Professional Documents
Culture Documents
Solution Manual-Chemical Engineering Thermodynamics - Smith Van Ness
Uploaded by
Surya Budi WidagdoCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Solution Manual-Chemical Engineering Thermodynamics - Smith Van Ness
Uploaded by
Surya Budi WidagdoCopyright:
Available Formats
c ~_~_c
Instructor's Soltutions
JlLlluJu,,'lion to
CHElV1lCAL ENGINEERING THERrvl0DYN AMICS
Sixth Edition
p
J. ~'L Snlith • H. C. Voln Nt'5S • 1\1.1\<1. Abbott
___ J
--."'-.;
Chapter 1 - Section A - Mathcad Solutions
1.4 The equation that relates deg F to deg Cis: t(F) = 1.8 t(C) + 32. Solve this equation by setting t(F) ;;;; t(C).
Guess solution: t := 0
Given
t = 1.8t + 32
1.5 By definition:
F P= - A
F = mass·g Note: Pressures are in gauge pressure.
F:= P·A
m g = 9.807- 2 s
A := ~. 02 A = 12.566mm2 4
mass i= F :·w~r¥'·:~'~~.,*:~~, . .- ADs. g
P:;;:::3000bar
1.6 By definition:
F P= - A
F = massg
F:= P·A
ft g = 32.174- 2 sec
A := 2.D2 A = O.023in2 4
mass := F;~~-"E:~Ppq~~lgP1 ADs. g
P ,:= ,3000atm
D:;;::: O.17in
1.7 Pabs = p-g-h + Patm
, ""gm
P':;;:::,13.535·-', ,-, , ,3 ·em
m g:= 9.832·- 2 s
h := 56.38cm
Patm:= 101.78kPa
Pabs := p-g-h + Patm
1.8
, ',gtn
P := 13.535·-,'-, 3 em
'ft g:= 32243·-- 2 s
h:= 25.62in
Patnl:~;29.86in_ Hg
Pabs := p-g-h + Patm
1
1.10
.•... gm Assume the following: p" .:= 135-.-' . '3 -em
. ··.m
g':= 9.8- . '. 2
s
P :;;;;; 400bar
p h:=p.g
Ans.
1.11 The force on a spring is described by: F = K x where K is the spring constant. First calculate K based on the earth measurement then gl\1ars based on spring measurement on Mars.
On Earth:
F = mass-g = K-x
mass :=O.40kg
.' .... m
g r= 9.81'---' . '., .... 2
s
x:= 1.08cll1
F := mass-g On Mars:
F = 3.924N
K'= F x
K = 363.333 N m
x r= OAOem FMars gMars:= -mass
FMars = 1.453N
Ans.
1.12 Given:
d
-p:::;: -p.g
dz
and:
M·P p=R·T
Substituting:
d M·P
-p = -_.g
dz R·T
Separating variables and integrating:
fPDenver 1 Jzoenver (M.g)
-dP= - - dz
P R·T
Psea 0
After integrating:
(PDenver) _ -M·g
In - --'ZDenver
Psea R·T
Taking the exponential of both sides and rearranging:
(-M-g )
_ ~'ZDenver
PDenver - psea·e
Psea:= latm
M:=29gm mol
2
1.15
- .3
.' .. cm-arm
R:= 82;06 .
. . mol- K
'r:={IO + 273.15)K
M.g
--'ZDenver = 0.194 R-T
(-M.g )
R- T - ZOenver
PDenver := psea·e
ZI>enver.:= ··1, mi
1.13 The same proportionality applies as in Pb. 1.11.
....... ... .. ..ft
·gearth :=32.186,~ . 2
·s
.... . .. :ft
gmoon .;= 5.32~2 s .
gearth ~learth := ~lmoon'-gmoon
Ll1earth = 113.498
Wmoon := M'gmoon
1.14
5.00dollars hr
costbulb := ·10-
1000hr day
O.ldollars hr
costelec := ·IO-·70W
kW·hr day
dollars costbulb = 18.262-yr
Altnooh :=18.76
Ans.
ADS.
dollars costeJec = 25.567-yr
:illlij~I~~~Ans.
costtotal := costbulb + costelec
D := 125ft
mass :=. 2501bm
Patm :::::30.12in_Bg
n 2 A :=-·D
4
3
. __ ._-----
. ft
g .::;= 32.169 ~ 2 s
A = 1.227ft2
(a) F:= Patm·A + mass·g
ADS.
F (b) Pabs:=A
ADS.
(c) L\.l:= L 7ft
Work := F·6.1
ADs.
ADs.
1.16
D:~ 0.47m
mass:= 150kg
m
g :=·9.&13;."..; '2 s
Patm.:= 101.57kPa
11: 2 A :=-·D 4
~~·:;~.~:'i:~p~~~:~t~Q!:;~
2 A = 0.173m
(a) F:= Patm·A + mass-g
ADS.
F (b) Pabs:=A
ADS.
(c) 6.1 :=O.&3rn
Work : .... F·L\.I
.~gt~·~·~t::t~'~~:~~~l~ ADS. '~g~:'E!'tt.:~%1·~ ADS.
1.18
mass:» 1250kg
ill u :=40-
s
1 2
EK := +rnass-u
2
Work:= EK
g~,[~~':il."R;"!~~E:W ADS. W~rk·~·i:·~.,io~J(J ADS.
1.19 Wdot = mass·g·bh .0.91.0.92
time
Wdot:=200W
m
g :"" 9.&'_;" .' 2
s
L\.h:=5Om
Wdot mdot := ----g·b,h·0.91·0.92
ADS.
4
Chapter 2 - Section A - Mathcad Solutions
2.1 (a)
Mwt:= 35·kg
m g:= 9.8·- 2 s
Az:= 5'm
(b)
AUtotal := Work
Ans.
(c) By Eqs. (2.14) and (2.21):
dU + d(PV) = Cp·dT
Since P is constant, this can be written:
MH20·Cp·dT = MH20·dU + MH20·P·dV
Take Cp and V constant and i~tegrate: MH20·CP·(t2 - t1) = AUtotal kJ
t1 :=20·degC Cp := 4.18· .- MH20 :=25·kg
kg·degC
AUtotal t2 := t1 + ~--MH20'Cp
(d) For the restoration process, the change in internal energy is equal but of opposite sign to that of the initial process. Thus
Q := -AUtotal
(e) In all cases the total internal energy change of the universe is zero.
2.2 Similar to Pb. 2.1 with mass of water == 30 kg.
Answers are:
(a) W = 3.43 kJ
(b) Internal energy change of the water = 2.86 kJ
(c) Final temp. = 20.027 deg C
(d) Q = -2.86 kJ
5
2.4 The electric power supplied to the motor must equal the work done by the motor plus the heat generated by the motor.
i .= 9.7amp
E:= HOV
Wdotmech:= L25hp
Wdotelect .= j·E
3 Wdotelect = 1.067 x 10 W
Qdot := W dote1ect ~ W dotmech
2.5
t
Eq. (2.3): L1U = Q + W
Step 1 to 2: AUt12. := ..,..2001
Q12 := L\Ut12 ~ W 12
W12 ::=-6000J
'~!~a.·~"~~~{*j:t~~~J\
Ans.
L1Ut34 := Q34 + W34
W34:= 3001
'~~!~~J;j;~~~~~g.J,~,j
Ans.
Step 3 to 4: Q34:;;:;: -8001
Step 1 to 2 to 3 to 4 to 1: Since L\Ut is a state function, L\Ut for a series of steps that leads back to the initial state must be zero. Therefore, the sum of t
the AU values for all of the steps must sum to zero.
Step 2 to 3:
3 L1Ut23 = -4 x 10 J
Q23:= -38001
il'1~?::~·§t~igI~;·.
Ans.
For a series of steps, the total work done is the sum of the work done for each step.
6
Ans.
Step 4 to 1: LlUt41:= 47QOJ
3 W41 = 4.5x 10 J
Q41 := L\Ut41 - W41
Note: Q12341 = -W12341
Ans.
2.11 The enthalpy change of the water = work done.
M:= 20;kg
, '" ,'. kJ
Cp.i= 4.18·_;__.;..-
, 'kg·degC
. . ' . . -
At::::: .1 O·degC
Wdot :=0.25·kW
M·Cp·Llt L\'t:= ---
Wdot
2.12
Q :=7.5·kJ
AU :=-J2·kJ
W:== AU """'-' Q
':W:;~tts:i~i$kJ Ans,
'~~~':fgt2@ ADs.
AU := -12·kJ
Q := liU
2.l3 Subscripts: c, casting; w, water; t, tank. Then
IDe·liUe + mw·t\..Uw + mt·t\..Ut = 0 Let C represent specific heat,
C = Cp = Cv
Then by Eq. (2.18)
me:= 2·kg
mw:= 40·kg
kJ Cc := 0.50· "', ,', ,,'
" kg-degC
kJ
Ci := 0.5: ",' ", ... ,,',
,kg·degC
',,', 'kJ
Cw:=4.18· """"
" ' '"kg·degC
tc := 500· degC
tl := 2s·degC
(guess)
Ans.
7
2.15 mass:» J-kg
(a) AT :=lK
m
(b) g:= 9.8-
2
s
AEp
Az:=
mass-g
(c) AEK:= AUt 2.17
Ai:::::. SOm
D:=2m
mdot := pu-A
. kJ
Cy:= 4.18-~
. kg·K
fo,Ut := mass.Cv·AT~y~::\~c'4fJ$~ ADS.
AEp:= AUt
~~]>~:~~'§t§J)l~ ADS.
u:~ j ~~::"
Ans.
.kg p :=1000- ..
m3
m u:=5~ S
IT 2 A :=-D 4
2 A = 3.142m
4 kg mdot = 1.571 x 10 -
S
2.18 (a)
.... . kJ U 1 :=762.0· kg
(b)
.' kJ
U2 := 2784.4·-.' .'
, .. k
. g
PI :"" lQ02.7~kPa
.' '. ->. 3
: .r- em' VI := 1,128·-: ··gm
ADS.
P2:= 1500·kPa
. 3
, ·em
V2:= 169.7·-'
. gm
AH := H2 - HI
ADS.
ADS.
8
- .. ~''''--
2.22
D1 :=2.5cm
·····.m uj :=2.....: s
. Di· ~= 5cm
(a) For an incompressible fluid, p=constant. Bya mass balance, mdot = constant = UtAtP = uZA2p.
mdotj-Hj - (mdotl·Hl + mdot2-H2) = Qdot mdotj - mdot 1 - mdot; = 0
mdotl·(H3 - HI) + mdot2·(H3 - H2) = Qdot mdot.Cp.(T3 - r.) + mdot2·Cp.(T3 - T2) = Qdot T3·Cp.(mdotl +mdot2) = Qdot+ mdotj-Cp-Tj +rndotj-Cp-Tj
(b)
2.23 Energy balance:
Mass balance:
Therefore:
or
kg rridotj t= 1.0- '.
. s
kJ Qdott> ....:.30_:_
s
Ans.
II := 25degC
.. . kg mdot2 := 0.8-.
. . s
T:2.:=·75degC
kJ Cp:= 4.18-·.-kg·K
2.25 By Eq. (2.32a):
By continuity, incompressibility
Mf = Cp·AT
kJ Cp:= 4.18· ...........••. .. .. kg·degC
9
SI units:
Dl.::=.2,S·cm
D2 :=3.8·cm
Ans.
D2 :=7.5cm
L1T := ~.[ 1 _ (01)4J
2·Cp D2
Ans.
