Professional Documents
Culture Documents
First Law of Thermodynamics
Uploaded by
affeena100%(1)100% found this document useful (1 vote)
162 views3 pages2nd Law Of Thermodynamics states that the entropy of the universe increases in a spontaneous process and remains unchanged in an equilibrium process. Energy cannot be created nor destroyed, only change the form and type through work and heat.
Original Description:
Original Title
FIRST LAW OF THERMODYNAMICS.doc
Copyright
© © All Rights Reserved
Available Formats
DOC, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Document2nd Law Of Thermodynamics states that the entropy of the universe increases in a spontaneous process and remains unchanged in an equilibrium process. Energy cannot be created nor destroyed, only change the form and type through work and heat.
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
100%(1)100% found this document useful (1 vote)
162 views3 pagesFirst Law of Thermodynamics
Uploaded by
affeena2nd Law Of Thermodynamics states that the entropy of the universe increases in a spontaneous process and remains unchanged in an equilibrium process. Energy cannot be created nor destroyed, only change the form and type through work and heat.
Copyright:
© All Rights Reserved
Available Formats
Download as DOC, PDF, TXT or read online from Scribd
You are on page 1of 3
FIRST LAW OF THERMODYNAMICS
State that energy cannot be created nor destroyed, only change the
form and type through work and heat
Equation
Where
q= heat transfer into/ out of the system
w= work done by/ on the system
Sign Convention For Heat
qsys < 0 = heat transfer from system
qsys > 0 = heat transfer from surrounding
surrounding
system
Sign Convention For Work
wsys < 0 = work done from system
surrounding
wsys > 0 = work done from surrounding
Example of Application
Isochoric
Volume constant (
Isobaric
Pressure constant,
Adiabatic
system
No heat transfer,
Diabatic/ Diathermic
Isothermal
Temperature constant, (
2nd Law Of Thermodynamics
State that the entropy of the universe increases in a spontaneous
process and remains unchanged in an equilibrium process
Equation
For Spontaneous Process
Entropy
Entropy ( S )
is a measurement of disorder or
randomness of a system
The greater the dispersal,the greater the
entropy
Entropy changes =
Unit =J/k @ J/K.mol (molar entropy)
You might also like
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Determine Your Skin UndertoneDocument15 pagesDetermine Your Skin UndertoneaffeenaNo ratings yet
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- DISCOVER YOUR UNDERTONEDocument17 pagesDISCOVER YOUR UNDERTONEaffeenaNo ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- Prepared by Nur Affeena Qa DepartmentDocument14 pagesPrepared by Nur Affeena Qa DepartmentaffeenaNo ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Prepared by Nur Affeena Qa DepartmentDocument15 pagesPrepared by Nur Affeena Qa DepartmentaffeenaNo ratings yet
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- DISCOVER YOUR UNDERTONEDocument17 pagesDISCOVER YOUR UNDERTONEaffeenaNo ratings yet
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Misconcept: Fair-Skinned Girls Can't Have Warm Undertones or Dark-Skinned Women of Color Can't Have Cool TonesDocument11 pagesMisconcept: Fair-Skinned Girls Can't Have Warm Undertones or Dark-Skinned Women of Color Can't Have Cool TonesaffeenaNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- How to Identify Your Skin Undertone and Choose Flattering Jewelry ColorsDocument8 pagesHow to Identify Your Skin Undertone and Choose Flattering Jewelry ColorsaffeenaNo ratings yet
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Prepared by Nur Affeena Qa DepartmentDocument15 pagesPrepared by Nur Affeena Qa DepartmentaffeenaNo ratings yet
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Determine Your Skin Undertone and Best ColorsDocument17 pagesDetermine Your Skin Undertone and Best ColorsaffeenaNo ratings yet
