These processes are typically called
once through, partial recycle and total recycle . In the total recycle process, which is employed
widely, all the ammonia leaving the synthesis section is recycled to the reactor and the overall
conversion of ammonia to urea reaches 99% . Stamicarbon and Snamprogetti processes are the
most common examples of such process . Since urea has became almost the most widely used
fertilizer and its production is important in the petrochemical industry, there has been many
attempts to model and simulate the reactor of urea production as the heart of the process . In the
present work the entire urea synthesis section based on the of stamicarbon process (including
urea reactor, stripper, scrubber , rectifying column and flash separator) is modelled. Urea
production consists of reaction between ammonia and carbon dioxide react to form urea and
water .The urea synthesis is considered to occur in heterogeneous phase. In stamicarbon process
compressed carbon dioxide feed passes through the stripper along which ammonia and carbon
dioxide are stripped off from the liquid phase to the gas phase . The gas flow from the rectifying
column which carries the stripped off ammonia and carbon dioxide is mixed with pumped
ammonia feed and gas flow from srubber and on further heating , compression and cooling is fed
to the reactor. The liquid mixture in the reactor overflows into the stripper. The gas phase exiting
the reactor contains free ammonia and carbon dioxide as well as inert gas and is discharged into
the scrubber. In the scrubbing part, remaining gases are scrubbed with effluent from flash
separator. This stream is an aqueous solution of unreacted carbon dioxide and originates from
flash separation of urea from liquid outlet of CO2 stripper.
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
2. HISTORICAL BACKGROUND
Urea was first noticed by Hermann Boerhaave in the early 18th century from evaporates of urine.
In 1773, Hilaire Rouelle obtained crystals containing urea from human urine by evaporating it
and treating it with alcohol in successive filtrations. This method was aided by Carl Wilhelm
Scheele's discovery that urine treated by concentrated nitric acid precipitated crystals. Antoine
Franois, comte de Fourcroy and Louis Nicolas Vauquelin discovered in 1799 that the nitrated
crystals were identical to Rouelle's substance and invented the term "urea." Berzelius made
further improvements to its purification and finally William Prout, in 1817, succeeded in
obtaining and determining the chemical composition of the pure substance. In the evolved
procedure, urea was precipitated as urea nitrate by adding strong nitric acid to urine. To purify
the resulting crystals, they were dissolved in boiling water with charcoal and filtered. After
cooling, pure crystals of urea nitrate form. To reconstitute the urea from the nitrate, the crystals
are dissolved in warm water, and barium carbonate added. The water is then evaporated and
anhydrous alcohol added to extract the urea. This solution is drained off and allowed to
evaporate resulting in pure urea. In 1828, the German chemist Friedrich Whler obtained urea
artificially by treating silver cyanate with ammonium chloride.This was the first time an organic
compound was artificially synthesized from inorganic starting materials, without the
involvement of living organisms.The basic process for urea synthesis, developed in 1922, is
called the BoschMeiser urea process after its discoverers. Commercial production started in
1922 Germany, 1932 USA and 1935 UK . The stripping concept developed in 1966 by
Stamicarbon in The Netherlands improved heat recovery and reuse in the synthesis process. The
stripping concept proved to be such a major advance that competitors such as Snamprogetti
now Saipem (Italy), the former Montedison (Italy), Toyo Engineering Corporation (Japan) and
Urea Casale (Switzerland) all developed their own versions of it. Today effectively all new urea
plants use the principle, and many total recycle urea plants have been converted to stripping
processes. No radical alternative to it has been proposed; the main thrust of technological
development today, in response to industry demands for ever larger individual plants, is directed
at reconfiguring and reorientating major items in the plant to reduce their size and the overall
height of the plant, as well as at meeting ever more challenging environmental performance
targets.
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
3. PROCESS DESCRIPTION
3.1.Urea synthesis
Two reactions are involved in the manufacture of urea. First ammonium carbamate is formed
under pressure of 14 MPa by reaction between carbon dioxide and ammonia at 180 oC .
2NH3+ CO2 H2NCOONH4
H= -155MJ/kg.mol.
This highly exothermic reaction is followed by an endothermic decomposition of the
ammonium carbamate.
H2NCOONH4 H2NCONH2 + H2O H = +42MJ/kg.mol.
Both are equilibrium reactions. The formation reaction goes to virtual completion under
usual reaction conditions; the decomposition reaction is less complete. Rate of carbamate
decomposition reaction increases with temperature. It is slow at temperature less than 150oC
at stoichiometric proportion of ammonia to carbon dioxide and quite rapid at 210oC.
Therefore optimum temperature for urea synthesis is 180-210C with a retention time of 0.3
to 1 hr. At high temperature corrosion rate is high. The preferred pressure for urea synthesis
process is 140-250 atm. The stoichiometric ratio of NH3/CO2 conversion to urea is 2:1 but
excess ammonia above the stoichimetric ratio favors the rate of reaction. So optimum mole
ratio of NH3 to CO2 is 3.1-4.1 is preferred for urea synthesis. The presence of water
decreases conversion to urea. So the feedstock is made moisture free before charged to urea
reactor. Carbamate is highly corrosive and its corrosiveness can be minimized using small
amount of oxygen.
At now urea synthesis process are of three following types:
(a) Once through process: The once through process is simplest and least expensive
(both capital and operating cost) among the three processes. It is least flexible and cannot be
operated unless some provision is made to utilize large amount of unconverted ammonia and
off-gas.The unchanged ammonia is converted to ammonium compounds like ammonium
nitrate but this proves to be expensive and markets for secondary products are problematical.
(b) Partial recycle process: In this process part of the off gas is recycled back to the
reactor. The amount of ammonia is reduced to 15% to that of once through that must be
3
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
used in other
processes. Investment cost is somewhat lower than the total recycle
process, this advantage apparently does not compensate the inflexibility arising from the
necessity to operate a co-product plant with mutual interdependency problems. However
it finds application in UAN co-product plants.
(c) Total recycle process: In this process all unconverted NH3 and CO2 is recycled back to
the reactor (99% conversion) and therefore no nitrogen co-product is necessary.It is most
flexible urea process as it depends only NH3 and CO2 supply but incurs investment and
operating cost. Basically Total recycle process are of five types namely hot-gas mixture
recycle, separated gas recycle, slurry recycle, carbamate solution recycle and stripping.
Among them stripping process is the modern process developed by various urea process
licencors Stamicarbon, Snamprogetti and ACES .