Maximum T change occurrs for infinite D2:
L1T := ~.[ 1 _ (DI)4]
2·Cp D2
Ans,
2.26 T 1:= 300K Tz:= 520K
... m
uI:= lO~ s
m li2 := 3.5-
. s
. k
molwt:= 29~ krnol
Wsdoti= 98.8kW
kinoI ndot .= 50 -.-
. .. hr
7 Cp :=-·R 2
L1H := Cp.(Tz - TI) By Eq. (2.30):
Qdot :~ [ ill I + [ u~ 2 - un molwt J ndot - W sdotC,llt~t,;cfj:9 ;<)</4k WAn s.
3 kJ Llli = 6.402 x 10 --
kmol
2.27 By Eq. (2.32b):
2 L1U L1H =-- 2·ge
also
VI T1 P2
By continunity, constant area
V2 Uz = li1'VI
2 2 2
L1U = li2 - ul
10
P}:=lQO·psi
II :=,579_67;ral1kine
fl·lbf
R = 3.407 -"",--. -'---~ mol-rankine
T2 := 578·rankine
" ... gm
molwt.r= 28- .. - r: . . mol
(guess)
7 Ul2 ~(T2 PIJ2 ]
-.R.(T2 - r.) = -_. -._ -] -molwt
2 2 T] P2
T2 .= Find(T2) ii!~··~1$Y~1'filirtRih~
,'-.':. - .,-;:,',.--.>-.-.", ;"~:: ."_ .
Given
'. m 2.28 Ul:::::: 3---s
By Eq. (2.32a):
. ill 2.29 lil := 30·--s
m ti2 := 500·s
Ans.
(119.1S·degF)
.' '. m U2::::;:; 200;-s
-, .... . ..•...... 'kJ Hj ;=334.9·-.· ..
. kg
.... , .kJ
H1:== 2726.5--. ' .•. kg
2 2 u2 - ul
Q:= H2-HI +--- 2
Ans.
.'. . .... kJ
H .. '1 := 311205'-
. kg
.: .: .. kJ
H2 .= 2945.7· _.' • kg
(guess)
By Eq. (2.32a): Given
Continuity:
3 em V 1 :,;" 388.61·-'. - gm
3
. .. em
V2 :=667.75-- •.. _'. gm
Ans.
11
2.30 (a) tt :,,;,'30.degC
12 := 250· degC
, J "
Cv:= 20.8· ,',
, mol-degC
By Eq. (2.19): Q := n.Cy.(t2 - tI)
n:~ 3·mol
Take into account the heat capacity of the vessel; then
my:= 100-kg
, kJ c,,:= O.s.~,.;._' - kg·degC
(b) tl :=200~degC
tz .= 40· degC
'Cp:=29.1. Joule
, mol-degf:
By Eq. (2.23):
2.31 (a) t1:= 70·degF
t2 := 350·degF
BTU CV:=5·.','" """ ' mol-degf
By Eq. (2.19):
Take account of the heat capacity of the vessel:
my := 200· Ibm
BTU Cy := 0.12·-~___;,
, Ibm·degF
ri i= 4·mol
Ans.
(b) tl:= 400·degF
" BTU
Cp:= 7·" ",
, .mol-degf
Q := n.Cp.(t2 - t1)
By Eq. (2.23):
(9~;~;.:~ZR9~~11~
12
n:::::o 4·mol
Ans.
Ans.
Ans.
Ans.
2.33
2.34
. . BTU
HI :=1322.6·___.;.Ibm
.' . . .... ft3 V 1:::.::3.058·-··.·_.
Ibm
rc 2 _·Dl ,uI
4
mdot:=---
VI V2
U2 := mdot·-rc 2 _·D2
4
'. . . BTU
H2 :=1148.6·-.- ... c:
.' ' .. 'lbnl
·.it ul:==10·~ s
.. " • ft3
V2:= 78.14·-··Ibm
4 Ib mdot = 3.463 x 10 - sec
ft uz = 22.997- sec
2 2
u2 - ul
Eq. (2.32a): Ws:= H2 - HI + --- 2
Wdot .= -Ws·mdot
BTU Ht:= 307·- .. :-.' :
. . '" Ibm
ft?
VI := 9.25·Ibm
rc 2 _·DI 'UI 4
mdot:=---
VI V2
U2 := mdot·-rc 2 -·D2 4
BTU Ws = -173.99-Ib
ADs.
.. '<. I1TU H2 :== 330"-"'-, .. lb
m
DI := 4·in
Ib mdot = 679.263- hr
ft U2 = 9.686- sec
BTU Ws:=5360,-' . _-_ ..
. Ibrnol
2 2
il2 -UI Ws BTU
Eq. (2.32a): Q:= H2 - HI + - -- Q = -98.82--
2 molwt Ibm
Qdot r= mdot-Q c~ili~'llli~ Ans.
13
._-- -----
2.36 T I := 300· K
P := I-bar
n:=
I·kg
n = 34.602mol
gm 28.9·mol
bar·cm3 r,
VI := 83.14· .-
mol-K P
3 em
VI = 24942- mol
Whence
W := -n·P·2· V 1
Ans.
Given:
Whence
. joule
Cpt= 29." .
·moi'K
Q:= n·~H
n
Ans.
zu := _Q_+_W_
2.37 Work exactly like Ex. 2.10: 2 steps, (a) & (b). A value is required for PVff, namely R.
J
R = 8.314-rnol-K
TI:= 293.1S·l( PI ::;: lOOO·kPa 7 .
Cp := ~~R 2
T2 ::;:::333.15·K P2 ::;::: lOO·kPa 5 .
Cy:=--R . 2
(a) Cool at const VI to P2 (b) Heat at const P2 to T2
Ta2 = 29.315K
14
3 J ~Hb = 8.841 x 10 -
mol
3 J ~Ua = -5.484 x 10 - mol
R·TI VI:= -PI
3 -3 m VI = 2.437 x 10 - mol
3 m
V2 = 0.028- mol
3 J ~Ha = -7.677 x 10 -.mol
3 J ~Ub = 6.315 x 10 -. mol
Ans.
Ans,
15
Chapter 3 - Section A - Mathcad Solutions
3.1 ~ = ~tT p) p
At constant T, the 2nd equation can be written:
dp
- = K·dP
P
~ := _1n_(_1._01_)
~p = 225.2bar
K
Since
-_ 3 _ em
c.:= 0.125·-- - gm
fV2
Work = - PdY
VI
3.4 b :=2700·bar
-- -r- 6 -_ 1
.1( := 44.1S.IO -bar P2 = 1.01· P 1
PI := I-bar
P2 := 500·bar
a bit of algebra leads to
fP2
Work:= c- _P- dP
P +b
PI
Alternatively, formal integration leads to
3.5 K = a + b-P
- - - -~6- -1
a:= 3;9·10·atm -
P2 := 3000'atm
Combine Eqs. (1.3) and (3.3) for const. T:
fP2
Work .= y. (a + b·P}P dP
PI
16
Ans.
b := -O.I.1O-9~atm~2 3
V := l-ft (assume const.)
ADS.
----------
1 . m3 VI := -_ .. - .. _ ... _.
1590 kg
. kJ
Cp:=O:84·· .
. . kg-degt:
M:,..5·kg
P:= l-bar
tl := O·degC
t2 := 20· degC
With beta independent of T and with P=constant,
dV
- = l3·dT V
Li Vtotal := M·LiV
Ans.
Work := -P·Li Vtotal (Con st. P)
3.8 PI:= 8.bar
Q := M.Cp.(t2 - tl)tfs'J1:)q Ans.
Lllitotal := Q tlil~:~,~~,~!~ ADS •
P2 := l-bar
Tl .= 600·K
7 Cp:="..·R 2
. ·5 .. Cy:=_·R
2
(a) Constant V:
P2 T2:= Tl·_
PI
LiU := Cy-LiT
Llli := Cp·LiT
(b) Constant T:
Work := RTlm(:~J
(c) Adiabatic:
W = 0 and
LiU = Q = Cy·LiT
LiT = -525K
Q and
Ans,
Ans.
LiU = LiH = 0
and
Q=W
Q and
ADS.
Q=O
and
L\.U = W = CV·L\.T
17
Cp y.=Cv
Wand
3.9 P 4:== 2bar
PI:= lObar
T2 = 331.227K
.6.U := Cv·.6. T
~~i~;~~~f~ii! ADS,
•.....•... 7
Cp :=-R 2
5 Cv:= -R 2
Tt:== 600K
R·Tl VI :=-PI
R
T4:~ Tl{;:t
Step 41: Adiabatic
.6.U41 := CY·(TI - T 4) i1H41 := Cp.(TI - T4) J
Q4I:= 0-
mol
T2:=600K
Step 12: Isothermal
J .6.U12 := 0-
mol
18
L'lH := Cp- L'l T
ADs.
3 -3 m VI = 4.988)( 10 -
mol
T4 = 378.831K
3 J i1U41 = 4.597 x 10 -
mol
3 J i1H4l = 6.436 x 10 -
mol
J Q4I = 0- mol
3 J W4I = 4.597 x 10 - mol
3 m V2 = 0.017- mol
J i1U12 = 0- mol
J ilH12:= 0·mol
Step 23: Isochoric
J W23:= 0- mol
J ':\H12 = 0- mol
3 J Q12 = 6.006 x 10 -
mol
3 J W12 = -6.006 x 10 -
mol
T3 =400K
3 J .:\U23 == -4.157x]0 -
mol
3 J .:\H23 = -5.82 x 10 -
mol
3 J Q23 = -4.157 x 10 -
mol
J '
W23 = 0-
mol
T4 = 378.831 K
R·T4 ,m3
V4:= -- V4 = 0.016-
P4 mol
Step 34: Isobaric
J ,:\U34 = -439.997- mol
J ilH34 = -615.996- mol
J Q34 = -615.996- mol
J W34 = 175.999- mol
3.10 For all parts of this problem: T2 = T] and
6.U = ilH = 0 Also Q = -Work and all that remains is
to calculate Work. Symbol V is used for total volume in this problem.
19
Pjr=vl-bar
P2:= 12·bar
(a)
(b) Step 1: adiabatic compression to P2
P2'Yi-PI'YI Wl:=----y - 1
Step 2: cool at const P2 to V2
(c) Step 1: adiabatic compression to V2
Work:~ hVlm(:~)
.W()tK~/:g~.?~<~~ ADs.
(intermediate V)
3 Vi = 2.702m
(intermediate P)
Pj·V2-PI,VI WI :=----y - 1
Step 2: No work.
(d) Step 1: heat at const VI to P2 WI = 0
Step 2: cool at const P2 to V2
20
Work:= WI
Work:= W2
WI zz: 3063kJ
W2 = 2042kJ
Pi = 62.898 bar
WI = 7635kJ
(e) Step 1: cool at const PI to V2
WI = ] IOOkJ
Step 2; heat at const V 2 to P2
Work:= Wi
'waHz:i':'j,f6hkJ
.- .-'- .- c' ' •• _.' • - .:.-(. __ ":~':_:., : ••
Ans.
3.17 (a) No work is done; no heat is transferred.
t
6.U = .6.T= 0
T2 = r. = IOO-degC
Not reversible
(b) The gas is returned to its initial state by isothermal compression.
