Professional Documents
Culture Documents
Section 3 HSC Chemistry Notes
Uploaded by
focuc98Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Section 3 HSC Chemistry Notes
Uploaded by
focuc98Copyright:
Available Formats
PRELIMINARY CHEMISTRY THE CHEMICAL EARTH
Elements in Earth materials are present mostly as compounds because of interactions at the atomic
level
Identify that matter is made of particles that are continuously moving and interacting
Matter
o Occurs in three states depending on the energy of the particles solids, liquids and
gases
Property
Arrangement of
particles
Solid
Close together and
vibrating in fixed
positions (orderly
arrangement)
Liquid
Close together and
moving more freely
Gas
Far apart and moving
freely
Shape
Volume (space
occupied)
Ability to be
compressed (be pushed
into a smaller volume)
Ability to diffuse
(spread through
another substance)
Kinetic energy of
particles
Definite shape
Definite volume
Shape of container
Definite volume
Depends on container
Fills all available shape
Cannot be
compressed
Cannot be
compressed
Can be compressed
Cannot diffuse
Can diffuse
Can diffuse
Least
More than solids, less
than gases
Greatest kinetic
energy
Describe qualitatively the energy levels of electrons in atoms
Energy levels of electrons
o Electrons in an atom exist in discrete energy levels or shells
o The further away from the nucleus the shell is, the higher the energy of the e- in it
Electronic configuration
o Refers to the particular order in which electrons are arranged on the shells of an atom
o Maximum number of electrons in each shell is given by: 22
Energy level
1st shell
2nd shell
3rd shell
4th shell
-
Maximum number of electrons
2
8
18 or 8e32e-or 8e-
Valence electrons
o Electrons in the outer shell are called valence electrons
o Elements can have between 1-8 electrons on the outer shell, if the outer shell has 8
electrons the atom is stable
o All atoms want to have 8 electrons on their outer shell [to do this, atoms may give
away or take electrons from other atoms i.e. undergo a chemical reaction]
o Only the valence electrons can be moved to other atoms
PRELIMINARY CHEMISTRY THE CHEMICAL EARTH
Elements in Earth materials are present mostly as compounds because of interactions at the atomic
level
Describe atoms in terms of mass number and atomic number
o
o
o
. () = no. of protons + no. of neutrons
. () = no. of protons
In a neutral atom, the no. of protons = no. of electrons
Describe the formation of ions in terms of atoms gaining or losing electrons
Achieving Noble Gas Configurations
o Atoms can achieve noble gas electron configurations [i.e. full outer shell] in two ways
1. Ionic bonding outright transfer of
electrons from one atom to another
2. Covalent bonding sharing electrons
between pairs of atoms
You might also like
- Chemstudy Finals IBDocument7 pagesChemstudy Finals IBRiley Jensen100% (1)
- The Chemical Earth-Ahmad ShahDocument46 pagesThe Chemical Earth-Ahmad ShahYouseffNo ratings yet
- EASA Module 2 - NotesDocument24 pagesEASA Module 2 - NotesSteven J. SelcukNo ratings yet
- Sci2Q Reviewer PDFDocument5 pagesSci2Q Reviewer PDFFire RobloxNo ratings yet
- Basic Terminology in ChemistryDocument4 pagesBasic Terminology in ChemistryHaider JalalNo ratings yet
- CHEMDocument31 pagesCHEMSheena Ann L. LLarenasNo ratings yet
- Atoms, Elements and Compounds: Part TwoDocument45 pagesAtoms, Elements and Compounds: Part TwoBerylNo ratings yet
- Inorganic Chemistry: Metallurgical Engineering Lecture SeriesDocument39 pagesInorganic Chemistry: Metallurgical Engineering Lecture SeriesAlvin Garcia PalancaNo ratings yet
- Chem NotesDocument51 pagesChem NotesHannah RossNo ratings yet
