Professional Documents
Culture Documents
Brownian Motion The Random Motion of
Uploaded by
Sujay BhattacharyaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Brownian Motion The Random Motion of
Uploaded by
Sujay BhattacharyaCopyright:
Available Formats
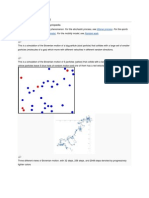
Brownian motion the random motion of particles suspended in a fluid (a liquid or a gas)
resulting from their collision with the quick atoms or molecules in the gas or liquid. The term
"Brownian motion" can also refer to the mathematical model used to describe such random
movements, which is often called a particle theory.[1]
This transport phenomenon is named after the botanist Robert Brown. In 1827, while looking
through a microscope at particles found in pollen grains in water, he noted that the particles
moved through the water but was not able to determine the mechanisms that caused this motion.
Atoms and molecules had long been theorized as the constituents of matter, and many decades
later, Albert Einstein published a paper in 1905 that explained in precise detail how the motion
that Brown had observed was a result of the pollen being moved by individual water molecules.
This explanation of Brownian motion served as definitive confirmation that atoms and molecules
actually exist, and was further verified experimentally by Jean Perrin in 1908. Perrin was
awarded the Nobel Prize in Physics in 1926 "for his work on the discontinuous structure of
matter" (Einstein had received the award five years earlier "for his services to theoretical
physics" with specific citation of different research). The direction of the force of atomic
bombardment is constantly changing, and at different times the particle is hit more on one side
than another, leading to the seemingly random nature of the motion.
You might also like
- Brownian Poster FinalDocument1 pageBrownian Poster Finalbecheanu13No ratings yet
- Brownian MotionDocument31 pagesBrownian Motionsujatha ramkiNo ratings yet
- Brownian MotionDocument25 pagesBrownian Motionsujatha ramkiNo ratings yet
- Karthikeya XII-A Physics Project PDFDocument23 pagesKarthikeya XII-A Physics Project PDFkarthikeya kakarlapudiNo ratings yet
- Brownian Motion: From Wikipedia, The Free EncyclopediaDocument21 pagesBrownian Motion: From Wikipedia, The Free EncyclopediarayroyNo ratings yet
- Atomic TheoryDocument9 pagesAtomic TheoryFriska ApriantiNo ratings yet
- Atomic Theory - Docx MoreDocument15 pagesAtomic Theory - Docx MoreAubrey MillerNo ratings yet
- Eduardo V. Flores - Ether and The Theory of RelativityDocument2 pagesEduardo V. Flores - Ether and The Theory of RelativityOppekeeNo ratings yet
- Fatematun Nur Tofa (20201128)Document1 pageFatematun Nur Tofa (20201128)Ariful Hassan SaikatNo ratings yet
- A Ether DetectionDocument164 pagesA Ether Detectionquicksilver2000No ratings yet
- Summary Of "The Roots And The Fruits: Topics Of Philosophy Of Science" By Eduardo Flichman: UNIVERSITY SUMMARIESFrom EverandSummary Of "The Roots And The Fruits: Topics Of Philosophy Of Science" By Eduardo Flichman: UNIVERSITY SUMMARIESNo ratings yet
- 3rd Quarter Science NotesDocument10 pages3rd Quarter Science Notescasey lNo ratings yet
- BrownianDocument15 pagesBrownianDeepak Prakash JayaNo ratings yet
- Gen. ChemistryDocument1 pageGen. ChemistryMargie PostoriosoNo ratings yet
- Ideas of The Ancient Greeks On AtomDocument2 pagesIdeas of The Ancient Greeks On AtomThalia Diosenne ArabesNo ratings yet
- Aether (Classical Element)Document5 pagesAether (Classical Element)j.miguel593515No ratings yet
- The Meaning of Relativity: Including the Relativistic Theory of the Non-Symmetric Field - Fifth EditionFrom EverandThe Meaning of Relativity: Including the Relativistic Theory of the Non-Symmetric Field - Fifth EditionNo ratings yet
- A 16 Unsubstantiated Relativity Models DR Burniston BrownDocument14 pagesA 16 Unsubstantiated Relativity Models DR Burniston BrownAnonymous i71HvPXNo ratings yet
- The Virtual EtherDocument7 pagesThe Virtual Ethermadbaking100% (1)
- The Science: Orbital Mechanics: Kepler's Laws of Planetary MotionDocument7 pagesThe Science: Orbital Mechanics: Kepler's Laws of Planetary MotionDeeksha TripathiNo ratings yet
- Brownian MotionDocument76 pagesBrownian MotionPraveen KumarNo ratings yet
- 04 - Background On GravityDocument14 pages04 - Background On GravityAdrianNo ratings yet
- Wave Particle DualityDocument7 pagesWave Particle DualityRahmatullahNo ratings yet
- Invisible Teacher PDFDocument15 pagesInvisible Teacher PDFpraiseakande250No ratings yet