3.2. Comparison of three types of total recycle stripping urea process
Table 01: Comparison of three types of total recycle stripping urea process
Process parameters
Stripping agent
Reactor temperature,oC
Reactor pressure,atm
Molar NH3/CO2 ratio
CO2 conversion,%
NH3 conversion,%
No. of high pressure vessels
Recirculation stages
NH3 consumption, t/t urea
CO2 consumption, t/t urea
Import steam,t/t urea
Cooling water,t/t urea
Electricity, kWh/t urea
Stamicarbon
Snamprogetti
Carbon dioxide Initially ammonia
now switch to steam
183
188
140
156
2.95
3.3-3.6
60
64
36
41
4
5
1
2
0.566
0.566
0.733
0.735
0.920
0.950
70
75
15
21-23
TEC ACES process
Carbon dioxide
190
175
4.0
6.8
34
5
2
0.568
0.735-0.740
0.80
80
15
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
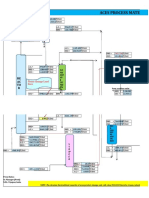
4. PROCESS DIAGRAM
4.1. Process block diagram
Reactor vent
High pressure feed
Feed compressor
Reactor
Wet gas
Reactor products
CO2
compressor
High
Rectifying
pressure
Column
stripper
Discharge gas
Moisture
Discharge
Mixer
CO2 feed
NH3 feed
Dry gas
Feed
NH3 pump
Mixed feed
Mixer
Vent gas
Scrubber
Recycle
Separator Effluent
Flash
Liquid products
Urea
Separator
Figure 01: Process Block diagram for Total recycle Stamicarbon CO2 stripping urea synthesis process
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
4.2.Process flow diagram
Figure 02: Process Flow diagram for Total recycle Stamicarbon CO2 stripping urea synthesis process
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
5. SIMULATION
5.1 Software package
Simulation refers to the application of computational models to the study and prediction of
physical events or the behavior of engineered systems. With the depth of its intellectual
development and its wide range of applications, computer simulation has emerged as a
powerful tool, one that promises to revolutionize the way engineering and science are
conducted in the twenty-first century. In engineering, simulation is important because
description of system behavior by experimentation might not be feasible due o inaccessible
inputs and outputs, experiment may be too dangerous or too costly, experimental behavior
might be obscured with disturbances. Simulation-based engineering science provides the
scientific and mathematical basis for the simulation of engineered systems. It facilitates the
engineers to be better able to predict and optimize systems.
ASPEN HYSYS is a commercially available process simulator for process analysis. It is a
powerful engineering simulation tool that has been uniquely created with respect to the
program architecture, interface design, engineering capabilities and interactive operation. It
contains a rigorous thermodynamic and physical property database and provides
comprehensive built-in process models, offering a convenient and time saving means for
chemical process studies, including system modeling, integration and optimization. The
original purpose of this software is for supporting the chemical engineering of crude oil
refineries. Process components of the simulation were implemented in ASPEN HYSYS using
standard, built-in unit operation modules and functions including all the components and
functions contained in the process.
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
5.2. Methodology
The simulation is carried out using UNIQUAC fluid package. It is an ideal model fit for
inorganic and organic reactive system. Prior to simulation ammonia, carbon dioxide, urea, water
and nitrogen are selected as simulation model components. The basis for simulation is selected
taking into account production rate of urea 1000kgmol/hr. To maintain such production rate of
urea 860kmol/hr of carbon dioxide at 1000C and 115kPa is compressed to 253.9kPa such that its
temperature rises to 185 0C and then fed to a high pressure stripper at the bottom where it strips
off unreacted ammonia and carbon dioxide present with urea solution in liquid reaction products
fed to the top of stripper column. The stripped gas passes to a rectifying column where the gas is
dried and water content is reduced to nil. The dry gas from rectifying column is passed to a
mixer where it is mixed with incoming ammonia feed of 1672 kmol/hr at -2100C which is
pumped to raise its pressure from 50.66kPa to 101.3 kPa. Meanwhile these two streams are
mixed with a recycle stream from scrubber and the combined
feed undergoes heating,
compression and cooling before entering the reactor such that its temperature is maintained at
1800C and pressure is kept at 506.6 kPa . The mole ratio of ammonia to carbon dioxide in the
combined feed is maintained at 4.44. In the reactor fractional conversion of CO2 is specified at
0.80 and cooling energy recovery is set up to keep reactor temperature at 75 0C. The reactor vent
gas then passes to the scrubber where it is scrubbed with aqueous solution of unreacted CO2 that
come from the flash separator through cooling. The liquid reaction product is fed to the high
pressure stripper from where the rich urea product is preheated to 1500C in order to be flashed in
a flash separator where remaining carbon dioxide is removed and urea solution is concentrated
to produce fairly concentrated urea solution. In both the scrubber and rectifying column inert
nitrogen, water vapor and slight amount of carbon dioxide is vented out. The simulation
maximize urea production through complete recycle of ammonia and almost total reuse of
carbon dioxide.
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
5.3. Procedure
1. Beginning the simulation: Select New Case from the File Menu>Press the New Case button.
The Simulation Basis Manager will appear. The next step is to create a Fluid Package.
2. Creating a Fluid Package: Press the Add button and the property view for new Fluid Package
appear>For Property Pkg select UNIQUAC by scrolling down the available property package
and then click on it.
3.Selecting components: Click View in Fluid Package . Then component list view will
appear>Type ammonia in Match box > Press Add Pure; the component will appear in Selected
Components list. Repeat for other components CO2 , urea, water and nitrogen.
4.Selecting physical coefficient: Move to Binary Coeffs>Click on UNIFAC VLE>Press
Unknowns only button. The unknown coefficients will appear.>Close the Fluid Package
5. Creating the reaction: Press Reactions tab in Simulation Basis Manager>Press Add
Rxn>Select Conversion type>Press Add Reaction tab. The Conversion Reaction Rxn-1 will
appear> Click on Add comp>Select ammonia from the Drop-Down arrow. Repeat for other
components. In the Stoichiometric Coeff corresponding to Ammonia type -2> Type -1 for
CO2>Type 1 for Urea >Type 1 for H2O> Balance Error will be noticeable 0.0>Move to the
Basis tab>Choose CO2 for Base Component>Leave Rxn Phase Overall>Type 80 for Co
>Close the Conversion Reaction property view > Press the Add Set button in the Reaction Sets
group> In the Active List for the cell called <empty> from the Drop-Down arrow select Rxn1>Press Close>Click on Set-1 in the Reaction sets on the Reactions tab>Press the Add to FP
button, the Add Set-1 view will appear>Press The Add Set to Fluid Package button.
6. Entering Simulation environment: Press the Enter Simulation Environment button on the
Simulation Basis Manager view . The PFD-Case(Main ) will appear. Click on File>Select
Save As>Type STAMICARBON UREA PROCESS SIMULATION>Click on Save> Click
on Tools> Press Variables> Set Unit to SI>Close.