- The Periodic Table, Electron Shells, and OrbitalsDocument13 pagesThe Periodic Table, Electron Shells, and OrbitalsCandyAnonymousNo ratings yet
- 2.1 Matter: 1 Chapter 2: The Structure of The AtomDocument6 pages2.1 Matter: 1 Chapter 2: The Structure of The AtomThaatchayani MuralleNo ratings yet
- CHEMISTRY NOTES Class 9 CBSEDocument13 pagesCHEMISTRY NOTES Class 9 CBSERamRakh YadavNo ratings yet
- Elements 2Document12 pagesElements 2Rahul KhatriNo ratings yet
- Chemistry For PhysiciansDocument14 pagesChemistry For PhysiciansenzlibraryNo ratings yet
- ChemistryDocument6 pagesChemistryArt FlameNo ratings yet
- Organic Chemistry Week 2Document18 pagesOrganic Chemistry Week 2Nur AmaninaNo ratings yet
- EASA Module 2 Questions and ExplanationsDocument6 pagesEASA Module 2 Questions and ExplanationsSteven J. SelcukNo ratings yet
- CH 2-The Chemistry of LifeDocument65 pagesCH 2-The Chemistry of LifeB-27 Rodero, Howard VincentNo ratings yet
- General Chemistry 2Document8 pagesGeneral Chemistry 2Almira MejoradaNo ratings yet
- Mod 2 Book 1 PhysicsDocument41 pagesMod 2 Book 1 Physicsranjit prasadNo ratings yet
- Chapter 4-Student Reading: Parts of The AtomDocument12 pagesChapter 4-Student Reading: Parts of The AtomShimmy LimmyNo ratings yet
- Chemistry: Atomic Number / Proton NumberDocument15 pagesChemistry: Atomic Number / Proton NumberZeynep AkıNo ratings yet
- Protons, Neutrons & Electrons: Relative Atomic MassDocument19 pagesProtons, Neutrons & Electrons: Relative Atomic MassAiza ImranNo ratings yet
- Chemistry Mod 1 + 2 SummaryDocument25 pagesChemistry Mod 1 + 2 SummaryLilyNo ratings yet
- ChemistryDocument17 pagesChemistryPhương Mai Nguyễn LêNo ratings yet
- Angels-Ricarro-General Chemistry 1Document7 pagesAngels-Ricarro-General Chemistry 1Maria Andrea Eunice RicarroNo ratings yet
- Structure of AtomDocument57 pagesStructure of Atomnurulakmal mohd kamalNo ratings yet
- Chapter 7Document11 pagesChapter 7Hend HamedNo ratings yet
- Reach Staars Science Review Booklet - ComboDocument14 pagesReach Staars Science Review Booklet - Comboapi-249360364No ratings yet
- Particle Nature of MatterDocument11 pagesParticle Nature of Matteramora eliNo ratings yet
- ChemistryDocument65 pagesChemistryDan Sebastian TilaoNo ratings yet
- Chemistry Aspect of General Science: Learning ObjectivesDocument18 pagesChemistry Aspect of General Science: Learning ObjectivessannaNo ratings yet
- Course: BIO 101: Introduction To Biology Matter and ElementsDocument9 pagesCourse: BIO 101: Introduction To Biology Matter and ElementsAhamadul Islam OnonnoNo ratings yet
- Electron ArrangementDocument5 pagesElectron ArrangementAries SeguiNo ratings yet
- Material Chapter OneDocument13 pagesMaterial Chapter OneTeshale AlemieNo ratings yet
- Reading Material 2 Basic ChemistryDocument15 pagesReading Material 2 Basic ChemistryCj IsoNo ratings yet
- ChemDocument58 pagesChemashwin.sp.004No ratings yet
- Chapter 2: The Chemical Context of LifeDocument35 pagesChapter 2: The Chemical Context of LifeJustin RobenyNo ratings yet
- Matter Is Everything Around You.: Proton ProtonDocument2 pagesMatter Is Everything Around You.: Proton ProtonMercy Clapano-Artazo MirandaNo ratings yet
- Grade 9-Chem. Atomic Structure and Periodic TableDocument10 pagesGrade 9-Chem. Atomic Structure and Periodic TableMusfira zaibNo ratings yet
- The Chemical Context of LifeDocument13 pagesThe Chemical Context of LifeAngel Gaddi LarenaNo ratings yet
- Presentation C.elementsDocument17 pagesPresentation C.elementsEsterkaKraljikNo ratings yet
- Tutorial 1.1Document6 pagesTutorial 1.1FirmansyahNo ratings yet
- Topic 1 - Atomic StructureDocument4 pagesTopic 1 - Atomic Structurejulian maltoNo ratings yet
- Fundamental of Gas Measument 1Document6 pagesFundamental of Gas Measument 1resureNo ratings yet