Work = nRTln(~~J
but
, 3 VI := 4·m
4 3 V2:= -·m 3
3.18 (a) Pj := lOO·kPa 7
Cp ::='..;.·R
2
P2;= 50Q·kPa
T1,:= 303.15·K Cp y:=-
Cv
,5 Cv:=-·R 2
Adiabatic compression from point 1 to point 2:
kJ Q12:= 0-mol
6.U12 := Cy-(Tz - Tl)
,~~})i~,i~\~~~~~;
6.H12 := Cp'(T2 - r.)
;~,l1,.·".".:,},~.~.i~,.";,,,·.,·· .• ·,~~i~;~
.- .- c_ .- • r' _-.- -0- "-"-" '~, .-.-
Ans .
21
Cool at Pz from point 2 to point 3:
kJ L1H23 = -5.15- mol
Ans.
kJ Q23 = -5.15- mol
Ans.
Isothermal expansion from point 3 to point 1:
"'U31 = "'H3I = 0 P3 r= P2 W31 :~ R.T3.ln(~:J
Q31 := -W31
Ans.
FOR THE CYCLE: L1U = 1I.H = 0
Q := Q12 + Q23 + Q31 Work:= W12 + W23 + W31
i~I~!~!~i~~h~~i~~j~;I~
(b) If each step that is 80% efficient accomplishes the same change of state, all property values are unchanged, and the delta H and delta U values are the same as in part (a). However, the Q and W values change.
Step 12:
W12 W12:= -- 0.8
Q12 := 1I.U12 - W12
Step 23:
W23 W23:= -- 0.8
22
--- ~----.----
Step 31: W31:= W31,O,8
FOR THE CYCLE:
Q := Q12 + Q23 + Q31
Work:= W12 + W23 + W31
IIlll£!lt
3.19 Here, V represents total volume.
Pf:~lOOO.kPa
V ····3
.. i:= I-rn
... joule Cp:=21·-mol·K
Cy:= Cp - R
Cp y:=Cv
Work ~ nRT,ln( ~~)
(a) Isothermal:
T2 := Tl
ADs.
(b) Adiabatic:
P2'Y2-Pl'Yl Work := ----y - I
23
3.20
(c) Restrained adiabatic:
Pext :"" 100·kPa
PI,VI
n:=--
KTI
Work T2:= --+T] n·Cy
VI T2 P2:= P1'-'V2 TI
Tl:= 423.15·K
. 7 Cp :=-·R 2
Step 12:
If
VI V]
r=-=-
V2 V3
kJ W12 = -2.502- mol
Step 23:
kJ Q23 = -2.079- mol
Process:
Work = L1U = -PexrL1 V
AU = n·Cv·AT
Ans.
Ans.
P3 := 3·bar
5 Cv r= -·R 2
. kJ
L~llt2 .= 0·- .. - ".
. . mol
T3 := 323.15·K
·kJ AU 12 :=0'-· - .,
.. mol
Then
TI P3
r:=-·-
T3 PI
kJ Q12 = 2.502- mol
kJ W.2··3.· t= 0·-. - . .... .. mol
kJ AU23 = -2.079- mol
kJ Mi23 = -2.91- mol
Work:= W12 + W23
ADS.
Q ;= Ql2 + Q:n
24
LllI := i1H 12 + i1H23
Ans.
Ans.
3.21 By Eq. (2.32a), unit-mass basis:
molwt := 2Sgm
. mol
1 2
i1H + -·Au = 0 2
But
i1H = Cp·AT
Whence
.7 R Cp :='2' rnolwt
· ..... ····m
til:= 2.5--'s
2 2 u2 - li1
tz > t1---- 2·Cp
3.22
7 Cp:;:::: ~~R
2
... 5
Cv:=-'R 2
Tl := 303.15·K
PI := l-bar
P3 := lO·bar
i1U := Cy.(T3 - Tl) .····.······•··· •• "f··'.·····' •• ·· C·i:'.·, (:.1tJi
. ·i\U-==2~n79~
, . ·'·'·-mol
Ans .
i1H := Cp.(T3 - r.)
~)~!~i":
ADs.
Each part consists of two steps, 12 & 23. T2 P2:= PI'-
Tl
Work:= W23
'$~~~~~i Ans,
25
Q := llU - Work
~s~~t~~~~i Ans,
Q12 :"'" M112
kJ W12 = -0.831- mol
kJ W23 = 7.718- mol
Q := llU - Work
.: ---; .-:'- - kJ'
W!~k- :,' 6:.~8~.~~I
~.~~t~i~
ADS.
Work:= W12 + W23
ADS.
(c)
WI2:= RT"~{~~J
Q23 := ~H23
Work:= W12 + W23
11'~1i~i Ans.
", kJ
Q = -2.894.-'-' Ans.
. mol
Q := llU - Work
For the second set of heat-capacity values, answers are (k.J/mol):
ilU = 1.247
llU = 2.079
(a)
Work = 6.762
Q = -5.515
26
(b) (c)
3.23
PI := l-bar
For the process:
Step 12:
kJ W12 "" 5.608- mol
Step 23:
For the process:
Q := Q12 + Q23
Work = 6.886
Q = -5.639 Q = -3.725
Work = 4.972
T3 := 393.l5·K 7
Cp;= ..;;;.,R
, 2
5 Cv:='":"·R 2,
~u := CV·(T3 - Tl)
~WJ~'rf;~~\(:~r
~H := Cp.(T3 - Tt)
',.') 'i;-:-"id
Llli = '2.619 _- - Ans.
- r - '-' - -. - niol
• < .' -~?'."
Q12 := -W12
kJ Q12 = -5.608-'-, mol
kJ W23 := 0·-- 'mol
Q23 := ~u
Work:= W12 + W23
ADS.
T3 = Tj So ...
W= R.h1n(;J
But
Also
In(_P ) = _T_2_-_T_1
PI r,
Therefore
r, := 800·K
27
ADS.
3.25
3 VA:= 256·cm
r:= -0.0639
Define:
Assume ideal gas; let V represent total volume:
-VA"Cr+ 1) VB:=----
-----
r
Ans.
Cp y:=· .. CV
P(final) = P2
The process occurring in section B is a reversible, adiabatic compression. Let
TB(fmaI) = TB
Since the total volume is constant,
2·nA·R·Tl nA·R-(TA + TB)
=------
PI
or
--=---
(a)P2:= 125'atm
y-I
(P2J Y
TB:=TI' Pt
Q = nA{1..U A + ~UB)
q := CY·(TA + TB - 2.TI)
Define
- Q q--
nA
28
(1)
(2)
(3)
Ans.
(b)
Combine Eqs. (1) & (2) to eliminate the ratio of pressures:
TA :=42S·K
(guess) TB := 300·K
y~l
Given
(1)
(e)
By Eg. (2),
TB:= 325·K
(1)
TB := Find(TB)
:~t~{~~~l:~~9~i{ ADs. c~Z~0.J~~~~@
Ans.
Ans.
c_pi;¥,"':1'&23'~im ADs .
• c ._. • ••• ~ ;,-~ _;-':'~'-._ .,~_ c:.
:fX-;~f16~j(
~ ~-~:' ; 0-.:,2 ._ .. ~
ADS.
Ans.
(d)
Eliminate
TA + TB from Eqs. (1) & (3):
kJ q:=3·mol
(2)
(1)
29
'T~ .. ::in$19:66E.: Ans.
. ,," -. . ... ~,' . - ~ , ..... -,; ", ..
3
..... em
3.30 B:=~242.5·-··-·
mol
. 6 .... ···..cm
C :=25200·_····_·
. '; 2
mol"
T:= 373.lS·J(
P2 r= 55·bar
B
B':=-
R·T
-3 1 B' = -7.817 x 10 - bar
c- 82
C':=---
C' = -3.492 x 10- 5 _1_ bar2
(a) Solve vi rial eqn. for initial V.
Guess:
R·T Vl:=p]
Given
B C = 1+-+-
Vl V/
Vl := Find(VI)
3 em VI = 30780- mol
Solve virial eqn. for final V.
Guess:
Given
PZ'Y2
---
R·T
B C 1+-+-
V2 vl
3 em V2:-:: 241.33- mol
Eliminate P from Eq. (1.3) by tbe virial equation:
Ans.
(b) Eliminate dV from Eq. (1.3) by the vlrial equation in P:
30
ADS.
Note: The answers to (a) & (b) differ because the relations between the two sets of parameters are exact only for infinite series.
3.32 T c .= 282.3' K
T := 298.15·K
Tr = 1.056
P;=12·bar
p Pr;= - Pc
Pr = 0.238
01:=0.087
(guess)
(a)
3 . em.
B := -140--·· _ ...
mol
6
. .. em
c:= 7200·_· ._.
mor
R·T V:=P
3 em V = 2066- mol
Given
p.y B C
-=1+-+R.T Y y2
<~·,g'·tj§'I~S~~
. ·····mol
Y := Find(V)
(b)
0,422 BO .= 0.083 - _T 1.6
r
BO = -0.304
0.172 Bj := 0.139 ---
T 4.2 r
-3 B, = 2.262 x 10
Z·R·T V:=--
p
(c) For Redlich/Kwong EOS:
o.r= 1
E := 0
Q ::::: 0.08664
'¥ := 0,42748
Table 3.1
-05 a(Tr) ;= Tr .
Table 3.1
Eq. (3.51)
31
Eq. (3.50)
Calculate Z
Guess:
Z:= 0.9
Z := Find(Z) Z = 0.928
Z·R·T Y:=-p
ADs.
(d) For SRK EOS:
0: :.=;:1
E:= 0
o := 0.08664
'l' :== 0.42748 Table 3.1
Table 3.1
q(Tr) '. __ 'l"CX (r, , ill)
Eq. (3.51)
or,
Eq. (3.50)
Calculate Z
Guess:
Z:= 0.9
Given Eq. (3.49)
Z := Find(Z) Z = 0.928
Z·R·T V:=--
ADs.
p
(e) For Peng/Robinson EOS:
a:= 1 +[2
E:= 1 - [2 n := 0.07779
'P := 0.45724 Table 3.1
a (r-, (0) := [ 1 + (0.37464 + 1.54226", - 0.26992,}). [1 - T) ) J 32
Table 3.1
q{Tr);= tp.a{Tr,co) Q·Tr
Calculate Z
Eq. (3.51)
Eq. (3.50)
Guess:
Z:= 0.9
Given Eq. (3.49)
Z := Find(Z)
Z = 0.92
v:= _Z_'R_'_T P
ADS.
3.33 Tc :=305.3;K
T:=323.l5·K
T, ;;;;; 1.058
Pc := ,48.72·bar
P :~ I5·bar
p
Pr:= - Pc
P, = 0.308
co :=0.100
(guess)
(a)
3
, ,em
B := "-156.7·-' -,' mol
,6 , ,em
G:= 9650·-' -" 2 mol
R·T Y'=p
3 em V = 1791-,,,-
mol
Given
p.y B C
-=1+-+R·T V y2
:~~~J,t1t~
p.y Z:=R·T
y := Find(V)
(b)
0.422 Bo := 0.083 - -~
T, 1.6
BO = -0.302
0.172 Bl := 0.139--T 4.2
r
-3 Bl = 3.517x 10
33
ADs.
(e) For Redlich/Kwong EOS:
rr:= 1
E: := 0
!1 := 0.08664
'Y:= 0,42748
Table 3.1
-0.5 a(Tr) := T,
Table 3.1
q (r.) '. __ \}I. a ( T r)
Eq. (3.51)
Q·Tr
Eq. (3.50)
Calculate Z
Guess:
Z:= 0.9
v:= _Z_.R_·T_ P
ADs.