7. Installing CO2 compressor: From the Object Pallete onto the PFD choose the
Compressor>Double Click the Compressor. In the Connections Page rename it CO2
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
compressor>In the Inlet type CO2 feed> In the Outlet type Compressed CO2 feed> In the
Energy type Compressor duty.
8. Installing High pressure stripper: From the Object Pallete onto the PFD choose the
Absorber>Double Click the Absorber. In the Connections Page rename it High pressure
stripper>In the Top Stage Inlet type Reaction Products> In the Bottom Stage Inlet select
Compressed CO2 feed by scrolling Drop-Down arrow>In the Ovhd Vapour Outlet type Wet
gas> In the Bottom Liquid Outlet type Liquid outlet> Keep the Number of stages default
10>Press Next to page 2 > Enter 405.3kPa and 506.6kPa at the Top Stage Pressure and
Bottom Stage Pressure respectively > Press Next to page 3> Press Done.
9. Installing Rectifying column : From the Object Pallete onto the PFD choose the Component
Splitter>Double Click the Component Splitter. In the Connections Page rename it Rectifying
column>In the Inlet select Wet gas by scrolling down Drop-Down arrow> In the Overhead
Outlet type Dry gas> In the Bottoms Outlet type Moisture> In the Energy Streams type
Column duty> Move to Parameters page> Click on Equalize all stream pressures > Move to
Splits page > Set Ammonia split in Dry gas column 1> Set CO2 split in Dry gas column
0.97190 > Set Urea split in Dry gas column 1> Set H2O split in Dry gas column 0> Set
Nitrogen split in Dry gas column 1.
10. Installing Centrifugal pump: From the Object Pallete onto the PFD choose the
Pump>Double Click the Pump. In the Connections Page rename it Centrifugal pump>In the
Inlet type Ammonia feed> In the outlet type Pumped Ammonia feed> In the Energy type
Pump duty>Move to the Parameters page and specify the Adiabatic Efficiency of 75.
11. Installing Feed mixer: From the Object Pallete onto the PFD choose the Mixer>Double
Click the Mixer. In the Connections Page rename it Feed mixer>In the Inlet select Dry gas and
Pumped Ammonia feed by scrolling down Drop-Down arrow and then type Recycle> In the
outlet type Mixed feed.
12. Installing Feed heater : From the Object Pallete onto the PFD choose the Heater>Double
Click the Heater. In the Connections Page rename it Feed heater >In the Inlet select Mixed
feed by scrolling down Drop-Down arrow> In the Outlet type Hot feed> In the Energy type H
duty>Move to the Parameters page and specify Delta P 0 kPa .
10
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
13. Installing Feed compressor: From the Object Pallete onto the PFD choose the
Compressor>Double Click the Compressor. In the Connections Page rename it Feed
compressor>In the Inlet type Hot feed> In the Outlet type feed> In the Energy type FCduty
14. Installing Feed cooler : From the Object Pallete onto the PFD choose the Cooler>Double
Click the Cooler. In the Connections Page rename it Feed Cooler >In the Inlet select feed by
scrolling down Drop-Down arrow> In the Outlet type Cooled feed> In the Energy type FeC
duty>Move to the Parameters page and specify Delta P 0 kPa .
15. Installing Balance: From the Object Pallete onto the PFD choose the Balance>Double Click
the Balance. In the Connections Page rename it Balance>In the Inlet Streams type Cooled
feed> In the Outlet streams type Combined feed> Move to the Parameters page and select
Balance Type as Mole.
16. Installing Reactor : From the Object pallete onto the PFD choose and click the General
Reactors > Double Click the Conversion Reactor. In the Connections Page rename it
Reactor>In the Inlets
select Combined feed by scrolling down Drop-Down arrow> In the
Vapour Outlet type Reactor vent> In the Energy type Coolant> In the Liquid Outlet select
Reaction Products by scrolling down Drop-Down arrow>Move to the Reactions tab>Select
Set-1 for the reaction>Click Conversion (%) and set Co as default >Move to Parameters page
> In the Delta P type 0 kPa > Set Duty to Cooling.
17. Installing Vaporizer: From the Object Pallete onto the PFD choose the Heater>Double
Click the Heater. In the Connections Page rename it Vaporizer >In the Inlet select Liquid
outlet by scrolling down Drop-Down arrow> In the Outlet type Hot liquid> In the Energy
type V duty>Move to the Parameters page and specify Delta P 30 kPa .
18. Installing Flash Separator : From the Object pallete onto the PFD choose the
Separator>Double Click the Separator. In the Connections Page rename it Flash Separator>In
the Inlets select Hot liquid by scrolling down Drop-Down arrow > In the Vapour Outlet type
Off gas> In the Liquid Outlet type Urea .
11
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
19.Installing Effluent Cooler: From the Object Pallete onto the PFD choose the Cooler>Double
Click the Cooler. In the Connections Page rename it Effluent Cooler >In the Inlet select feed
by scrolling down Drop-Down arrow> In the Outlet type Cooled feed> In the Energy type
FeC duty>Move to the Parameters page and specify Delta P 0 kPa .
20. Installing Scrubber: From the Object Pallete onto the PFD choose the Component
Splitter>Double Click the Component Splitter. In the Connections Page rename it S>In the
Inlet select Wet gas by scrolling down Drop-Down arrow> In the Overhead Outlet type
Moisture> In the Bottoms Outlet type Dry gas> In the Energy Streams type Column duty>
Move to Parameters page> Click on Equalize all stream pressures > Move to Splits page >
Set Ammonia split in Moisture column 0> Set CO2 split in Moisture column 0.02810> Set
Urea split in Moisture column 0> Set H2O split in Moisture column 1> Set Nitrogen split in
Moisture column 0.
21. Installing Valve : From the Object pallete onto the PFD choose the Valve>Double Click the
Valve. In the Connections page rename it Valve>In the Inlet select Reactor vent by scrolling
down Drop-Down arrow> In the Outlet select Relief gas by scrolling down Drop-Down arrow>
Click on the Parameters page and specify Delta P 30kPa.
22 Installing Discharge Mixer: From the Object Pallete onto the PFD choose the
Mixer>Double Click the Mixer. In the Connections Page rename it Discharge mixer>In the
Inlet select Moisture and Vent gas by scrolling down Drop-Down arrow > In the outlet type
Reaction discharge.