- Chapter 2: The Chemistry of Biology: 2.1 Atoms: Fundamental Building Blocks of All Matter in The UniverseDocument50 pagesChapter 2: The Chemistry of Biology: 2.1 Atoms: Fundamental Building Blocks of All Matter in The UniverseShemmy Delotina DadulaNo ratings yet
- ChemistryDocument27 pagesChemistryErica LeNo ratings yet
- Chemistry NotesDocument70 pagesChemistry NotesAngelina SandifordNo ratings yet
- Unit 1 General Chemistry PDFDocument29 pagesUnit 1 General Chemistry PDFchuchu maneNo ratings yet
- GRADE 8 2021 2022 G8 Module 1 Q3 2023 2024Document3 pagesGRADE 8 2021 2022 G8 Module 1 Q3 2023 2024chartreusevermilionNo ratings yet
- Atomic Structure and Chemical Bonding: Model of An AtomDocument9 pagesAtomic Structure and Chemical Bonding: Model of An AtomSoumya Ranjan SahooNo ratings yet
- Atomic StructureDocument5 pagesAtomic StructureMuhammadAbutalibKazmiNo ratings yet
- AP Bio Big Study GuideDocument33 pagesAP Bio Big Study GuideHayden CaseyNo ratings yet
- Fundamental of Organic ChemistryDocument11 pagesFundamental of Organic ChemistryBernie Suarez100% (1)
- Anh Văn Chuyên Ngành Hóa Học 1Document26 pagesAnh Văn Chuyên Ngành Hóa Học 1Nguyen TuanNo ratings yet
- The Periodic Table Atoms, Elements and IsotopesDocument10 pagesThe Periodic Table Atoms, Elements and IsotopesRubén De Gracia SantoNo ratings yet
- Science NotesDocument36 pagesScience NotesYippiyippi Lala100% (1)
- A-Level Chemistry Revision: Cheeky Revision ShortcutsFrom EverandA-Level Chemistry Revision: Cheeky Revision ShortcutsRating: 4 out of 5 stars4/5 (5)
- GCSE Chemistry Revision: Cheeky Revision ShortcutsFrom EverandGCSE Chemistry Revision: Cheeky Revision ShortcutsRating: 4.5 out of 5 stars4.5/5 (3)
- 1.1 Define The Role of Marketing in OrganisationsDocument2 pages1.1 Define The Role of Marketing in Organisationsfocuc98No ratings yet
- Aluminium: Atomic Number Symbol NameDocument1 pageAluminium: Atomic Number Symbol Namefocuc98No ratings yet
- MenuDocument7 pagesMenufocuc98No ratings yet
- Determining Main Idea Chart PDFDocument1 pageDetermining Main Idea Chart PDFfocuc98No ratings yet
- Quadratic Inequalities: Preliminary Mathematics Extension 1 (3unit)Document1 pageQuadratic Inequalities: Preliminary Mathematics Extension 1 (3unit)focuc98No ratings yet
- Average My Result Beep Test 4.6 4Document22 pagesAverage My Result Beep Test 4.6 4focuc98No ratings yet
- Variable Characteristic Observations: Ypes OF Data Concept AND JargonsDocument2 pagesVariable Characteristic Observations: Ypes OF Data Concept AND Jargonsfocuc98No ratings yet
- Question: Calculate The Chances of A Couple Who Are Heterozygous For Albinism Having An AlbinoDocument1 pageQuestion: Calculate The Chances of A Couple Who Are Heterozygous For Albinism Having An Albinofocuc98No ratings yet
- Scrib MssDocument1 pageScrib Mssfocuc98No ratings yet
- Ument For Free Download Access.: Select Files From Your Computer or Choose Other Ways To Upload BelowDocument1 pageUment For Free Download Access.: Select Files From Your Computer or Choose Other Ways To Upload Belowfocuc98No ratings yet
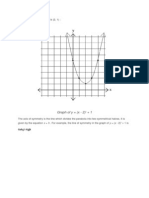
- Graph of y (X - 2) + 1: Gukg I RTGJHDocument1 pageGraph of y (X - 2) + 1: Gukg I RTGJHfocuc98No ratings yet
- My Name Is Shiela Ki JawaniDocument1 pageMy Name Is Shiela Ki Jawanifocuc98No ratings yet
- Non-Linear FunctionsDocument1 pageNon-Linear Functionsfocuc98No ratings yet
- Maths in Focus - Margaret Grove - ch10Document52 pagesMaths in Focus - Margaret Grove - ch10Sam Scheding100% (2)
- Adaptation of An Echidna and SkunkDocument9 pagesAdaptation of An Echidna and Skunkfocuc98No ratings yet
- Grade 3 Math FractionsDocument11 pagesGrade 3 Math Fractionsfocuc98No ratings yet
- Math FractionsDocument11 pagesMath Fractionsfocuc98No ratings yet