- G12 PHY SCI - How We Came To Realize That Earth Is Not The Center of The UniverseDocument26 pagesG12 PHY SCI - How We Came To Realize That Earth Is Not The Center of The UniverseKlienberg Ferenal CancinoNo ratings yet
- Ahmed Bahri Omar 1Document7 pagesAhmed Bahri Omar 1l3gsdNo ratings yet
- Wikipedia Atoms TheoryDocument9 pagesWikipedia Atoms TheoryKim TaehyungNo ratings yet
- Occult Chemistry: Clairvoyant Observations on the Chemical ElementsFrom EverandOccult Chemistry: Clairvoyant Observations on the Chemical ElementsNo ratings yet
- Theories of RelativityDocument292 pagesTheories of RelativityMark A. FosterNo ratings yet
- Ahmed Bahri Omar 1Document7 pagesAhmed Bahri Omar 1l3gsdNo ratings yet
- RelativityDocument2 pagesRelativityDoniahlNo ratings yet
- Brief Overview of TorsionDocument9 pagesBrief Overview of TorsionGustavo Gabriel CiaNo ratings yet
- Uncertainty Einstein, Heisenberg, Bohr, and The Struggle For The Soul of Science by David Lindley Richard A Blasband (Vol 18 No 2)Document10 pagesUncertainty Einstein, Heisenberg, Bohr, and The Struggle For The Soul of Science by David Lindley Richard A Blasband (Vol 18 No 2)Cambiador de MundoNo ratings yet
- GRAVITYDocument8 pagesGRAVITYveronicaNo ratings yet
- Luminiferous AetherDocument111 pagesLuminiferous AetherPraveen KumarNo ratings yet
- Take Home Exam Chemistry Essay RevisedDocument3 pagesTake Home Exam Chemistry Essay RevisedDanita AllenNo ratings yet
- Theories of LightDocument2 pagesTheories of Lightdela2100% (2)
- Elementary Particles : The Building Blocks of the Universe - Physics and the Universe | Children's Physics BooksFrom EverandElementary Particles : The Building Blocks of the Universe - Physics and the Universe | Children's Physics BooksNo ratings yet
- Chemistry SucksDocument4 pagesChemistry SucksNadia KhanNo ratings yet
- From Newton To Einstein: The Origins of General RelativityDocument5 pagesFrom Newton To Einstein: The Origins of General RelativityHammad MisbahNo ratings yet
- Fundamentals of the General Theory of the UniverseFrom EverandFundamentals of the General Theory of the UniverseNo ratings yet
- A Brief History of Relativity - Stephan Hawkings 1999Document6 pagesA Brief History of Relativity - Stephan Hawkings 1999TNT1842No ratings yet
- Water: A Portfolio of Thirty Original Watercolours Exploring the Sensory and Aesthetic Properties of Water Through the Lens of Science and Ancient Philosophy, Expounding the Art of Being CuriousFrom EverandWater: A Portfolio of Thirty Original Watercolours Exploring the Sensory and Aesthetic Properties of Water Through the Lens of Science and Ancient Philosophy, Expounding the Art of Being CuriousNo ratings yet
- Tombe - Einstein Big MistakeDocument10 pagesTombe - Einstein Big Mistakerr100% (1)
- The Einstein Theory of Relativity A Concise StatementFrom EverandThe Einstein Theory of Relativity A Concise StatementRating: 3.5 out of 5 stars3.5/5 (38)
- The Great Einstein Relativity Hoax and Other Science Questions, Hypotheses, and ImprobabilitiesFrom EverandThe Great Einstein Relativity Hoax and Other Science Questions, Hypotheses, and ImprobabilitiesNo ratings yet
- Ether VibrationsDocument67 pagesEther Vibrationsmira003No ratings yet
- The Changing Conceptions of the Universe - From Newton to Einstein -From EverandThe Changing Conceptions of the Universe - From Newton to Einstein -No ratings yet
- Get Cracking With ANSYS Workbench 19.2 - Digital Engineering 24 - 7Document15 pagesGet Cracking With ANSYS Workbench 19.2 - Digital Engineering 24 - 7Sujay BhattacharyaNo ratings yet
- Concave MirrorsDocument5 pagesConcave MirrorsSujay BhattacharyaNo ratings yet
- Analytical Geometry: Do You Know: The Equation of Straight Line IsDocument2 pagesAnalytical Geometry: Do You Know: The Equation of Straight Line IsSujay BhattacharyaNo ratings yet
- Theoretical Models For EdmDocument1 pageTheoretical Models For EdmSujay BhattacharyaNo ratings yet
- Methods of Improving MRRDocument1 pageMethods of Improving MRRSujay BhattacharyaNo ratings yet
- RotaionalDocument19 pagesRotaionalSujay BhattacharyaNo ratings yet
- Hotel Jayasyam Irctc DetailsDocument2 pagesHotel Jayasyam Irctc DetailsSujay BhattacharyaNo ratings yet
- Calculation of Two-Temperature Thermodynamic and Transport Properties For Argon, Oxygen, Nitrogen and Air Plasmas at Atmospheric PressureDocument4 pagesCalculation of Two-Temperature Thermodynamic and Transport Properties For Argon, Oxygen, Nitrogen and Air Plasmas at Atmospheric PressureSujay BhattacharyaNo ratings yet