23. Entering Process Specification: Press Workbook button on the button bar> In the CO2 feed
enter Temperature 100oC , Pressure 115kPa , Molar Flow Rate 860 kgmol/hr>Move to
Compositions tab>In the CO2 feed enter Comp Mole Frac(CO2) 0.9999 and Comp Mole
Frac(Nitrogen) 0.0001>Click Normalize>Click OK>Press Material Streams> In the
Compressed CO2 feed enter Temperature 1850C>In the Reactor vent enter Temperature
750C >In the Ammonia feed enter Temperature -210oC and Pressure 50.66kPa > In the
Pumped Ammonia feed Enter Temperature -2100C and Pressure 101.3kPa > In the
Combined feed enter Temperature 1800C , Pressure 506.6kPa and Molar Flow Rate set to
12
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
5688kgmol/hr>Move to Compositions Tab>In the Combined feed enter Comp Mole
frac(Ammonia) 0.8162, Comp Mole frac(CO2) 0.1837
and Comp Mole frac(Nitrogen)
0.0001>Click Normalize>Click OK> Return to Material streams > Enter Temperaure for Hot
feed , Moisture, Dry gas, Hot liquid, Cooled feed and Liquid effluent as 180 oC, 75 oC, 150
o
C, 150 oC,180 oC and 74.98oC respectively>In the feed enter Pressure 506.6kPa.
24. Converging High pressure stripper: Return to the PFD>Double Click High pressure
Stripper> Press Run to begin the calculation> The Converged box appear green and Simulation
is set to Solver Active mode and all Unit Ops, Material Streams and Energy Streams appear
green.
13
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
6. OPTIMIZATION
6.1. Methodology
Optimization is carried out taking profit as objective function. The basis for optimization is 1 hr
of operation .Profit is defined as excess of Sales over Operating Cost . Sales price of Urea is
taken to be $1.1/kg and total Sales is computed from production rate of urea , urea composition
and Sales price of Urea. Operating Cost is divided into two types: raw material cost and energy
cost.Raw material cost is computed from total cost of ammonia feed and total cost of carbon
dioxide where ammonia feed cost is the product of ammonia feed rate , its composition and
price and similarly carbon dioxide feed cost is the product of carbon dioxide feed rate , its
composition and price. Price of ammonia is set at $ 0.2/kg while that of CO2 is $ 0.02/kg. The
energy cost is derived from the summation of the product of heat duties of different unit
operations with their respective costs. Below given the energy costs of different operations:
Cost of puming/kWh - $ 0.500
Cost of compression /kWh-$ 0.600
Cost of heating/Kwh-$ 0.7370
Cost of Cooling/Kwh =$0.4710
Cost of Cooling in reactor/kWh = $0.25
Cost of heating and cooling in rectifying Column =$0.30
Incorporating the cost factors the profit function comes to be:
Profit = Urea sales priceUrea production rateUrea composition-Ammonia priceAmmonia
feed rateAmmonia composition-CO2 priceCO2 feed rateCO2 composition-Pump dutyCost
of pumping-(Feed compressor Duty+CO2 compressor duty)Cost of compression-Cost of
heating(Heater duty+ Vaporizer duty)-Cost of Cooling(Feed cooler duty+Effluent cooler
duty)-Cost of cooling in reactorReactor Coolant duty-Cost of heating and cooling in rectifying
columnRectifying Column energy duty.
The material flow rate and heat duties are imported from Hysys. Two modifying variables are
selected for optimization. They are:
CO2 feed rate = 850 to 870kgmol/hr and Combined flow rate = 5680 to 5690 kgmol/hr.
14
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
6.2. Procedure:
1. Click on Simulation on Menu bar
2. Press Optimizer
3. Configuration page appear
4. Press Spreadsheet tab
5. Fill up the specifications in spreadsheet
6. To import variable Right click on the mouse. A scroll list appear . Select Import
variable > Select Case (Main) from Flowsheet > Select desired Object >Select
desired Variable> Click OK
7. Define profit function.
8. Return to Simulation and Press Optimizer
9. Press Variables tab>Press Add > Select Object>Select Variables> Set Low Bound
and High Bound values.
10. Move to Parameters page> Set Maximum Iterations to 300> Set Tolerance to 1e6
11. Move to Functions tab> Select the Profit cell from scroll list > Click Maximize>
Press Start to begin the iteration. Then Proceed box appears green with Optimum
Found(Small Delta X) written.
15
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
7. GRAPHICAL TREND ANALYSIS
1. Effect of reactor temperature on Urea Production: Urea production increases with reactor
temperature at a hig rate up to 40oC and then slower rate perceived between 40oC and 80oC
maximum production found at 110oC.
Figure 03: Effect of reactor temperature on Urea Production
2.
Effect of reactor pressure on Urea production: Urea production increases exponentially
with reactor pressure with optimum found at 325kPa
Figure 04: Effect of reactor pressure on Urea Production
16
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
3. Effect of reactor temperature on unconverted ammonia and CO2: With increase in reactor
temperature unconverted CO2 decreaseses exponentially attaining steady conversion at
100oC. On the contrary unconverted ammonia increases up to 20oC reaching minimum
conversion at 40oC before reaching steady state conversion at 110oC.The minimum
conversion at 40oC induces slower rate of urea production between 40oC and 80oC .
Figure 05 : Effect of reactor temperature on unconverted ammonia and CO2
17
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
4. Effect of reactor pressure on unconverted ammonia and CO2 : With increase in reactor
presssure conversion of both ammonia and carbon dioxide increases with ammonia
converted faster to urea than CO2. The peak conversion for both chemical species is found at
325kPa. Onward conversion remains steady.
Figure 06: Effect of reactor pressure on unconverted ammonia and CO2
5. Effect of reactor temperature on reactor duty: Lesser energy consumption on reactor at
higher temperature.
Figure 07: Effect of reactor temperature on reactor duty
18
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
6. Effect of fractional conversion of CO2 on fraction of non-reacted ammonia: It is evident
from the graph that ammonia conversion decreases with increase in CO2 conversion.
Figure 08: Effect of fractional conversion of CO2 on fraction of non-reacted ammonia
19
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
7. Effect of mole ratio of NH3 to CO2 on rate of urea synthesis chemical species : Increasing
the mole ratio of NH3 to CO2 increases conversion of both ammonia and carbon dioxide
with carbon dioxide converted to urea faster than ammonia and optimum urea production
occurs at mole ratio 4.25.