- Structural Adaptations of Plants: Plant Adaptations - Honors BiologyDocument1 pageStructural Adaptations of Plants: Plant Adaptations - Honors Biologyfocuc98No ratings yet
- ENGINE COOLANT - CHANGE - Fiat - GRANDE PUNTO - Elearn - 4CarDataDocument1 pageENGINE COOLANT - CHANGE - Fiat - GRANDE PUNTO - Elearn - 4CarDataDevs AbdouNo ratings yet
- Newtons Rings Formal ReportDocument8 pagesNewtons Rings Formal ReportSammy BennettNo ratings yet
- 2.head SluiceDocument18 pages2.head SluiceDesktop NepalNo ratings yet
- Power Train: - 170 - STMGXXX 4/97Document66 pagesPower Train: - 170 - STMGXXX 4/97Evan AT YoelNo ratings yet
- Calcium CarbonateDocument116 pagesCalcium CarbonatemichaelNo ratings yet
- Outcome1 T1 PDFDocument12 pagesOutcome1 T1 PDFM RoyNo ratings yet
- Fixed Bed ReactorDocument43 pagesFixed Bed ReactorMaher Al-busaidi100% (2)
- Photo-Electric Effect and ComptonDocument7 pagesPhoto-Electric Effect and ComptonMohammad Irfan YousufNo ratings yet
- Nozzle TestDocument9 pagesNozzle TestDarshan PanchalNo ratings yet
- Soundblox: Key Features and BenefitsDocument2 pagesSoundblox: Key Features and BenefitsBogdan ŞerbănescuNo ratings yet
- Phase Behavior of Discotic Liquid Crystalline Polymers and Related Model CompoundsDocument5 pagesPhase Behavior of Discotic Liquid Crystalline Polymers and Related Model CompoundsLeoNo ratings yet
- 1 Frequently Asked Questions Diffusion-Weighted Imaging-00012754Document4 pages1 Frequently Asked Questions Diffusion-Weighted Imaging-00012754Maria MiguensNo ratings yet
- Mastery Test MT1 and MT2 Solution: None of TheseDocument6 pagesMastery Test MT1 and MT2 Solution: None of TheseU-line Anne Roque VillafloresNo ratings yet
- High Performance Concrete (HPC)Document3 pagesHigh Performance Concrete (HPC)ghazal nusairNo ratings yet
- Lesson Notes About SoundsDocument6 pagesLesson Notes About Soundsmark joseph cometaNo ratings yet
- Cambridge ANSWERSDocument29 pagesCambridge ANSWERSdevansh sharmaNo ratings yet
- Sheet 7 Thermal Analysis PDFDocument2 pagesSheet 7 Thermal Analysis PDFAbdel Azim MohamedNo ratings yet
- X-Ray DiffractionDocument26 pagesX-Ray DiffractionZain Ali KidwaiNo ratings yet
- Bent RuleDocument24 pagesBent Rulesuka11blyatNo ratings yet
- Optimizing Materials Cost and MechanicalDocument8 pagesOptimizing Materials Cost and MechanicalKaleem UllahNo ratings yet
- BH-TN-1: Total Stress Hydrostatic PressureDocument5 pagesBH-TN-1: Total Stress Hydrostatic PressureJarugit PoonchaiNo ratings yet
- Cutting Tool Materials and Cutting FluidsDocument21 pagesCutting Tool Materials and Cutting FluidsRam27092003 GermanNo ratings yet
- TPTG620 - Teaching Practice (Long Term) : Assignment Lesson Plan (Fall 2020) Total Marks: 20 - +instructionsDocument6 pagesTPTG620 - Teaching Practice (Long Term) : Assignment Lesson Plan (Fall 2020) Total Marks: 20 - +instructionsnisar aliNo ratings yet
- Effects of Temperature On The Hydration Characteristics of Free LimeDocument5 pagesEffects of Temperature On The Hydration Characteristics of Free LimeRendotian AnugrahNo ratings yet
- APCoasterProjectBookV2 PDFDocument60 pagesAPCoasterProjectBookV2 PDFguyNo ratings yet
- Behavior of SS-316 in Engine Oil Simulated EnvironmentDocument5 pagesBehavior of SS-316 in Engine Oil Simulated EnvironmentAnush RajNo ratings yet
- Ceramic Nanoparticle SynthesisDocument40 pagesCeramic Nanoparticle SynthesisXavier Jones100% (1)
- Conexion Viga - PilarDocument5 pagesConexion Viga - PilarAlejandro OspinaNo ratings yet
- Considerations For The Weldability of Types 304L and 316L Stainless SteelDocument8 pagesConsiderations For The Weldability of Types 304L and 316L Stainless Steelluisgonzalezf95No ratings yet
- Tie Back WallDocument13 pagesTie Back WallMinhLêNo ratings yet