- Discover: Prepared by Nur Affeena Qa DepartmentDocument12 pagesDiscover: Prepared by Nur Affeena Qa DepartmentaffeenaNo ratings yet
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- How to Find Your Skin Undertone and the Best Colors for YouDocument9 pagesHow to Find Your Skin Undertone and the Best Colors for YouaffeenaNo ratings yet
- Noise Hazard Identification Checklist PDFDocument2 pagesNoise Hazard Identification Checklist PDFaffeenaNo ratings yet
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- DISCOVER YOUR UNDERTONEDocument17 pagesDISCOVER YOUR UNDERTONEaffeenaNo ratings yet
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- How To Identify Your Undertone: Vein Test Jewelry Test Plain Paper TestDocument10 pagesHow To Identify Your Undertone: Vein Test Jewelry Test Plain Paper TestaffeenaNo ratings yet
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- Discove R Your Underto Ne Color: Prepared by Nur Affeena Qa DepartmentDocument13 pagesDiscove R Your Underto Ne Color: Prepared by Nur Affeena Qa DepartmentaffeenaNo ratings yet
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Discove R Your Underto Ne Color: Prepared by Nur Affeena Qa DepartmentDocument14 pagesDiscove R Your Underto Ne Color: Prepared by Nur Affeena Qa Departmentaffeena100% (1)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- JKLDocument13 pagesJKLaffeenaNo ratings yet
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- Discover Your Undertone ColorDocument16 pagesDiscover Your Undertone ColoraffeenaNo ratings yet
- DEFDocument15 pagesDEFaffeenaNo ratings yet
- GHIDocument14 pagesGHIaffeenaNo ratings yet
- PQRDocument12 pagesPQRaffeenaNo ratings yet
- STUDocument10 pagesSTUaffeenaNo ratings yet
- MNODocument12 pagesMNOaffeenaNo ratings yet
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Abc 1Document16 pagesAbc 1affeenaNo ratings yet
- MMMDocument7 pagesMMMaffeenaNo ratings yet
- Undertone ColorDocument17 pagesUndertone ColoraffeenaNo ratings yet
- TKKKKDocument10 pagesTKKKKaffeenaNo ratings yet
- SSSSDocument9 pagesSSSSaffeenaNo ratings yet
- DSDDDocument9 pagesDSDDaffeenaNo ratings yet
- DSDDDocument9 pagesDSDDaffeenaNo ratings yet
- Chapter 03 Volumetric Properties of Pure Fluids 4 Slides Per PageDocument8 pagesChapter 03 Volumetric Properties of Pure Fluids 4 Slides Per PageHana Atalia100% (1)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Model Question: Third Semester M.Sc. (Physics)Document1 pageModel Question: Third Semester M.Sc. (Physics)Sagar RawalNo ratings yet
- The Emergence of Quantum Physics: - Some "Problems" With Classical PhysicsDocument19 pagesThe Emergence of Quantum Physics: - Some "Problems" With Classical PhysicsAnita Puspita Handayani0% (1)
- Heat Transfer Intro-Chapter 1Document41 pagesHeat Transfer Intro-Chapter 1yaqoobNo ratings yet
- Chapter 2 Matrices MATV101 UpdatedDocument35 pagesChapter 2 Matrices MATV101 Updated1jabujohnshibambo12No ratings yet
- ANSYS CFX-Solver Theory GuideDocument372 pagesANSYS CFX-Solver Theory GuideBhaskar NandiNo ratings yet
- CIS Multivariate Calculus CSDocument6 pagesCIS Multivariate Calculus CSwaleedmateen81No ratings yet
- EPQ Final Version v3Document23 pagesEPQ Final Version v3Aryan GadhaveNo ratings yet
- De Broglie Matter Waves and Quantum Mechanics ConceptsDocument31 pagesDe Broglie Matter Waves and Quantum Mechanics ConceptsSai Printers83% (18)
- Lecture Powerpoints: Physics: Principles With Applications, 6 EditionDocument11 pagesLecture Powerpoints: Physics: Principles With Applications, 6 EditionKarren Ferrer-Mora HandayanNo ratings yet
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Design and Experimental Analysis of PipeDocument7 pagesDesign and Experimental Analysis of PipeMustafa VatanseverNo ratings yet
- Perturbation Theory: Supplementary Subject: Quantum ChemistryDocument42 pagesPerturbation Theory: Supplementary Subject: Quantum ChemistryrittenuovNo ratings yet