Effect of mole ratio on rate of urea synthesis
chemical species
7.00E+04
6.00E+04
5.00E+04
Mass flow rate
/kg/h
4.00E+04
unreacted CO2
3.00E+04
urea
Unreacted ammonia
2.00E+04
1.00E+04
0.00E+00
1
Mole ratio of ammonia to CO2
Figure 09: Effect of mole ratio of NH3 to CO2 on rate of urea synthesis chemical species
20
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
8. Effect of reactor temperature on reactor chemical species distribution: Urea distribution in
reactor increases with reactor temperature while that of ammonia and carbon dioxide
decreases with temperature . Water distribution increases up to 40oC and remains steady
between 40oC and 70oC and then decreases due to vaporization. This shows that presence of
high amount of water between 40oC and 70oC slows down urea production.
Reactor Chemical species distribution at
reactor temperature
1.1
1
0.9
0.8
0.7
Reactor
0.6
Chemical species
Distribution 0.5
0.4
Urea
ammonia
Carbon dioxide
0.3
water
0.2
0.1
0
0
20
40
60
80
100
120
Reactor temperature/C
Figure 10: Effect of reactor temperature on reactor chemical species distribution
21
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
9. Effect of Separator temperature on rate of Off gas: Graph shows that Off gas kicks of
separation at 148oC and then increases constantly. It may be the fact that CO2 is partially
dissolved in urea solution and that its solubility decrease with increase in temperature with
all of CO2 start to leave liquid phase at 148oC and partly due to the fact that water vaporizes
making urea solution concentrated.
Figure 11: Effect of Separator temperature on rate of Off gas
22
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
10. Effect of Price of urea synthesis raw materials on Profit: Both ammonia and CO2 high
pricing decreases profitability through enhancing operating cost but that of ammonia price
has higher affect on net return than that of CO2. So efficient use of ammonia helps to keep a
Urea plant profitable.
Profit Vs Price of Urea synthesis raw materials
4.2
4.1
4
Profit(hundred 3.9
thousand
dollars)
3.8
Carbon dioxide
Ammonia
3.7
3.6
3.5
0
0.2
0.4
0.6
0.8
1.2
Price of raw materials ($/kg)
Figure 12: Effect of Price of urea synthesis raw materials on Profit
23
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
11. Effect of Sales price of Urea on Profit : Profit increases linearly with increase in Urea sales
price.
Figure 13: Effect of Sales price of Urea on Profit
24
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
8. RESULTS AND DISCUSSION
Table 02: Comparison between Process Specifications And Simulation findings
Case Variables
Process Specifications
Simulation findings
Reactor Temperature/ C
180
110 (optimum)
Reactor Pressure/kPa
506.6
325 (optimum)
Mole ratio of NH3 to CO2
4.44
4.25 (optimum)
CO2 conversion(%)
80
80
NH3 conversion(%)
36
35.997
NH3 consumption t/t urea
0.566
0.536
The simulation performed for Stamicarbon urea synthesis brings some factors for consideration.
Evidently, urea production increases at high temperature though it slows down between 40 oC and
80oC. From reactor species distribution graph it is found that water content in reactor is
considerably high that shifts equilibrium to left for urea formation reaction stated in section 3.1.
Within this temperature interval ammonia conversion reaches minimum resulting in high
percentage of ammonia left over and furthermore CO2 conversion is also steady in this
temperature interval . The matter can be explained by ammonium carbamate formation and
decomposition though carbamate is not considered in the simulation. Ammonium carbamate
formation is exothermic and reversible and its decomposition to urea is endothermic and also
reversible. High temperature increases the rate of both reactions and particularly favors the
decomposition of ammonium carbamate and so urea production increases. Meanwhile too high
temperature limits carbamate formation from ammonia and carbon dioxide which in turn slows
down urea production . Elevated reactor pressure also enhances urea production as high pressure
shifts equilibrium to right for urea synthesis that results in higher conversion of both CO2 and
ammonia with ammonia converted faster to urea than CO2.
Compared to process specification simulation provides optimum temperature and
pressure at low values. The mole ratio of ammonia to carbon dioxide found in simulation almost
matches that of process specification . CO2 conversion for simulation and process specification is
25
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
same. Ammonia conversion found from simulation varies very little from process specification
but the ammonia consumption per ton of urea is lesser for simulation than process specifications.
On the other hand optimization shows the economic aspects of simulated urea
synthesis process. Though profit is maximized using optimization tool the rate of net return is
found to be 8.5% that shows high operating expenses eats up the remaining revenues. High
operating expenses is contributed to raw material cost and energy expenditure where energy
expenditure predominates raw material cost by factor of 104 . This considerably high energy
expenditure arises from heating , compression and cooling of reaction feed . High coolant duty in
the reactor also adds up energy expenditure. To increase profitability of urea synthesis process
some improvements can be made:
To decrease coolant duty the feed can be charged at optimum temperature and pressure
prescribed by simulation or raising the reactor temperature.
Installing a condenser which will condense the dry gas from rectifying column using
incoming ammonia feed followed by mixing of condensate with ammonia feed and
subsequently pumped and preheated to be charged to the reactor. This will replace the
compression and cooling costs for reaction feed and heating costs will be kept to low as
possible.
Lower the mole ratio of ammonia to carbon dioxide to optimum mole ratio 4.25 to favor
more production of urea that will enhance profitability.
The liquid outlet from stripper can be heated to a lower temperature than 150 oC sothat
energy expenditure for flash separation can be reduced.
26
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
9. CONCLUSION
Finally the simulation and subsequent optimization of Stamicarbon urea synthesis process will
be useful for energy saving studies to improve economics of the plant , study of individual
pieces of equipment with a view of improving their performance and last of all
troubleshooting. The close concordance of simulation results with process specifications
indicate that AspenHysys can be accurately applied to simulate other urea synthesis processes.
The treatise presented can be extended to develop kinetic and thermodynamic models for urea
synthesis along with on-line control system of the plant.
27
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
10. REFERENCES
1. B. Claudel, E. Brousse, G. Shehadeh, Novel thermodynamics and kinetics investigation of
ammonium carbamate decomposition into urea and water, Thermochemica Acta 102
(1986) 357371.
2. Cline, J. E. , Manufacture of urea, a literature survey, Tennes-see Valley Authority
Research Section, p. 11; p. 26; p. 35; Wilson Dam, Alabama, Report No. 646, 1951.
3. Frjacques, M., Theoretical Basis of the Industrial Synthesis of Urea, Chim. Ind., 60, 2235 (1948).
4. H.A. Irazoqui, M.A. Isla, C.M. Genoud, Simulation of an urea synthesis reactor. 2.
Reactor model, Ind. Eng. Chem. Res. 32 (1993) 26712680.
5. Hamidipour, Mohsen; Mostoufi, Navid; Sotudeh-Gharebagh, Rahmat; Modeling the
synthesis section of an industrial urea plant, Chemical Engineering Journal 106 (2005)
249260
6. Inoue, S.; Kanai, K.; Otsuka, E., Equilibrium of Urea Synthesis. I. Bull. Chem. Soc.
Japan, 45, 13339-1345 (1972).
