Professional Documents
Culture Documents
PCS 181 Notes Class 2
Uploaded by
Marc MCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
PCS 181 Notes Class 2
Uploaded by
Marc MCopyright:
Available Formats
PCS181 Notes
The EM Spectrum:
Light:
o Mutually perpendicular oscillating electric and magnetic
fields
o Moves in wavelike pattern at a speed of c = 3 x 108 m/s
o c = speed of light
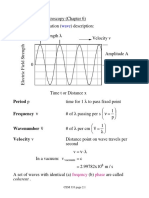
Wavelength (): Distance between peaks of a wave
Tachyons: Theoretical faster-than light particles
Electromagnetic Properties:
Wavelength -> colour
1 nanometer = 1nm = 1 m = 1 x 10-9m = 10-9m
1 x 109
Red = 700nm = 700 x 10-9m = 7 x 10-7m
Violet = 400nm = 400 x 10-9m = 4 x 10-7m
Yellow = 550nm
Visible Colours: ROYGBIV
Photons: bundles of energy -> light as a particle
The shorter the wavelength, the more dangerous they are (ie:
Ultraviolet, x-rays, gamma rays)
Frequency = f
Number of wave peaks passing a given point each second
Number of waves/second
Complete cycles/second
Cycles/s
Hertz (Hz)
f = c/ (speed of light/wavelength)
fred = 3 x 108 m/s
700 x 10-9m
**(m will cancel each other so put in meters rather than
nanometers)**
= 4.3 x 1014 cycles/s
= 4.3 x 1014 Hz
Planck Curve:
-273oC = 0 Kelvins
nothing colder than 0 Kelvins
WIEN Equation
o T = 0.0029 m*K
0.0029 m*K
max
550x10-9m
T = 5272.7 ~ 5300 K
Note: the surface temperature is ~ 5800 K
PCS181 Notes
Energy of Light:
E = hf
o h = Plancks constant
Ered = (6.625 x 10-34 j*s)(4.3 x 1014 cycles/s)
Ered = 2.85 x 10-19J
Note: shorter wavelength = higher frequencies and energies.
Hot low density element produces bright coloured lines on a dark
background = emission spectrum
When light having a continuous spectrum passes through a
cooler gas, the gas absorbs specific wavelengths = dark lines on
rainbow background = absorption spectrum
Hydrogen Emission Spectrum-> two green, blue, and red
You might also like
- Radiation 1Document18 pagesRadiation 1Amer JumahNo ratings yet
- Chapter 40 ProblemsDocument11 pagesChapter 40 ProblemsVeera Manoharan RNo ratings yet
- Chapter 1 SlidesDocument6 pagesChapter 1 SlidesJohn O'BrienNo ratings yet
- Photons and Matter WavesDocument34 pagesPhotons and Matter Wavesjaymart villartaNo ratings yet
- Atomic StructureDocument49 pagesAtomic StructureFatimaNo ratings yet
- Chem 242 DR - Salwa M. Al-Rashed: Associate Professor of Physical ChemistryDocument34 pagesChem 242 DR - Salwa M. Al-Rashed: Associate Professor of Physical ChemistryMark Carmelo A AzorNo ratings yet
- CHEM 101 - L4 - Atomic Structure and Periodicity of Elements - Part BDocument41 pagesCHEM 101 - L4 - Atomic Structure and Periodicity of Elements - Part BlentlebuisanyangNo ratings yet
- Atoms and LightDocument4 pagesAtoms and LightIdil WarsameNo ratings yet
- 3.3 BohrÆs Atomic ModelDocument35 pages3.3 BohrÆs Atomic ModelAnisha Syazwana Binti RoslyNo ratings yet
- The For Laws of Radiation 1Document15 pagesThe For Laws of Radiation 1أ. علي محمدNo ratings yet
- Advanced Lecture1 - Theoretical Introduction (28-02-2021)Document20 pagesAdvanced Lecture1 - Theoretical Introduction (28-02-2021)Ziad HassanNo ratings yet
- Quantum mechanics concepts and calculationsDocument4 pagesQuantum mechanics concepts and calculationsAnonymous 7CxwuBUJz3No ratings yet
- Structure of AtomDocument90 pagesStructure of Atomnazaatul aaklimaNo ratings yet
- X RaysDocument118 pagesX RaysBadri Narayan MishraNo ratings yet
- Identification of Organic and Inorganic Compounds Using Spectroscopy TechniquesDocument93 pagesIdentification of Organic and Inorganic Compounds Using Spectroscopy TechniqueskarinaNo ratings yet
- Chemistry NotesDocument64 pagesChemistry NotesCJ NgoNo ratings yet
- Laser PropertiesDocument13 pagesLaser PropertieszxcvuiopNo ratings yet
- Physics NotesDocument14 pagesPhysics NotesDeniz TopNo ratings yet