- Differential Geometry and Its Applications: A. PatákDocument4 pagesDifferential Geometry and Its Applications: A. PatákmiftahchoyNo ratings yet
- Cp502 Advanced Fluid Mechanics: University of Peradeniya Faculty of EngineeringDocument4 pagesCp502 Advanced Fluid Mechanics: University of Peradeniya Faculty of EngineeringPrabhasha JayasundaraNo ratings yet
- Application of Derivatives for JEE Main: Top 500 Question Bank on MathonGoDocument54 pagesApplication of Derivatives for JEE Main: Top 500 Question Bank on MathonGoThirunagari RohithNo ratings yet
- Ideal Gas Model: Review, Ideal Gas Model, Ideal Gas Equation of State, Thermodynamic Properties of Ideal GasesDocument34 pagesIdeal Gas Model: Review, Ideal Gas Model, Ideal Gas Equation of State, Thermodynamic Properties of Ideal GasesJake SyNo ratings yet
- Kuliah 12 Aliran KompresibelDocument67 pagesKuliah 12 Aliran KompresibelherawanadifNo ratings yet
- Transport PhenomenaDocument3 pagesTransport PhenomenawetcoNo ratings yet
- Lesson 1 - First Law of Thermodynamics and CalorimetryDocument4 pagesLesson 1 - First Law of Thermodynamics and CalorimetryJeff ValdezNo ratings yet
- Einstein's Theory of Relativity ExplainedDocument2 pagesEinstein's Theory of Relativity Explainedbrad bNo ratings yet
- Ilya PrigogineDocument5 pagesIlya PrigogineValentin MateiNo ratings yet
- AOD (IITian Notes - Kota)Document166 pagesAOD (IITian Notes - Kota)Varun Choudary KommalapatiNo ratings yet
- Energy Conservation Explained in 40 CharactersDocument17 pagesEnergy Conservation Explained in 40 Charactersozgurturunc4No ratings yet
- Einstein's Special Theory of Relativity and Its Futuristic AspectsDocument2 pagesEinstein's Special Theory of Relativity and Its Futuristic AspectsInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Supplee's ParadoxDocument3 pagesSupplee's Paradoxperception888No ratings yet
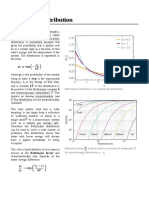
- Boltzmann DistributionDocument7 pagesBoltzmann Distributionhobson616No ratings yet
- Expt. 9 Natural ConvectionDocument5 pagesExpt. 9 Natural ConvectionPradeep DiwakarNo ratings yet
- Thermal EquilibriumDocument9 pagesThermal EquilibriumHarsh WaliaNo ratings yet
- Statistical MechanicsDocument208 pagesStatistical MechanicsAyush Kumar BhojrajNo ratings yet
- Lectures on 2D Gravity and 2D String TheoryDocument200 pagesLectures on 2D Gravity and 2D String Theorymanosmagicas7340No ratings yet
- A Brief History of Time: From the Big Bang to Black HolesFrom EverandA Brief History of Time: From the Big Bang to Black HolesRating: 4 out of 5 stars4/5 (2193)
- The Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifeFrom EverandThe Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifeNo ratings yet
- Summary and Interpretation of Reality TransurfingFrom EverandSummary and Interpretation of Reality TransurfingRating: 5 out of 5 stars5/5 (5)
- The Physics of God: How the Deepest Theories of Science Explain Religion and How the Deepest Truths of Religion Explain ScienceFrom EverandThe Physics of God: How the Deepest Theories of Science Explain Religion and How the Deepest Truths of Religion Explain ScienceRating: 4.5 out of 5 stars4.5/5 (23)
- When the Earth Had Two Moons: Cannibal Planets, Icy Giants, Dirty Comets, Dreadful Orbits, and the Origins of the Night SkyFrom EverandWhen the Earth Had Two Moons: Cannibal Planets, Icy Giants, Dirty Comets, Dreadful Orbits, and the Origins of the Night SkyRating: 3 out of 5 stars3/5 (7)
- Quantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessFrom EverandQuantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessRating: 4 out of 5 stars4/5 (6)
- The Tao of Physics: An Exploration of the Parallels between Modern Physics and Eastern MysticismFrom EverandThe Tao of Physics: An Exploration of the Parallels between Modern Physics and Eastern MysticismRating: 4 out of 5 stars4/5 (500)