7. Kawasumi, S., Equilibrium of the CO2-NH3-Urea-H2O System Under High Temperature
and Pressure. II. Liquid-Vapor Equilibrium in the Loading Mole Ratio of 2NH3 to CO2,
Bull. Chem. Soc. Japan, 26, 218-227 (1953).
8. Lemkowitz, S.M.; van Erp J.C.; Rekers, D.M.; van den Berg, P.J., Phase Equilibria in the
Ammonia-Carbon Dioxide Systems at and Above Urea Synthesis Conditions, J. Appl.
Chem.Biotechnol., 30, 85-101 (1980).
9. M. Dente, M. Rovaglio, G. Bozzano, A. Sogaro, Gas-liquid reactor in the synthesis of
urea, Chem. Eng. Sci. 47 (1992) 24752480.
10. M. Dente, S. Pierucci, A. Sogaro, G. Carloni, E. Rigolli, Simulation program for urea
plants, Comput. Chem. Eng. 21 (1988) 389400.
11. M.A. Isla, H.A. Irazoqui, C.M. Genoud, Simulation of an urea synthesis reactor.
1.Thermodynamic framework, Ind. Eng. Chem. Res. 32 (1993) 26622670.
12. M.J. Joncich, B.H. Solka, J.E. Bower, The Thermodynamic properties of ammonium
carbamate. An experiment in heterogeneous equilibrium, J. Chem. Educ. 44 (1967) 598
600.
28
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
13. M.A. Satyro, Y. Li, R.K. Agarwal, O.J. Santollani, Modeling urea processes, A new
thermodynamic model and software integration paradigm, from The Virtual Materials
Group,
Presented
at
the
Chemical
Engineers
Resource
Page,
http://www.virtualmaterials.com, 2003.
14. Otsuka, E. and K. Tanimoto, Conversion rate and reaction conditions in urea synthesis,
Journal of the Chem. Soc. of Japan, Ind. Chem. Section, 63, No. 2, 254-258, 1960.
(Translated)
15. Peters, Max M. and Timmerhaus, Klaus D.: Plant Design And Economics For Chemical
Engineers,4th edition,McGraw-Hill,Inc.,p 341-420(1958)
16. R.K. Agarwal, Y.-K. Li, O.J. Santollani, M.A. Satyro, Modeling of Urea Production
Processes, in: 52nd Canadian Chemical Engineering Conference, Vancouver, Canada,
2002.
29
Simulation and Optimization of Total Recycle Stamicarbon CO2 Stripping Urea Synthesis Process
11. APPENDIX
A. Workbook:Case(Main)-Material Streams
B. Workbook:Case(Main)-Composition
C. Workbook:Case(Main)-Energy Streams
D. Workbook:Case(Main)-Unit Operations
E. Optimizer Spreadsheet
30
You might also like
- Urea 2Document16 pagesUrea 2ginga716No ratings yet
- UREA PROCESSDocument8 pagesUREA PROCESSIshan HaiderNo ratings yet
- Snamprogetti Urea ProcessDocument106 pagesSnamprogetti Urea ProcessHeba Ramadan95% (19)
- Urea ProjectDocument17 pagesUrea ProjectAbdo Shaaban100% (2)
- Description of Various Urea Manufacturing ProcessDocument5 pagesDescription of Various Urea Manufacturing ProcessSameer Pandey100% (1)
- Comparing Manufacturing ProcessesDocument11 pagesComparing Manufacturing ProcessesMohit BayerNo ratings yet
- Urea Manufacturing Plant-StamicarbonDocument4 pagesUrea Manufacturing Plant-StamicarbonRadhika PillayNo ratings yet
- Flow Diagram of Urea ProductionDocument11 pagesFlow Diagram of Urea ProductionClaudine Beloso Castillo100% (1)
- Stamicarbon Urea Process Data PDFDocument1 pageStamicarbon Urea Process Data PDFPermata AdindaNo ratings yet
- Reactor Kinetics of Urea FormationDocument21 pagesReactor Kinetics of Urea Formationtitas5123100% (1)
- Stamicarbon (Holland)Document3 pagesStamicarbon (Holland)Mohd Hakimie100% (2)
- Designing Urea ReactorDocument20 pagesDesigning Urea ReactordcobasbNo ratings yet
- Draft Report For Urea ProductionDocument59 pagesDraft Report For Urea ProductionBryan Jesher Dela CruzNo ratings yet
- P Urea Smcarb 2018 Ok Ok OkDocument143 pagesP Urea Smcarb 2018 Ok Ok Okهشام حدودNo ratings yet
- Urea Process (Technical Report)Document42 pagesUrea Process (Technical Report)Heidi Adel100% (2)
- Safety Guidelines for Urea Plant OperationsDocument11 pagesSafety Guidelines for Urea Plant Operationsvariable26100% (1)
- Urea ReactorDocument11 pagesUrea ReactorKevin GonzalezNo ratings yet
- Urea PDFDocument11 pagesUrea PDFSteve WanNo ratings yet
- Urea Plant Material Balance (ACES Process)Document7 pagesUrea Plant Material Balance (ACES Process)muks19950% (2)
- Full Report UreaDocument103 pagesFull Report Ureanisasoberi100% (1)
- Report - 1 - 12.12.2011Document42 pagesReport - 1 - 12.12.2011rosha_friends4ever_noja100% (1)
- Stamicarbon Urea Process Data PDFDocument1 pageStamicarbon Urea Process Data PDFtreyzzztylerNo ratings yet
- Designing Urea ReactorDocument21 pagesDesigning Urea ReactorAdawiyah Al-jufri100% (4)
- Introduction to Urea: Properties, Production, and ApplicationsDocument70 pagesIntroduction to Urea: Properties, Production, and Applicationsravichem823No ratings yet
- Nirbhay Urea Final PDFDocument99 pagesNirbhay Urea Final PDFHimanshu vikram100% (1)
- Urea Synthesis With Pool CondenserDocument1 pageUrea Synthesis With Pool Condensersite commissing teamNo ratings yet
- Urea ModelingDocument20 pagesUrea ModelingekmagisNo ratings yet
- Urea Manufacturing Plant: CH 4200 - Comprehensive Design ProjectDocument124 pagesUrea Manufacturing Plant: CH 4200 - Comprehensive Design Projectwaqas83% (18)
- Urea Project ReportDocument107 pagesUrea Project Reportmuks19995% (57)
- How Green Is The Stamicarbon Urea Process v2 WDocument18 pagesHow Green Is The Stamicarbon Urea Process v2 Wtiadhafri100% (1)