Z := Find(Z) Z = 0.906
(d) For SRK EOS;
o := 1
8: := 0
Q:::: 0.08664
P:= 0.42748 Table 3.1
Table 3.1
q(Tr) '.= tp.a(Tr,co)
Eq. (3.51)
Q·Tr
Eq. (3.50)
Calculate Z
Guess:
Z:= 0.9
Given Eq. (3.49)
34
Z := Find(Z) Z =: 0,907
y:= _Z_'R_'_T p
(e) For Peng/Robinson EOS:
IT := 1 +{2
8:= 1 '-[2 n :=0,07779
ADS.
'l'. := 0,45724 Table 3.1
Table 3.1
Eq. (3.51)
Eq. (3.50)
Calculate Z
GuesS:
z .- 0.9
Given Eq. (3.49)
Z := Find(Z)
y:= _Z_,R_'T_ P
z = 0.896
3.34 Te:= 318.7·K
T T, :=-
Te
T := 348.l5·K
n, r= 37.6·bar
P ;= IS·bar
p Pr := - Pe
()) := 0.286
(a)
3 ··cm B:= -194---·
mol
.. 6 ... ·cm C:= 15300·-- 2 mol
Given
p.y B C
-=1+-+R.T Y y2
35
ADs.
Tf = 1.092
Pr = 0.399
(guess)
R·T
V:=-
p
3 Y = 1930 em mol
V := Find(V)
rv
Z:;;= -
R·T
(b)
0.422 BO := 0.083 - -T 1.6 r
BO = -0.283
0.172 Bj := 0.139 ---
Tr4.2
BI = 0.02
Z·R·T V:=-p
(e) For Redlich/Kwong EOS:
(J :=1
n := 0.08664
'P := 0.42748
Table 3.1
-0.5 c.(Tr) := T,
Table 3.1
Eq. (3.51)
Eq. (3.50)
Calculate Z
Guess:
Z:= 0.9
Z := Find(Z) Z = 0.888
Z·R·T V:=-p
Ans.
(d) For SRK EOS;
cr:= 1
E :=Q
Q:== 0.08664
T:=0.42748 Table 3.1
36
Table 3.1
Eq. (3.50)
Calculate Z
Guess:
Z:= 0.9
Given Eq. (3.49)
Z := Find(Z) Z = 0.895
V:= _Z_·R_._T P
(e) For PenglRobinson EOS:
o :=::: 1 +/2
E .= 1- f2 Q .= 0.07779
T ::::: 0.45724 Table 3.1
Table 3.1
Eq. (3.51)
Eq. (3.50)
Calculate Z
Guess:
Z:= 0.9
Given Eq. (3.49)
Z := Find(Z) Z = 0.882
Z·R·T V:=-p
Ans.
37
3.35 T:=523.l5·K
(a)
3 em B::::= ~152.5·-··- .. . mol
Given
p.y z·=. R·T
(b) Tc:= 647.1·K
T Tr:= -
Tc
Tr :;::: 0.808
P:= 1800·kPa
. 6
..... em
C ;= -5800·_· -
. ·mof
p.y B C
-=1+-+R·T V y2
Bj := 0.139 ~ 0.172 Tr4.2
Z·R·T Y:=-p
(c) Table F.2:
. 3 3.37 B:~ ..;.53A·cm ... mol
Pc := 220.55·bar
P
Pr:= -
Pc
Pr = 0.082
Bl = ~0.281
Z:;::: 0.939
molwt :« 18.015. gm mol
or
6 C:= 262(). qn .. 12 mo
38
R·T
V '= - (guess) . p
Y .= Find(Y)
Ans.
co := 0.345
BO :;::: ~0.51
Ans.
3 cm
Y := 124.99·_·molwt
gm
Ans.
9
..... .cm
D :==5000· _ ... ~...... n := mol
01013
T := 273.15·K
Given
p.y
RT
BCD 1 +-+-+Y y2 y3
i :"" 0 .. 10
( ~ 10 )
Pi:"" 10 + 20·j -bar
B,Pi zi. .- 1 +--
1 R.T
110 ·10
20
40
60
80
100
120
140
160
180
200 Eq. (3.12)
Eq. (3.37)
bar
1
0.953
0.906
f--
0.861
f--
0.819
f--
0.784
0.757
0.74
0.733
0.735
0.743 f(P ,Y) := Find(Y)
(guess)
Eq. (3.38)
1
0.951
0.895
0.83
0.749
0.622
0.5+0.179i
0.5+0.281i
0.5+0.355i
0.5+0.416i
0.5+0.469i Note that values of Z from Eq. (3.38) are not physically meaningful for pressures above 100 bar.
39
Zl j =
1
0.953
0.906
0.859
0.812
0.765
0.718
0.671
0.624
0.577
0.53 T .= 273. 15·K
Given
p.y
R-T
BCD 1+-+-+Y y2 y3
i := 0 .. 10
( - 10 )
Pj i= 10 + 20·j -bar
B,Pi Z1·::= 1 +-
1 R.T
1.10 ·10
20
40
60
80
100
120
140
160
180
200 Eq. (3.12)
Eq. (3.37)
bar
1
0.953
0.906
0.861
0.819
0.784
0.757
0.74
f---
0.733
f---
0.735
0.743 f(P , V) := Find(V)
R·T Yj:=--
Pi
(guess)
Eq. (3.38)
1
0.951
0.895
0.83
0.749
0.622
O.5+0.179j
0.5+0.281i
O.5+0.355i
0.5+0.416i
05+0.469j 0.906 0859 0.812 0.765 0.718 0.671 0.624 0.577
0.53
Note that values of Z from Eq. (3.38) are not physically meaningful for pressures above 100 bar.
39
0.9
0.8
Z2i
0.7
0.6
0.5 0
50
100
150
~1 Pi' bar
3.38 (a) Propane:
r, .= 369.8·K
Pc .= 42.48· bar
T := 313.15·K
P := B.7l·bar p
Pr :=-
Pc
T Tr :=Tc
Tr = 0.847
For RedlichlKwong EOS:
o := 1
.Q := 0.08664
q; := 0.42748
s := 0
-05 a(Tr) := Tr .
Table 3.1
Eq. (3.50)
Calculate Z for liquid by Eq. (3.53) Guess:
Z := 0.01
40
-. <,
200
0);= .OJ52
P, == 0.323
Table 3.1
Eq. (3.51)
Given
Z := Find(Z) Z = 0.057
Ans,
p
Calculate Z for vapor by Eq. (3.49) Guess:
Z:= 0.9
Given
Z := Find(Z)
Z = 0.789
v:= _Z_·R_·_T P
Ans.
Rackett equation for saturated liquid:
T Tr:= -
Tc
T, = 0.847
.' .· ... ·.3
. ' ... " .em·
Vc .::;:: 20Q.O·~ Ze ::;:;0.276
. . '.' mol
For saturated vapor, use Pitzer correlation:
BO := 0.083 _ 0.422 T 1.6
r
BO = -0.468
Bl := 0.l39 _ 0.172 Tr4.2
Bl = -0.207
R·T ( ) r,
V :=-+R- BO+ffi·Bl·-
P Pc
Ans.
41
Parts (b) through (t) are worked exactly the same way. All results are
summarized as follows. Volume units are cu.cm./mole.
RiK, Liq. RiK, Vap. Rackett Pitzer
(a) 108.1 1499.2 94.2 1537.8
(b) 114.5 1174.7 98.1 1228.7
(c) 122.7 920.3 102.8 990.4
(d) 133.6 717.0 109.0 805.0
(e) 148.9 1516.2 125.4 1577.0
(0 158.3 1216.1 130.7 1296.8
(g) 170.4 971.1 137.4 1074.0
(h) 187.1 768.8 146.4 896.0
(i) 153.2 1330.3 133.9 1405.7
(j) 164.2 1057.9 140.3 1154.3
(k) 179.1 835.3 148.6 955.4
(I) 201.4 645.8 160.6 795.8
(m) 61.7 1252.5 53.5 1276.9
(n) 64.1 1006.9 55.1 1038.5
(0) 66.9 814.5 57.0 853.4
(p) 70.3 661.2 59.1 707.8
(q) 64.4 1318.7 54.6 1319.0
(r) 67.4 1046.6 56.3 1057.2
(s) 70.8 835.6 58.3 856.4
(t) 74.8 669.5 60.6 700.5
42 3.39 (a) Propane Tc;;:;:369.S·k
T:=(40 + 273.15)·K
T ~ 313.1SK
P := 13.71· bar
T
Tr:=Tc
Tr = 0.847
P, = 0.323
From Table 3.1 for SRI(:
o := 1
E := 0
n := 0.08664
\{' := 0.42748
Eq. (3.51)
Eq. (3.50)
Calculate Z for liquid by Eq. (3.53) Guess:
Z := 0.01
Given
Z := Find(Z)
Z = 0.055
v:= _Z_·R_·_T P
Ans.
Calculate Z for vapor by Eq. (3.49) Guess:
Z:= 0.9
Given
Z := Find(Z) Z = 0.78
v:= _Z_·R_·T_ P
Ans.
43
Parts (b) through (t) are worked exactly the same way. All results are
summarized as follows. Volume units are cu.cm.!mole.
SRK, Liq. SRK, Vap. Rackett Pitzer
(a) 104.7 1480.7 94.2 1537.8
(b) 110.6 1157.8 98.1 1228.7
(c) 118.2 904.9 102.8 990.4
(d) 128.5 703.3 109.0 805.0
(e) 142.1 1487.1 125.4 1577.0
(I) 150.7 1189.9 130.7 1296.8
(g) 161.8 947.8 137.4 1074.0
(h) 177.1 747.8 146.4 896.0
(i) 146.7 1305.3 133.9 1405.7
(j) 156.9 1035.2 140.3 1154.3
(k) 170.7 815.1 148.6 955.4
(I) 191.3 628.5 160.6 795.8
(m) 61.2 1248.9 53.5 1276.9
(n) 63.5 1003.2 55.1 1038.5
(0) 66.3 810.7 57.0 853.4
(p) 69.5 657.4 59.1 707.8
(q) 61.4 1296.8 54.6 1319.0
(r) 63.9 1026.3 56.3 1057.2
(s) 66.9 817.0 58.3 856.4
(t) 70.5 652.5 60.6 700.5
44 3.40 (a) Propane
r, .= 369.8·K
P~ := 42.48·bar
0):=0.152
T :=(40+273.15)-K
T = 313.l5K
P :::: n.7l-bar
T Tr:=Te
T, = 0.847
P Pr:= - Pe
Pr = 0.323
From Table 3.1 for PR:
a(T"",) .= [ I + (0.37464 + 1.54226m - 0.269920,2).(1- T}) J
CJ := 1 + {2 E := I - fi n:= 0.07779 \}J:= 0.45724
Eq. (3.51)
Eq. (3.50)
Calculate Z for liquid by Eq. (3.53) Guess:
Z:=0.01
Given
Z := Find(Z)
Z = 0.049
V := _Z·_R._T P
ADs.
Calculate Z for vapor by Eq. (3.49) Guess:
Z:= 0.6
Given
Z := Find(Z) Z = 0.766
v:= _Z._R_·T P
ADS.
45
Parts (b) through (t) are worked exactly the same way. All results are
summarized as follows. Volume units are cu.cm.zmole.
