Professional Documents
Culture Documents
243B NMR Problem Set
Uploaded by
RushabhDaulatCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
243B NMR Problem Set
Uploaded by
RushabhDaulatCopyright:
Available Formats
LABORATORY 1B
lI
t.
Problenrs
Draw the NMR spectrum for each of the following compounds. Draw the peaks in the approximate positions where they would appear in a recorded spectrum. Also indicate the splitdng panern of the peaks.
a.
Acetone
b.
Propane
c-
Tohcne
d.
,-Buyl ethyl ether
e.
Ethyl acetate
974
I
NUCLEAR MAGNETTC RESONANCE SPECTROSCOPY: A SECOND VtStT
f.
Benzaldehyde
876543210
Common molecular moieties have easily distinguishable patterns in NMR
spectra. Match the
following molecular moieties with the generalized spectra shown below.
a.
xrcH-cHY,
b.
xrcH-cH2Y
c.
xcH2-cHrY
d.
xcH2crl3
LABORATORY 18
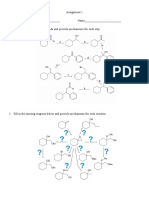
The following pairs of isomers can be easily distinguished by NMR.Identify the appropriate
spectrum for each isomer and explain all the peaks.
a.
r-Butyl ethyl ether and isopropyl propyl ether
b.
Methyl butanoate and isopropyl acetate
NUCLEAR MAGNETTC RESONANCE SPECTROSCOPy: A SECOND VtStT
These five spectra were recorded from different isomers with our molecular formula
CsHeClO.
They all contain a carbonyl functionaliry. Determine the structure of the different isomers.
LABORATORY 18
,
,
I,
I
I
I,
5.
The following spectra are from the isomers of CrHrOr. Identify the different compounds based
on the NMR spectra.
I
T
I
I,
I
I,
,
I,
,
,
,
D
t
I
,
t
I
t
,
,
D
D
101
You might also like
- MCAT Review SmithDocument47 pagesMCAT Review SmithMonu KadianNo ratings yet
- Carotenoids – 4: Main Lectures Presented at the Fourth International Symposium on Carotenoids, Berne, Switzerland, 25-29 August 1975From EverandCarotenoids – 4: Main Lectures Presented at the Fourth International Symposium on Carotenoids, Berne, Switzerland, 25-29 August 1975B. C. L. WeedonNo ratings yet
- PhenylpropanolamineDocument27 pagesPhenylpropanolaminepeterjohnsonuk100% (1)
- 4.11 Structure DeterminationDocument46 pages4.11 Structure DeterminationJaslinder Kaur Dhillon100% (1)
- Reductions in Organic ChemistryDocument13 pagesReductions in Organic ChemistryNikola PudjaNo ratings yet
- AromaticsDocument5 pagesAromaticskinepela853No ratings yet
- NMR Spectroscopy - Short NoteDocument6 pagesNMR Spectroscopy - Short Notecoolhemakumar100% (4)
- 2d NMRDocument32 pages2d NMRDelicz TanNo ratings yet
- Nuclear Techniques in Analytical Chemistry: International Series of Monographs on Analytical ChemistryFrom EverandNuclear Techniques in Analytical Chemistry: International Series of Monographs on Analytical ChemistryNo ratings yet
- Tutorial 3 (Mass Spectrometry & Infra-Red Spectroscopy)Document6 pagesTutorial 3 (Mass Spectrometry & Infra-Red Spectroscopy)Ahmed ZakyNo ratings yet
- Assignment@SEM I@NMRDocument3 pagesAssignment@SEM I@NMRSoumyadeep BarmanNo ratings yet
- III Semester SPECTROS PDFDocument11 pagesIII Semester SPECTROS PDFArangaNo ratings yet
- McMurry OC8e EV CH13 PDFDocument28 pagesMcMurry OC8e EV CH13 PDFCrizel Ricaro100% (1)
- 2425 Chapt 13Document10 pages2425 Chapt 13Ivan Alberto NinaNo ratings yet
- Guía de Estudio EspectrosDocument5 pagesGuía de Estudio EspectrosCésar CidNo ratings yet
- A-Level Chemistry NMR SummaryDocument23 pagesA-Level Chemistry NMR SummaryZubayr MoroNo ratings yet
- Chem273 Take Home LabDocument12 pagesChem273 Take Home LabMentari Permata HatiNo ratings yet
- Qualitative NMR (#1) : Matt Prater Group 4 Analysis LabDocument1 pageQualitative NMR (#1) : Matt Prater Group 4 Analysis LabMatt PraterNo ratings yet
- Questions For Assignment 1 CHM557Document8 pagesQuestions For Assignment 1 CHM557SITI NUR AFIQAH MAHAZANNo ratings yet
- Experiment 8Document8 pagesExperiment 8NathanianNo ratings yet
- Optional SpectrosDocument4 pagesOptional SpectrosPhil LiuNo ratings yet