- Electromagnetic Spectrum - Lecture 1Document14 pagesElectromagnetic Spectrum - Lecture 1Mvita HenocNo ratings yet
- Lecture 7: Radiation and Matter: Phys 275 Astrophysics I - Planets and Stars Lecture 7 January 25th 2011Document29 pagesLecture 7: Radiation and Matter: Phys 275 Astrophysics I - Planets and Stars Lecture 7 January 25th 2011Nick CoishNo ratings yet
- ThesisDocument54 pagesThesisJeon ManobanNo ratings yet
- Chapter 7 Atomic Structure and PeriodicityDocument77 pagesChapter 7 Atomic Structure and Periodicityabd jafNo ratings yet
- Higher Physics - Unit 3: 3.1 - WavesDocument41 pagesHigher Physics - Unit 3: 3.1 - Wavesr1meshb1lla1969No ratings yet
- Practice Problem Set 1 Electromagnetic RadiationDocument11 pagesPractice Problem Set 1 Electromagnetic RadiationEnaam MuhammadNo ratings yet
- Physics 208, Spring 08, Quiz Week 13 Apr. 14, 2008Document2 pagesPhysics 208, Spring 08, Quiz Week 13 Apr. 14, 2008Noone F. moonNo ratings yet
- Electromagnetic Waves Learning GuideDocument4 pagesElectromagnetic Waves Learning GuideKate Megan ApuliNo ratings yet
- New Microsoft Office Word DocumentDocument39 pagesNew Microsoft Office Word DocumentUadNo ratings yet
- Electromagnetic Wave09Document23 pagesElectromagnetic Wave09reemulyaNo ratings yet
- NCERT Solutions Physics Chapter 11 Dual Nature of Radiation and MatterDocument15 pagesNCERT Solutions Physics Chapter 11 Dual Nature of Radiation and MatterVidyakulNo ratings yet
- Electrical Engineering - Remote SensingDocument43 pagesElectrical Engineering - Remote SensingAnaStairsNo ratings yet
- Plancks Constant Worksheet 2 PDFDocument2 pagesPlancks Constant Worksheet 2 PDFSailokesh NagaruruNo ratings yet
- Chapter 20-22 Physics Problems All With Answer ADocument3 pagesChapter 20-22 Physics Problems All With Answer Adhruv1117No ratings yet
- Light as an electromagnetic radiationDocument16 pagesLight as an electromagnetic radiationAries Blado PascuaNo ratings yet
- Annex 3 - Delivery Format - Task 3 Miguel PamplonaDocument17 pagesAnnex 3 - Delivery Format - Task 3 Miguel Pamplonamiguelitux martinezNo ratings yet
- The Nature of Light Atomic Spectra The Wave - Particle Duality of Matter and Energy The Quantum - Mechanical Model of The AtomDocument31 pagesThe Nature of Light Atomic Spectra The Wave - Particle Duality of Matter and Energy The Quantum - Mechanical Model of The AtomwitriNo ratings yet
- Topic 10.quantumDocument34 pagesTopic 10.quantumNOR AZAM BIN ENDOT / FSNo ratings yet
- Atomic StructureDocument16 pagesAtomic StructureLuna eukharisNo ratings yet
- EdexcelASPhysicsRG 9781846905957 Pg70-78 WebDocument9 pagesEdexcelASPhysicsRG 9781846905957 Pg70-78 WebSabrizalNo ratings yet
- Week 02Document10 pagesWeek 02Arfin FardiansyahNo ratings yet
- Tutorial 25, NumericalDocument7 pagesTutorial 25, NumericalDYES Motion GraphicsNo ratings yet
- Electromagnetic WavesDocument5 pagesElectromagnetic WavesVishwanath BrungiNo ratings yet
- Introduction To Aerial Photographic Systems: Ies / Cee / For / Gle 301Document30 pagesIntroduction To Aerial Photographic Systems: Ies / Cee / For / Gle 301JJNo ratings yet
- Week 02Document10 pagesWeek 02Marina Rosa AnggraeniNo ratings yet
- Demo: Field From Accelerating Charge (Phy Java App) : em Waves Lecture Notes Electromagnetic Waves Units: Electric FieldDocument4 pagesDemo: Field From Accelerating Charge (Phy Java App) : em Waves Lecture Notes Electromagnetic Waves Units: Electric FieldVivek SaiNo ratings yet
- A Brief History of Light StudyDocument30 pagesA Brief History of Light StudyNiko DidicNo ratings yet
- Lecture12 EDMDocument13 pagesLecture12 EDMOmar Adnan AhmadNo ratings yet
- Physics 2075Document5 pagesPhysics 2075sujeet.jha.311No ratings yet
- Environmental ChemistryDocument1 pageEnvironmental ChemistryFajar NugrahaNo ratings yet
- Physical Quantum MechanicsDocument33 pagesPhysical Quantum MechanicsJerome ColicoNo ratings yet