PR, Liq. PR, Vap. Rackett Pitzer
(a) 92.2 1454.5 94.2 1537.8
(b) 97.6 1131.8 98.1 1228.7
(e) 104.4 879.2 102.8 990.4
(d) 113.7 678.1 109.0 805.0
(e) 125.2 1453.5 125.4 1577.0
(f) 132.9 1156.3 130.7 1296.8
(g) 143.0 915.0 137.4 1074.0
(h) 157.1 715.8 146.4 896.0
(i) 129.4 1271.9 133.9 1405.7
U) 138.6 1002.3 140.3 1154.3
(k) 151.2 782.8 148.6 955.4
(I) 170.2 597.3 160.6 795.8
(m) 54.0 1233.0 53.5 1276.9
(n) 56.0 987.3 55.1 1038.5
(0) 58.4 794.8 57.0 853.4
(p) 61.4 641.6 59.1 707.8
(q) 54.1 1280.2 54.6 1319.0
(r) 56.3 1009.7 56.3 1057.2
(s) 58.9 800.5 58.3 856.4
(t) 62.2 636.1 60.6 700.5
46 3.41 (a) For ethylene,
'. ,,,.... . . .: gin .' .' ":.' .
'molwt:= 28.054......,_1'.· .r, :=282.JK mo
Pc:;::: SOAO·bar
T
Tr:=-
Tc
co := 0.087 P
Pr:= -
Pc
T := 328.15·K
P·;=3S·bar
T, = 1.162
P, = 0.694
From Tables E.1 & E.2:
Zo:= 0.838
ZI:=0.033
Z := Zo + roZj
Z = 0.841
n'-
• -r- molwt
18~kg
Z·n·R·T Vtotal := --p
(b) T:= 323.15·K
P:= 11S:bar
3 Vtotal := O.25·m
T
T '-_
r .-
Te
T, = 1.145
p Pr:=Pe
Pr= 2.282
From Tables E.3 & E.4: Zo := 0.482
Z1 := 0.126
Z = 0.493
p. Vtotal
n = 2171 mol
n:=---
mass t= n-rnolwt
Z·R·T
_m~~i'~~,~2,~2'§*~:,:' Ans.
3.42 Assume validity of Eq. (3.37).
...
PI := .Ibar
Tr :=300K
3 em VI::::: 23000-' _ ..
. . mol
3 B = -I.942 x 103 em mol
With this B, recalculate at P2
47
B,P2
Z2:= 1 +-- Z2 = 0.611 R·T1
R·Tl,Z2 V2 :=--P2
3.43 T:= 753.15·K
Te:=' 513.9·K
P := 6000~kPa
P c := 61.48· bar
ro :=" 0.645
0.422 Bo :== 0.083 - --
T 1.6 r
0.172 B1:=0.139---
Tr4.2
For an ideal gas:
R·T V'=P
3.44 T := 320· K
P :=' l S-bar
ro::;= 0.152
3 em
V e:== 20Q--· .-. mol
T, = 0.865
..... 3 Vtank :== 0.35·m
0.8· Vtank
mliq:=--Vjjq
molwt
0.422
BO := 0.083 - -- Bo = -0.449
T/6
48
Ans.
T
T . __
r .-
Te
Tr = 1.466
P
Pr :=-
Pc
P, == 0.976
Bo = -0.146
Bl = 0.104
ADs.
Te:= 369.8·K
Pe:= 42.48·bar
Zc:= 0.276
gm molwt:» 44.097-.···
. . mol
Pr == 0.377
3 em
Vliq == 96.769- mol
Ans.
0.172 Bl:=0.139--- Bl=-0.177
Tr4.2
R.T r,
Vvap:= - + (BO + (O.Bl)·R.-
P Pc
3.45 T :::=, 298.15·K
P := 2.43· bar
0.422 BO := 0.083 - --
Tr 1.6
0.172 Bl := 0.139 ---
Tr4.2
0.2· V tank
mvap :=
Vvap molwt
Te:= 425J·K
Pc :::::37.96·bar
3 Vvap:= 16'm
BO = -0.661
Bl ::;: -0.624
R·T ( r,
V := - + Bo + (O.Bl)-R--
P Pc
Vvap
v
mvap :=
molwt
3.46 (a) T:= 333.15·K
P::: 14QOQ·kPa
0), :,=O.lDO
3 3cm Vvap = 1.318 x 10 -, -
mol
T Tr:= -
Tc
p Pr:= - Pc
molwtr= 58.123· gm
, mol
3 V = 9.469 x 103 cm mol
Tc := 305,3·K
Pc :== 48,.72·bar
V',' "'0' J "" total := " •. 15;m
49
Ans.
T Tr :=Tc
p Pr:= - Pc
Ans.
T, = 0.701
P, = 0.064
T, = 1.091
Pr = 2.874
, ' gm molwt.;= 30.07-'-,'
',' 'nio}
From tables E.3 & E.4: Zo:= 0.463
Z := Zo + (r}·ZI
methane :=
Ytotal V
rnolwt
(b)
V:= Vtotal 40~kg
or
U Tr= - Z
Whence
z, :=-'-0.037
Z = 0.459
Z·R·T Y:=--
3 V = 90.87 em mol
p
Ans.
P r= 20000·kPa
where
p·v
U'=--
R·Tc
mol U = 29.548- kg
0.889 Tr= -- at Z
P Pr:= -
Pc
Pr = 4.105
This equation giving T r as a function of Z and Eq. (3.54) in conjunction with Tables E.3 & E.4 are two relations in the same variables which must be satisfied at the given reduced pressu reo The intersection of these two relations can be found by one means or another to occur at about:
Tr := 1.283
Whence
T = 391.7K
3.47 VtotaI;= O.15.m3
r, := 282.3· K
Vtatal V := .-"'(-40~'k-g--:-.) . molwt
or
Pr = fl·Z
and
Z ;= 0.693
T := 298.15·K
Pc := SOAO·bar
.... gm molwt := 28.054 _ .. -. . mol
co := 0.087
where
R·T
(1'=--
pc·Y
o: = 4.675
50
Whence
P, = 4.675·Z at
T Tr :=Tc
r, = 1.056
This equation giving P r as a function of Z and Eq. (3.54) in conjunction with Tables E.3 & E.4 are two relations in the same variables which must be satisfied at the given reduced temperature. The intersection of these two relations can be found by one means or another to occur at about:
r, := 1.582
and Z:= 0.338
Ans.
mwater
3 em V = 26.667- gm
3.48 mwafer;= 15 -kg
VtotaI:= OA·m3
Vtotal V:=---
Interpolate in Table F.2 at 400 degC to find:g~j;':~2;g~i~~ ADS.
3.49 Tl:= 298.l5·K
r, := 305.3· K
Trl = 0.977
PI := 2200·kPa
Pc := 48.72· bar
Prl==0,452
3 Vtotal := O.35·m
rn r= 0.100
From Tables E.l & E.2: ZO:= .8105
ZI := ~0.0479
Z = 0.806
Z·R·TI VI:= --PI
3 em VI = 908- mol
T2:= 493.15·K
Tr2 ::: ] .615
Assume Eq. (3.37) applies at the final state.
0.422 BO := 0.083 - -T 1.6 r2
BO = -0.113
0.172 BI := 0.139 ---
. T 4.2
r2
BI=0.116
51
3.50 T:= 303.15· K
Tc;= 304.2·K
' ... ' . .3
V total':= 0.5- ill
P c;=·· 73;83' bar
BO = -0.341
0.172 B1 :"" 0.139 ---
Tr4.2
BI = -0.036
Vtotal V .= -(-lO-'k-g~J
molwt
3 3em V = 2.2x 10 - mol
R-T
P := --------
V - (Bo + ro.BI)-R. Tc Pc
T Tr:=-
Tc
ro:= 0.224
3.51 Basis: 1 mole of LIQUID nitrogen
Tn:= 77.J·K
Pc. :=34.d·bar
" '>. grn
m .. olwt::::;:28.014·-· .-,',
, , .. mol
0.422 BO := 0.083 - --
T, 1.6
Bo = -0.842
52
Tn Tr :=Tc
P
Pr:= - Pc
Ans.
Tr = 0.997
.' .' ..... gill .molwt.:« 44.01 '-. '-. . . . mol
Ans.
T, = 0.613
Pr == 0.03
V ', "'3'4 '7' .... 3
· ... I··:=·cm
lq, .... ' .... '.
B 1 :=: -1.209
Z :=: 0.957
p. Vliq
n '=---
vapor· Z.R. r,
-3 nvapor = 5.718 x 10 mol
Final conditions:
ntotal := I -rnol + nvapor
2· Vliq V:=-ntotal
3 em V = 69.005- mol
T:=298J5·K
T Tr:=-
Tc
T; :=: 2.363
R·T Pig:= - V
Pig = 359.2bar
Use RedlichlKwong at so high a P.
Q := 0.08664 q.t := 0.42748
- 5 ex (Tr) := Tr .
q'.a(lr)-R2'T!
a := (3.42)
Pc
3 a = 0.901 m3 bar·em
moll
Q·R·Tc b:=---
(3.43)
3 b = 26.737 em mol
Ans.
3.52 For isobutane: Tc := 408.1·K Pc := 36.48,bar
3 .cm VI .= ~ .824·_·· ._ ..
gm
Tl := 300·K PI := 4·bar T2 := 415·K
P2 := 75·bar
53
Tj Pj T2 P2
Trl :=- Prj :=- Tr2:"" - Pr2:= -
Te Pc Te Pc
Trl "" 0.735 Prj = O. I I Tr2 = 1.017 Pr2 = 2.056 From Fig. (3.17):
Prl .= 2.45
The final T > Tc, and Fig. 3.17 probably should not be used. One can easily show that
p·Ve Pr = Z·R·T
with Z from Eq. (3.54) and Tables E.3 and E.4. Thus
.. 3 .. em Yc<=262.7·-· .. :_.
. <>, mol
Zo ::=03356
·il:=~O.0756
Z := Zo + O)·Z]
Z = 0.322
P2,Ve Pr2 := Z.R.T2
Pr2 = 1.774
Eq. (3.65):
Prl V2:= Vj'-
Pr2
ADS.
3.53 For a-pentane:
Pc:= 33.7·bar
. ..... gm Pl:=0.63.-· _
-. ". 3
em
It:::: 291.J.5·K
Pl .• :=·l·bar
P2 : ~ 120·bar
P2 Pr2:= - Pc
Trl = 0.62
Prl = 0.03
Tr2 = 0.88
Pr2 = 3.561
From Fig. (3.17):
Prt:=2.69
Pr2 ;=2.27
By Eq. (3.65),
Pr2 P2:= Pr'-
Prl
Ans.
54
3.54 For ethanol: Tc:= 5I3~9.K
Pc :== 61.48·bar
. 1 .·cm Ve ::::.} 67·-· .- . . mol
From Fig. 3.17:
3.55 For ammonia:
Te:= 405.1'·K
Pc:=·112.8·bar
3 .cm Vc:=]25·- - _ •.. '
. mol
Pr:=2.28
P :=
T .= 4$J.15·K
P :=200·bar
T
Tr:= - Te
inolwt:=4ko69:<gIU . mol
Pr Vc molwt
T·:= 293.15·K
P:= 857·kPa
Zc:= 0.242
Eq. (3.63):
0.422 Bo := 0.083 - _-
Trl06
0.172 Bl := 0.139 - _-
Tr4.2
BO = -0.627
BI = -0.534
55
Pr P = PrPc = Vc
T Tr:=-
Tc
P
Pr:=-
Pc
(i) := 0253
3 cm
Vliquid = 27.11- mol
Tr = 0.882
Pr = 3.253
ADS.