- C 13 NMRDocument75 pagesC 13 NMRalwysyahNo ratings yet
- Assignment 1Document3 pagesAssignment 1鄒大安No ratings yet
- Assignment 1 CHM557Document15 pagesAssignment 1 CHM557Jibb BossNo ratings yet
- Topic 9 Exercise 1 - Analytical TechniquesDocument2 pagesTopic 9 Exercise 1 - Analytical TechniquesChryssa EconomouNo ratings yet
- Chapter 15 - NMR SpectrosDocument13 pagesChapter 15 - NMR SpectrosHepi NuriyawanNo ratings yet
- Chemistry 318: Ir, MS, Uv, NMR SpectrosDocument17 pagesChemistry 318: Ir, MS, Uv, NMR Spectrosaamer_shahbaaz0% (4)
- CH301 Tutorial IRDocument2 pagesCH301 Tutorial IRArjun MaharajNo ratings yet
- Chap 013Document29 pagesChap 013msoup21100% (1)
- RMN ProblemsDocument7 pagesRMN ProblemsAnonymous llSDP0tNo ratings yet
- ICH 501-May 2022Document3 pagesICH 501-May 2022Jagadeesh YNo ratings yet
- Chemistry Unit 4 Goodie BagDocument26 pagesChemistry Unit 4 Goodie BagJacob SalkinNo ratings yet
- Assignment 502Document1 pageAssignment 502Milan VadodariaNo ratings yet
- Optional Area Examination Analytical ChemistryDocument4 pagesOptional Area Examination Analytical ChemistryMohamed DahmaneNo ratings yet
- Spectroscopy Problems-MaleczkaDocument33 pagesSpectroscopy Problems-MaleczkaBruna SofiaNo ratings yet
- 1455877785CHE P12 M35 EtextDocument8 pages1455877785CHE P12 M35 EtextSaurav PaulNo ratings yet
- Chapter 9Document5 pagesChapter 9Stephen BrownNo ratings yet
- Biophysics: Nuclear Magnetic ResonanceDocument28 pagesBiophysics: Nuclear Magnetic Resonancejosito2003No ratings yet
- Exam 1 Spring 2016 PDFDocument14 pagesExam 1 Spring 2016 PDFMac BobNo ratings yet
- Chapter 24-Amines and Heterocycles: Short Answer Exhibit 24-1Document28 pagesChapter 24-Amines and Heterocycles: Short Answer Exhibit 24-1DONNA JEAN ACOJEDONo ratings yet
- No Card IcinDocument12 pagesNo Card IcinHazrati UmmiNo ratings yet
- Schrader 1999Document2 pagesSchrader 1999Pablo E-6034No ratings yet
- NMR SpectrosDocument29 pagesNMR Spectroshareesh13h100% (1)
- Hafiz M. Asad NAwaz's AssignmentDocument8 pagesHafiz M. Asad NAwaz's AssignmentArslan ElahiNo ratings yet
- Paper 12: Organic Spectroscopy: Subject ChemistryDocument12 pagesPaper 12: Organic Spectroscopy: Subject ChemistryFaiza AnsariNo ratings yet
- Cy4202 21-22 EndDocument3 pagesCy4202 21-22 EndAakash BanerjeeNo ratings yet
- Chem 112A Homework 1Document4 pagesChem 112A Homework 1Shyam BhaktaNo ratings yet
- Pharmaceutical Analysis: Mass SpectrometryDocument32 pagesPharmaceutical Analysis: Mass Spectrometryesie345No ratings yet
- MSC II SEM III Natural Product and Spectroscopy MOCK TESTDocument3 pagesMSC II SEM III Natural Product and Spectroscopy MOCK TESTEvgenia MakantasiNo ratings yet
- 2D NMR SpectrosDocument21 pages2D NMR SpectrosraisameongNo ratings yet
- NMR Spectroscopy LabDocument7 pagesNMR Spectroscopy LabBriana Halbert100% (2)
- 6spectroscopy and ChromatographyDocument15 pages6spectroscopy and ChromatographyThinaya JayarathneNo ratings yet
- ASSIGNMENT CHM557-mergedDocument3 pagesASSIGNMENT CHM557-mergedAhmad ZakwanNo ratings yet
- Lab Supplement: Fundamentals of Organic SpectrosDocument7 pagesLab Supplement: Fundamentals of Organic SpectrosChris MitrevskiNo ratings yet
- Looking at Infrared Spectra:: ExampleDocument4 pagesLooking at Infrared Spectra:: ExampleRamshaNo ratings yet
- Structural Analysis of Organic Compounds by Combined Application of Spectroscopic MethodsFrom EverandStructural Analysis of Organic Compounds by Combined Application of Spectroscopic MethodsNo ratings yet
- Progress in Analytical Atomic SpectroscopyFrom EverandProgress in Analytical Atomic SpectroscopyC L ChakrabartiNo ratings yet
- Ultraviolet Photoelectron and Photoion Spectroscopy, Auger Electron Spectroscopy, Plasma Excitation in Spectrochemical AnalysisFrom EverandUltraviolet Photoelectron and Photoion Spectroscopy, Auger Electron Spectroscopy, Plasma Excitation in Spectrochemical AnalysisNo ratings yet