- SP01 - Atomic Theory To Bohr - PresentationDocument29 pagesSP01 - Atomic Theory To Bohr - PresentationMarianNo ratings yet
- Find the Maximum Frequency, Minimum Wavelength, Momentum, and Speed of Electrons Emitted from MetalsDocument50 pagesFind the Maximum Frequency, Minimum Wavelength, Momentum, and Speed of Electrons Emitted from MetalsChetan ChhalaniNo ratings yet
- Chapter 1, 2 and 3Document79 pagesChapter 1, 2 and 3ashenafiNo ratings yet
- Solution:: 7.2.5 Photoelectric EffectDocument7 pagesSolution:: 7.2.5 Photoelectric EffectIka Fitria WatiNo ratings yet
- Using Wien's Law For Measuring The Temperature of An AsteroidDocument4 pagesUsing Wien's Law For Measuring The Temperature of An AsteroidAneesh GangalNo ratings yet
- Temu 1Document36 pagesTemu 1Farida UtamiNo ratings yet
- Electromagnetic RadiationDocument22 pagesElectromagnetic RadiationSelva Kumar0% (1)
- Feynman Lectures Simplified 2C: Electromagnetism: in Relativity & in Dense MatterFrom EverandFeynman Lectures Simplified 2C: Electromagnetism: in Relativity & in Dense MatterNo ratings yet
- Electronic Devices and Circuits: The Commonwealth and International Library: Electrical Engineering Division, Volume 1From EverandElectronic Devices and Circuits: The Commonwealth and International Library: Electrical Engineering Division, Volume 1Rating: 4.5 out of 5 stars4.5/5 (5)
- Interactions between Electromagnetic Fields and Matter: Vieweg Tracts in Pure and Applied PhysicsFrom EverandInteractions between Electromagnetic Fields and Matter: Vieweg Tracts in Pure and Applied PhysicsNo ratings yet
- Advances in Structure Research by Diffraction Methods: Fortschritte der Strukturforschung mit BeugungsmethodenFrom EverandAdvances in Structure Research by Diffraction Methods: Fortschritte der Strukturforschung mit BeugungsmethodenR. BrillNo ratings yet
- FIN300 Notes - Class 1Document1 pageFIN300 Notes - Class 1Marc MNo ratings yet
- CMN279 Notes - Class 4Document1 pageCMN279 Notes - Class 4Marc MNo ratings yet
- Finance Cheat SheetDocument2 pagesFinance Cheat SheetMarc MNo ratings yet
- FIN300 Notes: Financial Planning Process and ComponentsDocument1 pageFIN300 Notes: Financial Planning Process and ComponentsMarc MNo ratings yet
- COGS: Income Statement (Expense) Inventory SystemsDocument1 pageCOGS: Income Statement (Expense) Inventory SystemsMarc MNo ratings yet
- CMN279 Notes - Class 3Document2 pagesCMN279 Notes - Class 3Marc MNo ratings yet
- ECN Midterm ReviewDocument3 pagesECN Midterm ReviewMarc MNo ratings yet
- Learning Objectives:: Larger Slice of Sales ResultDocument2 pagesLearning Objectives:: Larger Slice of Sales ResultMarc MNo ratings yet
- FIN300 Notes Class 2Document1 pageFIN300 Notes Class 2Marc MNo ratings yet
- Introduction to Torts Chapter ObjectivesDocument4 pagesIntroduction to Torts Chapter ObjectivesMarc MNo ratings yet
- ECN 204 Notes Class 2Document3 pagesECN 204 Notes Class 2Marc MNo ratings yet
- ACC 100 Notes Sep 23Document1 pageACC 100 Notes Sep 23Marc MNo ratings yet
- CMN279 Notes - Class 2Document3 pagesCMN279 Notes - Class 2Marc MNo ratings yet
- CH 5 Law 122Document2 pagesCH 5 Law 122Marc MNo ratings yet
- Intentional Torts and Defences ExplainedDocument3 pagesIntentional Torts and Defences ExplainedMarc MNo ratings yet
- CMN279 Notes - Class 1Document2 pagesCMN279 Notes - Class 1Marc MNo ratings yet
- PCS181 Notes Class 5Document1 pagePCS181 Notes Class 5Marc MNo ratings yet
- PCS181 Class 3Document2 pagesPCS181 Class 3Marc MNo ratings yet
- PCS181 Notes Class 4Document3 pagesPCS181 Notes Class 4Marc MNo ratings yet
- PCS181 Notes Class 6Document3 pagesPCS181 Notes Class 6Marc MNo ratings yet
- PCS 181 Notes Class 1Document1 pagePCS 181 Notes Class 1Marc MNo ratings yet
- PCS181 Notes Class 7Document2 pagesPCS181 Notes Class 7Marc MNo ratings yet