Tr;:;; 0.723
Pr = 0.076
R·T ( ) r,
Vvapor ::0: -- + BO + ro-Bj ·R·-
P Pc
3 em
Vvapor = 2616-mol
L1Y ;= Yvapor- Vliquid
ADS.
Alternatively, use Tables E.1 & E.2 to get thevapor volume:
20;::;;; 0.929
21:== ~0.071
2 := Zo + co-Zj
Z =: 0.911
Z·R·T Yvapor:= -p
3 em
Vvapor = 2591-mol
L1 V := V vapor - Vliquid
ADS.
3.58 10 gal. of gasoline is equivalent to 1400 cu ft. of methane at 60 degF and 1 atm. Assume at these conditions that methane is an ideal gas!
ft3·atm
R = 0.7302---Ibmo I, rankine
T ':=519.67 -rankine
:3 V ':== 1400· ft
p.y
n:=--
R·T
n = 3.6891bmol
For methane at 3000 psi and 60 degF:
Tc:== IQO.6·L8·rankine
T:= 519.67·rankine
T Tr:=-
Tc
Tr = 1.515
Pe> 45.99·bar
p:~ 3000·psi
P
Pr:= -
Pc
P, == 4.498
ill := 0.012
From Tables E.3 & E.4:
20:=0.819
21:=0.234
Z := Zo + 0)·Z1
Z = 0.822
56
3.59 T := 25K P := 3213bar
Calculate the effective critical parameters for hydrogen by equations (3.55) and (3.56)
43.6 Tc:=----·K 21.8K 1+---
2.016T
T, = 30.435K
20.5
Pc := . bar
1 + 44.2K
2.016T
Pc = 1O.922bar
m:=O
T
Tr:= -
Tc
Initial guess of volume:
R·T Y'=-
P
3 V = 646.903 em mol
Use the generalized Pitzer correlation
0.422 BO := 0.083 - --
T/6
) Pr
Z := 1 + (BO +w·Bj .Tr
BO = -0.495
0.172
Bl := 0.139 - -- Bl = -0.254
Tr4.2
Ans. Experimental: Z = 0.7757
For RedlichlKwong EOS:
c := 1
~ := 0
n := 0.08664
'f' := 0.42748
Table 3.1
() - 0.5
a Tr t= r,
Table 3.1
Eq. (3.51)
57
Calculate Z
Eq. (3.50)
Guess:
Z::=c 0.9
Z :== Find (Z)
3.61 For methane: 0):= 0.012
Experimental: Z = 0.7757
Tc:= 190.6K
Pc :=45.99bar
At standard condition: T ""I(60:":l251+273:JS} Pitzer correlations:
T
Tr ::=c - Te
0.422 BO := 0.083 - --
Tr1.6
Pr ZO:= 1 +Bo'-
Tr
Z := Zo + (O·ZI
(a) At actual condition:
Pitzer correlations:
T Tr:= -
Tc
0.422 BO :== 0.083 - _-
Tr 1.6
T = 288.706K
Tr = 1.515
P
Pr:= - Pc
Pr = 0.022
BO = -0.134
0.172
Bj :== 0.139 - -~ B] = 0.109
Tr4.2
Zo = 0.998
ZI == 0.00158
Z == 0.998
Z·R·T VI :==-P
3 m VI == 0.024-mol
T :=[(50 ~ 3:2)0% '" 273.15}::
T == 283.15K
P:=300psi
It = 1.486
P Pr ::=c -
Pc
P, == 0.45
BO = -0.141
58
0.172 Bj :"" 0.139 ---
Tr4.2
Z := Zo + m·21
3
. 6 ft ql:= 150·10 .. -.-
day
(c) D := 22.624in
q2
u:=-
A
BI = 0.106
Zo = 0.957
Z "" 0.958
ZI = 0.0322
Z·R·T V2:=-p
3 m V2 = 0.00109-·mol
ADS.
1t 2 A :=-D
4
59
ADS.
2 A = 0.259m
ADS.
3.62
0.152 0.181 0.187 OJ9 OJ91
0;194 0.196
0.2 0.205 0.21 0.21 0.212 0.218 0.23
0.235 0.252 0.262 0.28 0.297 0.301
0.302 0.-303 O.3i .0.322 0.326·
0.266 0,266 0.263 0.263
0.26 0261
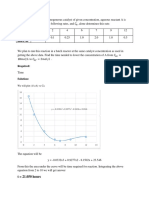
Use the first 29 components in Table B.1 sorted so that 0) values are in ascending order. This is required for the Mathcad slope and intercept functions.
ill:~ slope(ID,Zc) = ( -0.091} b := iri~rcept(ro;Zc) = (Q.291)
R:= corr(w,Zc) = (-0.878) R2= 0;771
0.29
Zc 0.28
000
mo-i-b 0.27
0.26
0
0.25 0 0.1 0.2 OJ 0.4 co
ili&1~lf~~! Ans,
60
Chapter 4 - Section A - Mathcad Solutions
4.1 (a) TO :;0;: 473.l5·K
T :"" 1373.15·K
n :"" IO'mol
For S02:
A = 5.699
-3
B=0.801·1O C=O.O
..... ·····5
D= -1.015·10
( ~ 3 5) 3
ICPH 473.15,1373.15,5.699,0.801·10 ,0.0,-1.015·10 = 5.654·10
1-.H := R·ICPH
Q := n·1-.H
:Q1~'~;~wi.Q£QZ4e;tq ADs.
(b) To :=523.15·K
T:"= 1473.15· K
For propane:
A = 1.213
B= 28.785.10';';3
..... : ... : . ·-6
C = ~8.824·10
( -3 -6) 4
ICPH 523.15,1473.15,1.213,28.785·10 ,-8.824·10 ,0.0 = 1.947·10
ICPH := 1.947.104.K 1-.H := R·ICPH Q := n·M{
~1~~i~~;~i~~~~1~ Ans.
4.2 (a) TO := 473.l5·K II := 10·moJQ:= 800·k]
... K
For ethylene:
A:= 1.424
14.394.10~J
B := -----"----"~
t .= 2 (guess) Given
Q = nOR{[ ATo(' - I) + ~T02(,2 -I)J + ~ oTl(,3 - I)J
t := Find(t)
t = 2.905
T := t·To
ADs.
(b) TO := 533.15·K
Q := 2500·kJ
For f-butene: A:= 1.967
. 31.630.10~ 3
B := -.~. -K--':-·· ~
. _9.873.10-6
C;= ... _. -'--,..;--..'-----
K2
61
t ;= 3 (guess) Given
Q = n.R{[ A·To·(' - I) + ~ Tl( / - 1)] + ~Tl(,3 - 1) J
't = 2.652
T ;= 't·To
Ans,
(c) TO :==500·degF
h:= 40·1bmol
. . 6
Q:==lO ·BTU
Values converted to SI units
TO := 533.l5K
4
n = 1.814 x 10 mol
For ethylene:
A::::; 1.424
't ;= 2 (guess)
Given
t .= Find( 't)
't = 2.256
T .= 't·TO
.··~~~£~i~~~~:· ]:ti;~l~;fJ~~~&~
Ans,
4.3 Assume air at the given conditions an ideal gas. Basis of calculation is 1 second.
p::= l-atm
TO ::;:: 122·degF
. . 3
V:::;: 250·ft
T;= 932·K
Convert given values to SI units
3 Y = 7.079m
T := (T - 32degF) + 273.15K T = 1187.37K
TO ::=: (To - 32degF) + 273.15K TO = 323.15K
rv
n:=--
R·TO
n = 266.985 mol
For air:
A.::J.355
'-3
8=0.575·10 .
C :==0.0
( - 3 5)
ICPH 323.15,773.15,3.355,0.575·10 ,0.0,-0.016·10 = 1648.702
62
ICPH :== 1648.702·K
L\H := R·ICPH Q:== n·L\H
~~;~~:~~':~~~~1'~~7g~~ Ans.
4.4 molwt.re 100.I.gm mol
TO :=323.15·J( T :=1153.15·K
IOOOO·kg
n:""--moIwt
4
n == 9.99 x 10 mol
For CaC03:
A := 12;572
. ··-3
B = 2.637~ 10
5 D.= -3.120-10
( - 3 5)
ICPH 323.15,1153.15,12.572,2.637·10 ,0.0,-3.120·10 = 11355.4
rCPR := 11355.4·K
Lill := R·ICPH
Q := n·L\H
,~;i~1~:~~·~~*·j:;r~~;~J Ans,
4.7 Let step 12 represent the initial reversible adiabatic expansion, and step 23 the final constant-volume heating.
Tl:=298.15·K
PI ::;:: 12L3·kPa
P2 .= IOL3·kPa
P2 T2:= T3'P3
T2 = 290.41 K
joule Cp :=30·-mo]·K
(guess)
Given
R
T2 = Tl{:~t
Cp ;= Find( c-)
4.9 72.150 469.7 33.70 309.2
86.177 507.6 30.25 341.9
78.114 gm Tc:= 562.2 ·K Pc := 48~98 . bar T ._ 353.2 ·K
M'-
.. - n'~
mol
92.141 591.8 41.06 383,8
84.161 553.6 40.73 353.9
63 --j.
Trl := (273.15 + 25)K Tn
Tc Tr2;= -
Tc
0.635 0.658
0.587 0.674
Trl = 0.53 Tr2 = 0.628
0.504 0.649
0.539 0.639 I1H is the value at 25 degC.
l1~xp is th e given value at the normal boiling point.
366.3 366.1 433.3 ·412.3. .
··392.5
357.2 336.7
J
. 4Hex.p .:-:;: ·3.93-~9 ~ -
gm
363.2
358,2
J
(a) By Eq. (4.13)
[ (1 - Tf2JO.38]
.1Hn:= .1H· --I - Trl
[ .1Hn - I1Hexp J
PCE:= ·100%
.1Hexp
(b) By Eq. (4.12):
This is the % error
I1Hn:= [R-Tn.rI.092·H~) -l~~~Jr
M 0.930 - Tr2 j
[I1Hn - .1Hexp J
PCE:= ·100%
.1Hexp
64
4.10 The In P vs. 1fT relation over a short range is very nearly linear. Our procedure is therefore to take 5 points, including the point at the temperature of interest and two points on either side, and to do a linear least-squares fit, from which the required derivative in Eq. (4.11) can be found. Temperatures are in rankines, pressures in psia, volumes in cu ft/lbrn, and enthalpies in Btu/Ibm. The molar mass M of tetrafluoroethane is 102.04. The factor 5.4039 converts energy units from (psia)(cu ft) to Btu.
(a) T;= 459.67 + 5
18.787 21.162 Data: P:= 23.767
26.617 29.726
AV:= 1.934-":0.012
i:= 1.. 5
o 5 10 15
1
Xj:=----
ti + 459.67
slope := slope(x, y) slope = -4952
(~P)3
dPdT := -_. slop, dPdT = 0.545
r
The remaining parts ofthe problem are worked in exactly the same way. All answers are as follows, with the Table 9.1 value in ():
(a) M-I = 90.078
(90.111 )
(b) AH = 85.817
(85.834)
(c) AH = 81.034
(81. 136)
(d) AH = 76.007
(75.902)
(e) AH = 69.863
(69.969)
65
~H is the value at o degC.
~Hexp is the given value at the normal boiling point.