- Urea Written ReportDocument64 pagesUrea Written Reportnitish100% (3)
- Production of Urea by ACES ProcessDocument157 pagesProduction of Urea by ACES ProcessAriel Sandoval100% (1)
- Urea Project Report 1 PDFDocument9 pagesUrea Project Report 1 PDFVirendra RathvaNo ratings yet
- Recent Developments in Urea Plant DesignDocument17 pagesRecent Developments in Urea Plant DesignSathish Kumar100% (1)
- Aces 4 Complete Urea ProjectDocument92 pagesAces 4 Complete Urea ProjectAyat100% (1)
- Final ThesisDocument33 pagesFinal Thesisusama100% (2)
- Material balance for nitric acid production processDocument4 pagesMaterial balance for nitric acid production processyogeshdama100% (1)
- Urea - Aspen TutrialDocument19 pagesUrea - Aspen TutrialAmanda BittencourtNo ratings yet
- Urea Prills Manufacturing FinalDocument31 pagesUrea Prills Manufacturing FinalGaurav VinayakNo ratings yet
- Snamprogetti Urea Process GuideDocument2 pagesSnamprogetti Urea Process GuideBalas43No ratings yet
- Report On Urea Production and Process AnalysisDocument102 pagesReport On Urea Production and Process AnalysisSameer SaxenaNo ratings yet
- Simulation of A Urea Synthesis Reactor. 1. ThermodynamicDocument10 pagesSimulation of A Urea Synthesis Reactor. 1. ThermodynamicYaraKanawatiNo ratings yet
- Urea PlantDocument26 pagesUrea PlantAbdullah Al-Riyami67% (3)
- Aces Process Material Balance: RE AC TO RDocument4 pagesAces Process Material Balance: RE AC TO Rwaheed ahmadNo ratings yet
- Urea Manufacturing 1Document46 pagesUrea Manufacturing 1Sho aibNo ratings yet
- Co2 StripperDocument5 pagesCo2 StripperOkta Ochan Chandra100% (1)
- 2000 Development of The ACES 21 ProcessDocument17 pages2000 Development of The ACES 21 ProcessTTaddictNo ratings yet
- Optimization of Urea PlantsDocument37 pagesOptimization of Urea PlantsShivakumar UmaraniNo ratings yet
- Engineers Guide: The Major Features of These Processes Are Described BelowDocument2 pagesEngineers Guide: The Major Features of These Processes Are Described BelowzeeshanNo ratings yet
- Ammonia ProductionDocument7 pagesAmmonia ProductionIkhtiander IkhtianderNo ratings yet
- UREA MANUFACTURING PROCESSDocument5 pagesUREA MANUFACTURING PROCESSIVAN SAMCRUZNo ratings yet
- Raw Materials and Reactions for Urea ManufactureDocument2 pagesRaw Materials and Reactions for Urea ManufactureTusharNo ratings yet
- Urea Fertilizer Manufacturing ProcessDocument25 pagesUrea Fertilizer Manufacturing ProcessMuhammad YasirNo ratings yet
- The Urea Manufacturing ProcessDocument5 pagesThe Urea Manufacturing ProcessJhonny Huanca ChampiriNo ratings yet
- Simulation Ammonia Plant On PRO IIDocument58 pagesSimulation Ammonia Plant On PRO IIFabrizio Dugo100% (1)
- AmmoniaDocument59 pagesAmmoniavcoolkrazy0% (3)
- cothu123Document34 pagescothu123nguyenvietphuoc22No ratings yet
- UreaDocument18 pagesUreaDian Anggraini PurbaNo ratings yet
- Industerial Chemistry Lecture 2Document37 pagesIndusterial Chemistry Lecture 2amirmisrNo ratings yet
- Income Tax Acknowledgement Receipt 21-22Document1 pageIncome Tax Acknowledgement Receipt 21-22Mir Hasib Ul LatifNo ratings yet
- Medicines For Common ColdDocument1 pageMedicines For Common ColdMir Hasib Ul LatifNo ratings yet
- Design BasisDocument9 pagesDesign BasisMir Hasib Ul LatifNo ratings yet
- Chemical Plant Design EnumerationDocument21 pagesChemical Plant Design EnumerationMir Hasib Ul LatifNo ratings yet
- Waste Management: Wei Zheng, Khamphe Phoungthong, Fan Lü, Li-Ming Shao, Pin-Jing HeDocument9 pagesWaste Management: Wei Zheng, Khamphe Phoungthong, Fan Lü, Li-Ming Shao, Pin-Jing HeMir Hasib Ul LatifNo ratings yet
- Design StudioDocument1 pageDesign StudioMir Hasib Ul LatifNo ratings yet
- Dames & Moore: Flr00037LDocument186 pagesDames & Moore: Flr00037LMir Hasib Ul LatifNo ratings yet
- Model Development and Simulation ProceduresDocument4 pagesModel Development and Simulation ProceduresMir Hasib Ul LatifNo ratings yet
- PMP (Etp)Document3 pagesPMP (Etp)Mir Hasib Ul LatifNo ratings yet
- Technology Licensor or Technology Vendor Identification: Explanation of Different Types of Engineering CompaniesDocument2 pagesTechnology Licensor or Technology Vendor Identification: Explanation of Different Types of Engineering CompaniesMir Hasib Ul LatifNo ratings yet
- UF Reactor Process Flow Costing and Environmental Factor: Reactor Design For Urea Formaldehyde Resin ProductionDocument2 pagesUF Reactor Process Flow Costing and Environmental Factor: Reactor Design For Urea Formaldehyde Resin ProductionMir Hasib Ul LatifNo ratings yet
- Bernoulli's theorem experiment resultsDocument1 pageBernoulli's theorem experiment resultsMir Hasib Ul LatifNo ratings yet
- ChE 6502 - Audit Class - 01-03 - Food MicrobiologyDocument21 pagesChE 6502 - Audit Class - 01-03 - Food MicrobiologyMir Hasib Ul LatifNo ratings yet
- On Wet Chemical Phosphorus Recovery From Sewage Sludge Ash by Acidic or Alkaline Leaching and An Optimized Combination of BothDocument12 pagesOn Wet Chemical Phosphorus Recovery From Sewage Sludge Ash by Acidic or Alkaline Leaching and An Optimized Combination of BothMir Hasib Ul LatifNo ratings yet