.. ( .r : 270;9 "J" .....
'. ' ... , J
AH :=.11.8 .. 9.5 ...••. _ .217.8·.gm
." . .' ,[. 246.9 "J
AHexp:= .•... : .•. 1 ... · .. 0.9. ···.9 •. 5 •. ·.·.·.·•• . J ...
............. gm
194.2 . ' .. '.
(a) By Eq. (4.13)
(mn -~Hexp J
PCE:"" ·100%
~Hexp
273.15K Trl:== --Tc
[0.509J
Trl = 0.533
0.491
[ (1 - Tr2]038
mn:= ~H·
1 - Td
This is the % error
Tn := .••. (~:~::J .. K
.. 349.8
[0.623J
Tr2:;:;; 0.659
0.629
i1Hn := r R.Tn.[I.092{ m({;) - I.DI3)JJ'>-
M 0.930 - Tr2 J
(b) By Eq. (4.12):
(M-I - LiH J
PCE := n exp .100%
~Hexp
66
(1) -2,732,016 J (s) -492,640 J
(g) -105,140 J (t) 109,780 J
(h) -38,292 J (u) 235,030 J
(i) 164,647 J (v) -132,038 J
(j) -48,969 J (w) -1,807,968 J
(k) -149,728 J (x) 42,720 J
(I) -1,036,036 J (y) 117,440 J
(m) 207,436 J (z) 175,305 J 4.22 The solution to each of these problems is exactly like that shown in Example 4.6. In each case the value of 8.H0298 is calculated in
Problem 4.21. Results are given in the following table. In the first column the letter in ( ) indicates the part of problem 4.21 appropriate to
the 8.Ho298 value.
--- .. --- ---··---··----··r----·---- .- ..... - ...... .. ____ .. _'C·'· ___ ~r _____
TIK 1l.A 1031l.B 1061l.C 10-5 LlD IDCPHJJ 1l.HoT/J
(a) ----- -_--_ ... _- - .. - .... - ...... --.--~ 1--._-_ ............. -- ........ - '.
873.15 -5.871 4.181 0.000 -0.661 -17,575 -109,795
_._
(b) 773.15 1.861 -3.394 0.000 2.661 4,729 -900,739
(ti 923.15 6.048 -9.779 0.000 7.972 15,635 -2,]16,381
(i) 973.15 9.811 -9.248 2.106 -1.067 25,229 189,876
(j) 583.15 -9.523 11.355 -3.450 1.029 -10,949 -59,918-
(1) 683.15 -0.441 0.004 0.000 -0.643 . ___ ,._ ... :2-l~J!L -1,038,452
--- i--- .. ~-- .. -- ... ---- .. -
(m) 850.00 4.575 -2.323 0.000 -0.776 13,467 220,903
(n) 1350.00 -0.145 0.159 0.000 0.215 345 -180"j45 .
(0) 1073.15 -1.011 -1.149 0.000 0.916 -9,743 "--
168,578
(r) 723.15 -1.424 1.601 0.156 -0.083 __ .~~~:1, I2L ~_ ~;T~03'7'-[
(t) t-----------~-~
733.15 4.016 -4.422 0.991 0.083 . L. 7,424 117,204
(u) 750.00 7.297 -9.285 2.520 0.16'6--'- --1.-=~1_t_~1?'l_1 __ ~~~~ 7,2_91~
(v) 900.00 2.418 -3.647 0.991 0.235 3,534 -128,504
(w) 673.15 2.586 -4.189 0.000 1.586 4,184 -1,803,784
(x) 648.15 0.060 0.173 0.000 -0.191 -125 -- ----42--845--
(y) 1083.15 4.175 -4.766 1.814 0-:083 --'-'I---'-J1, 18.fr·---·-i12~~~] 76
4.23 This is a simple application of a combination of Eqs, (4.18) & (4.19) with evaluated parameters. In each case the value of ~Ho298 is calculated in Pb. 4.21. The values of LiA, LlB, LiC and LiD are given for all cases except for Parts (e), (g), (h), (k), and (z) in the preceding table. Those missing are as follows:
Part No. .1.A 103 LlB 106 de 10-5.1.D
(e) -7.425 20.778 0.000 3.737
(~) -3.629 8.816 -4.904 0.114
(h) -9.987 20.061 -9.296 1.178
(k) 1.704 -3.997 1.573 0.234
(z) -3.858 -1.042 0.180 0.919 ftJ 5
4.24 q := 150.106 -d· .. T:= (60 - 32)·-K + 273.15K T = 288.71 K J>:~hitm
ay 9
The higher heating value is the negative of the heat of combustion with water as liquid product.
Calculate methane standard heat of combustion with water as liquid product:
Standard Heats of Formation:
... .J
MIfCH4:= ~ 74520 - .... -. . mol
.,J. AHft)2 := O~· mol
J LiHfC02:= -393509-. ._
. mol
.. J
AHffi20liq:==...;285830-. _.: mol
~e .= L\HfC02 + 2'~fH201iq - L\HfCH4 - 2·L\Hf02
HigherHeatingYalue .= -L\He
5 J MIe = -S.906x 10 - mol
Assuming methane is an ideal gas at standard conditions:
p n:=q-R·T
s mol n = 1.793 x 10 - day
77
4.25 Calculate methane standard heat of combustion with water as liquid produc1 Standard Heats of Formationt Cfl., + 202 --> CO2 +2H20
". . .... J
AHfCH4 :==.-74520mo1
". .J AH:te02::= ~393509-· .• -..
. mol
'. . .......J AHf02:== 0 _'.' -.' . mol
'. . j MItH2· 0" ·1'::;;:: -;:285830--
· .. lq ..... .' mol
AHcCH4 .= AHfC02 + 2·AHfH201iq - AHfCH4 ~ 2·tl.HfD2 J
.M-IcCH4 = -890649-
mol
Calculate ethane standard heat of combustion with water as liquid product:
Standard Heats of Formation: C2M.; + 7/2°2 --> 2C02 +3H20
..... . .' J
AHfC2H6:== -83820'-" .. -'.
. . .... '. mol
7 tl.HcC2H6 := 2tl.HfC02 + 3.tl.HfH201iq - tl.HfC2H6 - -·tl.HfD2 2
, '.' ".' ..... '1
MIcC2I-I6 :;;::~1560688J1101
Calculate propane standard heat of combustion with water as liquid product Standard Heats of Formation: C3HS + 502 --> 3C02 +4H20
, '.' J
AHfC3HS:= -104680~
. . mol
tl.HcC3H8 := 3tl.HfC02 + 4.tl.HfH201iq ~ tl.HfC3H8 ~ S·tl.Hf02 kJ
tl.HcC3H8 = -2219.167-
mol
Calculate the standard heat of combustion for the mixtures
a)
b)
c)
Gas b) has the highest standard heat of com bustion. A
ns.
78
~~----- ..
4.26
2H2 + 02 = 2H20(1)
C + 02 = C02(g) bHf2:=-'":393S()9·J
N2(g)+2H20(l)+C02(g)=(NH2)2CO(s)+3/202 Llli:=63166Q~J
N2(g)+ 2H2(g)+C(s)+ 1I202(g)=(NH2)2CO(s)
!~~~l~X,,;g.,""~-:.~~g~ Ans.
4.28 On the basis of 1 mole of CI0H18
(molar mass = 162.27)
Q := -43960· 162.27·J
This value is for the constant-volume reaction:
CI0H18(l) + 14.502(g) = 10C02(g) + 9H20(l)
Assuming ideal gases and with symbols representing total properties,
Q = <3.U = LllI - ~ CPy) = ~H - R· T·ililgas
T := 298.15·K
~ngas := (1 0-14.5)·mol Llli = -7.145 x 106 J
This value is for the constant-V reaction, whereas the STANDARD reaction is at con st. P.However, for ideal gases H = f(T), and for liquids H is a very weak function of P. We therefore take the above value as the standard value, and for the specified reaction:
CI0H18(l) + 14.502(g) = lOC02(g) + 9H20(l)
<3.H
9H200) = 9H20(g)
CIOH18(1) + 14.502(g) = lOC02(g) + 9H20(g)
<3.H298 ;= ~H + Lllivap , .. ~",:.k .• ·"~'§!'~~~~%4~4$'g .• ·.;~.J.:,. Ans .
... ~ ," .. ~ - .' - - .;,.' ~',.- .. ; ",",-,
79
4.29 FURNACE: Basis is 1 mole of methane burned with 30% excess air.
CH4 + 202 = C02 + 2H20(g)
Entering:
Moles methane
Moles oxygen
nz := 2·1.3 79 "3 :=2,6'21
Moles nitrogen
Total moles of dry gases entering
At 30 degC the vapor pressure of water is 4.241 kPa. Moles of water vapor entering:
Leaving:
4.241
n := -13.381
4 101.325-4.241
C02 --1 mol H20 -- 2.585 mol
02 -- 2.6 - 2 = 0.6 mol N2 -- 9.781 mol
By an energy balance on the furnace:
Q = bH = b.H298 + MIp
For evaluation of .1.Hp we number species as above.
i := 1 .. 4
R := 8.314
A ::::: 48.692 B = 0.010897
80
"2 = 2.6
ll3 = 9.781
n = 13.381
114 = 0.585
(1) (2) (3) (4)
·· .••. ·.0.121
--0.227· .0.046
4 D ::::: -5.892 x 10
The TOTAL value for MCPH of the product stream:
( - 3 5)
MCPH 303.15,1773.15,48.692,10.897· 10 ,0.0, -0.5892·10 = 59.89511
MCPH t= 59.89511
Lllip := R·MCPH·(1773.15 - 303.15)
From Example 4.7:
~H298 .= -802625
;,~~ffi1&;~~<Q~~6'1~~;J
Ans.
HEA T EXCHANGER: Flue gases cool from 1500 degC to
50 degC. The partial pressure of the water in the flue gases leaving the furnace (in kPa) is
ll2
pp := ·101.325
II I + n2 + ll3 + I14
pp = 18.754
The vapor pressure of water at 50 degC (exit of heat exchanger) is 12.34 kl'a, and water must condense to lower its partial pressure to this value.
Moles of dry flue gases:
n :== n1 + nj + 04
n = 11.381
Moles of water vapor leaving the heat exchanger:
12.34
n2 = 1.578
n2:= 101.325 _ 12.34·n
Moles water condensing:
M:= 2585~ J.578
Latent heat of water at 50 degC in J/moJ:
AHsO := 2382.9·18.015
Sensible heat of cooling the flue gases to 50 degC with all the water as vapor (we assumed condensation at 50 degC):
MCPHb23.15, 1773.15,48.692,10.897·10- 3,0.0, -0.5892.105) = 60.01086 MCPH:= 60.01086
Q := R·MCPH·(323.15 - 1773.15) - ~n.Llli5of:i~,~,fB~~~i~~\1.'J Ans.
81
4.30 4NH3(g) + 502(g) = 4NO(g) + 6H20(g)
BASIS: 4 moles ammonia entering reactor
Moles 02 entering = (S}(1.3) = 6.S Moles N2 entering = (6.5)(79/21) = 24.45
Moles NH3 reacting = moles NO formed = (4)(0.8) = 3.2 Moles 02 reacting = (5)(0.8) = 4.0
Moles water formed = (6)(0.8) = 4.8
ENERGY BALANCE:
~H = bHR +bH298 +MIp = 0 REACTANTS: I=NH3; 2=02; 3=N2
i := 1 .. 3
A= 118.161
-. (30020J: ...