- Pr8843 - Drawing and Equipment Tag NumberingDocument32 pagesPr8843 - Drawing and Equipment Tag Numberinggalih santosoNo ratings yet
- Examination: Gce A'Level Examining Body: Edexcel Course Specification: As Physics Course Instructor: Mir Hasib-Ul-LatifDocument2 pagesExamination: Gce A'Level Examining Body: Edexcel Course Specification: As Physics Course Instructor: Mir Hasib-Ul-LatifMir Hasib Ul LatifNo ratings yet
- Waste Management: Yuliya Kalmykova, K. Karlfeldt FedjeDocument8 pagesWaste Management: Yuliya Kalmykova, K. Karlfeldt FedjeMir Hasib Ul LatifNo ratings yet
- MSC Accident InvestigationDocument21 pagesMSC Accident InvestigationMir Hasib Ul LatifNo ratings yet
- Qualitative Inorganic AnalysisDocument17 pagesQualitative Inorganic AnalysisMir Hasib Ul LatifNo ratings yet
- Unit Operations in Food ProcessingDocument5 pagesUnit Operations in Food ProcessingMac JeffersonNo ratings yet
- ChE 6502 - Assignment 1Document7 pagesChE 6502 - Assignment 1Mir Hasib Ul LatifNo ratings yet
- Case StudyDocument33 pagesCase StudyMir Hasib Ul LatifNo ratings yet
- Quantum Chemistry On Graphical Processing Units. 1.Document10 pagesQuantum Chemistry On Graphical Processing Units. 1.Mir Hasib Ul LatifNo ratings yet
- To Plot The Thermo-Electromotive Force Vs Temperature (Calibration) Curve For Given TemperatureDocument2 pagesTo Plot The Thermo-Electromotive Force Vs Temperature (Calibration) Curve For Given TemperatureMir Hasib Ul LatifNo ratings yet
- SCHAUM Et Al Ny Phosphorus Recovery From Sewage Sludge AshDocument8 pagesSCHAUM Et Al Ny Phosphorus Recovery From Sewage Sludge AshMir Hasib Ul LatifNo ratings yet
- Chemical Engineering DrawingDocument3 pagesChemical Engineering DrawingNasrul ZA ST MT0% (1)
- Joining LetterDocument1 pageJoining LetterMir Hasib Ul LatifNo ratings yet
- Case StudyDocument33 pagesCase StudyMir Hasib Ul LatifNo ratings yet
- Chemical Plant DesignDocument4 pagesChemical Plant DesignMir Hasib Ul LatifNo ratings yet
- MSC Accident InvestigationDocument21 pagesMSC Accident InvestigationMir Hasib Ul LatifNo ratings yet
- GMAW Lesson PlanDocument77 pagesGMAW Lesson PlanKentDemeterioNo ratings yet
- Continuous Energy: Product CatalogDocument40 pagesContinuous Energy: Product CatalogNegash JaferNo ratings yet
- DT90 Digital Room ThermostatDocument8 pagesDT90 Digital Room ThermostatAbdullah YÜKSELNo ratings yet
- Machinery Breakdown InsuranceDocument20 pagesMachinery Breakdown InsuranceRumman Arshad DarNo ratings yet
- Asphalts Catalog Tcm14-55227Document54 pagesAsphalts Catalog Tcm14-55227Savaşer YetişNo ratings yet
- TSX ULT Freezers - NorthAmerica - 0719 v2 PDFDocument9 pagesTSX ULT Freezers - NorthAmerica - 0719 v2 PDFambitiousamit1No ratings yet
- FB77WPCC/T04: M-18 Iridium Industrial Spark PlugDocument11 pagesFB77WPCC/T04: M-18 Iridium Industrial Spark PlugAlexanderNo ratings yet
- E Beam ManualDocument7 pagesE Beam ManualMarco SalvatoriNo ratings yet
- Maruti 800 LPG - ManualDocument120 pagesMaruti 800 LPG - Manuala_grassl1920No ratings yet
- Gulf of Mexico Oil Spill Explosion RuptureDocument203 pagesGulf of Mexico Oil Spill Explosion RuptureVincent J. CataldiNo ratings yet
- DIY Audiophile Bookshelf Speaker PlansDocument11 pagesDIY Audiophile Bookshelf Speaker Plansleonardo osmelNo ratings yet
- VFR by Michael AshamDocument8 pagesVFR by Michael AshamHany Elsawy AbdelrahmanNo ratings yet
- Liquid Crystal ColloidsDocument313 pagesLiquid Crystal ColloidsyyyyyyyNo ratings yet
- 05Document17 pages05Andreas StathatosNo ratings yet
- Energy Conservation and ManagementDocument2 pagesEnergy Conservation and ManagementkannanchammyNo ratings yet
- Dynamic Balancing of Hydronic SystemsDocument65 pagesDynamic Balancing of Hydronic Systems黃偉哲100% (4)
- The Chernobyl Accident Research PaperDocument7 pagesThe Chernobyl Accident Research Paperapi-401778753100% (2)
- Cat c7 DiagramaDocument2 pagesCat c7 DiagramaJose F Rivera Morales89% (27)
- Basic Hydraulic Systems Components GuideDocument67 pagesBasic Hydraulic Systems Components Guidesba98No ratings yet
- Ebara DW VoxDocument2 pagesEbara DW Voxmarckalhi100% (1)
- B-3801 IOM - Rev - 2011-09-02 (1) MidlandDocument23 pagesB-3801 IOM - Rev - 2011-09-02 (1) MidlandLucas MonteNo ratings yet
- Cre S16Document4 pagesCre S16vikas patheNo ratings yet
- Manufacturers in Oil and Gas Industry EgyptDocument107 pagesManufacturers in Oil and Gas Industry Egyptsachin0% (1)
- 4.1 Site Roads Crane Spec 3MW-DFIG-120 130 137-xxHz EMEA EN r08Document44 pages4.1 Site Roads Crane Spec 3MW-DFIG-120 130 137-xxHz EMEA EN r08Georgescu MirceaNo ratings yet
- IPENZ Engineers New Zealand Magazine (July 2008, Issue 72)Document12 pagesIPENZ Engineers New Zealand Magazine (July 2008, Issue 72)Harold TaylorNo ratings yet
- Head Loss Lab ReportDocument15 pagesHead Loss Lab ReportMajak MarialNo ratings yet
- Lecture 7.3 - SolidsDocument33 pagesLecture 7.3 - SolidsAdamNo ratings yet
- Magazine-ETC (SANCHAR-2023)Document38 pagesMagazine-ETC (SANCHAR-2023)Ajit PatraNo ratings yet
- 21 - Heavy HaulDocument131 pages21 - Heavy HaulIrwan JoeNo ratings yet
- Ficha Tecnica Ultramid B3WG5Document2 pagesFicha Tecnica Ultramid B3WG5manaswin404No ratings yet