' ......•. ·-3
B:::; ••.. · .. 0.50. 6 ... :.010 0;593·
B = 0.02987
5 D = -1.242 x 10
TOTAL mean heat capacity of reactant stream:
MCPH(J48.15 ,298.15,118.161,0.02987,0.0, -1.2420105) = 126.61632
MCPH := 126.61632
bHR := R·MCPH·(298.15 - 348.15)
The result ofPb. 4.21(b) is used to get AHz98:=O.8,{,""905468)
PRODUCTSi=NH3; 2=02; 3=NO; 4=H20; 5=N2
0.8 3.578 3;020
82
-0.186 .,....0 .. 227
i .= 1 .. 5
A = 119.65
B = 0.027
4 D = 8.873 x 10
By the energy balance and Eq. (4.7), we can write:
TO := 298.15 T := 2
(guess)
Given
1: := Find( T)
T = 3.283
T:= TO·KT
4.31 C2H4(g) + H20(g) = C2H50H(l)
BASIS: 1 mole ethanol produced
Energy balance: M1 = Q = 1l.HR + 1l.H298
1l.H298:= -277690-(52510-241818)
4 fl.H298 = -8.838 x 10
Reactant stream consists of 1 mole each of C2H4 and H20.
i ;= I.. 2 n:= G)
.... ( ..... 1. .. 42.4)··. '. ( .: 1.4 .. 3 ... 94 .... ) .. -.3 ... ('"-'4 . .3.9.2) ... ··· ... 7"""6 (. 00.)' 5
A:= 3.470 B::= 1.450 .·10 .' C:= '. 0,0 '·10 D:=O.;21·10·
A :::: 4.894
B = 0.01584
C = -4.392 x 10- 6 0 = 1.21 x 104
MCPH(298.15, 593.15,4.894,0.01584,-4.392.10- 6,0.121'105) = 11.1192
83
MCPH ::0: 1 L 1192
4.32 One way to proceed is as in Example 4.8 with the alternative pair of reactions:
CH4+ H20 = CO + 3H2
M-l298a::::::205813
CH4 + 2H20 = C02 + 4H2
MI298b;:O: 164647
BASIS: 1 mole of product gases containing 0.0275 mol C02; 0.1725 mol CO; & H20 0.6275 mol H2
Entering gas, by carbon & oxygen balances:
0.0275 + 0.1725 = 0.2000 mol CH4
0.1725 + 0.1725 + 2(0.0275) = 0.4000 mol H2O
&H298 := 0.1725·&H298a + 0.0275·&H298b
4 AH298 :0: 4.003 x 10
The energy balance is written
REACTANTS: 1=CH4; 2=H20
n;=(~:}]
A;" G::~) B ;~(;::~~)l O~} C;= t~;4}10 ~6D ;=C)~;~jllO~
i ::0: 1 .. 2
D;= I nj·Dj
A = L728 B = 2.396 x 10-3 C::: -4.328 x 10-7 D = 4.84x 103
ICPH( 773.15,298.15, 1.728,2.396·10- 3 , -4.33·10- 7,4.84.103) = -1377.435
ICPH := -1377.435
&HR := R· ICPH
4 AHR = -1.145x 10
PRODUCTS: 1=C02; 2=CO; 3=H20; 4=H2
84
0.0275
n:=
i := 1 .. 4
A:=
0.422
.lO~3
.. 5 ,10
0,083 0:= Lni·Oj
A = 3.37
-4 B = 6.397x 10
3 D=3.579xlO
( -4 3)
ICPH 298.15,1123.15,3.370,6.397·]0 ,0.0,3.579'10 = 3164.293
ICPH := 3164.293
Mfp := R·ICPH
4.33 CH4 + 202 = C02 + 2H20(g)
C2H6 + 3.502 = 2e02 + 3H20(g)
4 LlHp= 2.631 x 10
Ans.
AH298a:=-802625
AH298b:=-1428652
BASIS: 1 mole fuel (0.75 mol CH4; 0.25 mol C2H6) burned completely with 80% xs. air.
02 in = 1.8[(0.75)(2) + (0.25)(3.5)] = 4.275 mol N2 in = 4.275(79/21) = 16.082 mol
Product gases: C02 = 0.75 + 2(0.25) = 1.25 mol H20 = 2(0.75) + 3(0.25) = 2.25 mol 02 = (0.8/1.8)(4.275) = 1.9 mol
N2 = 16.082 mol
Energy balance: Q = LlH = Mf298 + LlHp PRODUCTS: 1=C02; 2=H20; 3=02; 4=N2
1.25 2.25
n.t=
1.9
16.082
5.457 3,470 3.639 3.280
A . ._c.
.-
B·..,;.·
.-
85
5 Q := -8·10
0.040
i := 1..4
D:= Lnj.Dj
A = 74.292
B = 0.015
4 o = ~9.62x 10
By the energy balance and Eq. (4.7), we can write:
TO:= 303.15
1 .= 2 (guess)
Q ~ 6.H298 = R·
Given
1 := Finder)
1 = 1.788
T := To·KI:
Ans.
4.34 BASIS: 1 mole of entering gases containing 0.15 mol S02; 0.20 mol 02; 0.65 mol N2
S02 + 0.502 = S03 Conversion = 86%
S02 reacted = S03 formed = (0.15)(0.86) = 0.129 mol
02 reacted = (0.5)(0.129) = 0.0645 mol
Energy balance: .:1H773 = L1HR + L1H298 + L1Hp
Since L\HR and MIp cancel for the gas that passes through the converter un reacted, we need consider only those species that react or are formed. Moreover, the reactants and products experience the same temperature change, and can therefore be considered together. We simply take the number of moles of reactants as beinz negative. The energy balance is then written: .:1H773 = .:1H298 + L1t1net
1: S02; 2: 02; 3: S03
[' "'J'" ['"
-0.129 ,'5.699
n:f . ~t_:;;~'.~r~~~J
86
i := 1 .. 3
A = 0.06985
-7 B = 2.58 x 10
4 D = -1.16x 10
( -7 4)
MCPH 298.15,773.15,0.06985,2.58·10 ,0.0,-1.16·10 = 0.019666
MCPH := 0.019666
~Hnet := R·MCPH(773.15 - 298.15)
Ans.
4.35 CO(g) + H20(g) = C02(g) + H2(g)
BASIS: 1 mole of feed consisting of 0.5 mol CO and 0.5 mol H2O.
Moles CO reacted = moles H2O reacted = moles C02 formed = moles H2 formed = (0.6)(0.5) = 0.3
Product stream:
moles CO = moles H20 = 0.2 moles C02 = moles H2 = 0.3
Energy balance:
Llih9S:= 0.3·[-393509 ~ (-110525 -214818)1 Reactants: 1: CO 2: H20
. . '.(' •. 0.5.,) .. (3 . .3.7.6 .. ) .•...
n:= .. '. A:=," ' ..
0.5' '3.470
4 ~298 = -2.045 x 10
'. .'.(" - 0.0. 3.1 )'., ···.·.·,· 5
D:= .. '. . ·10
.. 0]21 .r . '.'
i:= 1..2
A = 3.423 B = 1.004 x 10- 3 D = 4.5 x 103
MCPH(298.15 ,398.15,3.423,1.0035·10- 3 ,0.0,0.045.105) = 3.8103 MCPH := 3.8103
AHR := R·MCPH·(298.15 - 398.15)
3 AHR == -3.168x 10
Products:
1: CO 2: H2O 3: C02 4: H2
87
A'::=
0.083
0557
}.450 3
B:= '10-
1.045
·0;422
~0~031
i:"" 1..4
A = 3.981
-4 B = 8.415x 10
4 D = -3.042 x 10
MCPH(298.15 ,698.15,3.981,0.8415.10-3,0.0,-0.3042.105) = 4.25405
MCPH := 4.25405
L'.Hp := R·MCPH·(698.15 - 298.15)
Q := (MIR + L'.H298 + L'.Hp}J
4 MIp = 1.415 x 10
Ans.
4.36 BASIS: 100 lbmol DRY flue gases containing 3.00 lbmol C02 and 11.80 lbmol CO x Ibmol 02 and 100-(14.8-x)= 85.2-x lbmol N2. The oil therefore contains 14.80 Ibmol carbon;a carbon balance gives the mass of oil burned:
12.011
14.8· ·Ibm = 209.133 Ibm
0.85
The oil also contains H20: 209.133·0.01
----·lbmol = 0.1161bmol 18.015
Also H2O is formed by combustion of H2 in the oil in the amount
209.133·0.12
----·lbmol = 12.4481bmol 2.016
Find amount of air entering by N2 & 02 balances. N2 entering in oil:
209.133·0.02
----·lbmol = 0.1491bmol 28.013
Ibmol N2 entering in the air={8S.2-x)-O.149 =8S.0S1-x
88
Ibmol 02 in flue gas entering with dry air =
3.00 + 11.8/2 + x + 12.448/2 = 15.124 + x Ibmol (C02) (CO) (02) (H20 from combustion)
Total dry air = N2 in air + 02 in air = 85.051 - x + 15.124 + x = 100.1751bmol
Since air is 21 mol % 02,
15.124 + x 0.21 = ---- 100.175
x i= (0.21·}00.175 - 15.124)·lbmol x::= 5.9131bmol
02 in air = 15.124 + x = 21.037 Ibmols N2 in air = 85.051 - x = 79.138 Ibmoies
N2 in flue gas = 79.138 + 0.149 = 79.2871bmols
[CHECK: Total dry flue gas
= 3.00 + 11.80 + 5.913 + 79.287 = 100.00 Ibmol]
Humidity of entering air, sat. at 77 degF in Ibmol H20llbmol dry air, P(sat)=0.4594(psia)
0.4594 = 0.03227
14.696 - 0.4594
Ibmol H2O entering in air:
0.03227·100.175·lbmol = 3.2331bmol
If y = Ibmol H2O evaporated in the drier, then Ibmol H20 in flue gas = 0.116+ 12.448+3.233+y = 15.797 + Y
Entering the process are oil, moist air, and the wet material to be dried, all at 77 dcgF. The "products" at 400 degF consist of:
3.00 Ibmol C02 11.80 Ibmol CO 5.913 Ibmol 02 79.287 Ibmol N2
(15.797 + y) Ibmol H20(g)
Energy balance:
where Q = 30% of net heating value of the oil:
89
· ..... . BTU
Q := ~0.3. ,19000--· _··209.13·lbrn
lbpJ. .
Q == -1.192 x 106BTU
Reaction upon which net heating value is based:
OIL + (21.024)02 = (14.8)C02 + (12.448 + 0.116)H20(g) + (0.149)N2
MlZ98a:= -19000·209.13·BTU
6 ,1.HZ98a = -3.973 x 10 BTU
To get the "reaction" in the drier, we add to this the following:
(11.8)C02 = (11.8)CO + (5.9)02
Ml298b:= 1 L.f(.-:11 0525 + 393509)·QA2993·.atV
(y)H20(I) = (y)H20(g)
Guess: y:= 50
L\FI 298c(y):2 44012; 0 .42Q93·Y' 13TV
[The factor 0.42993 converts from joules on the basis of moles to Btu on the basis of Ibmol.]
Addition of these three reactions gives the "reaction" in the drier, except for some 02, N2, and H20 that pass through unchanged. Addition of the corresponding delta H values gives the standard heat of reaction at 298 K:
~H298(Y) := ~H298a + ,1.H298b + ilH298c(Y)
For the product stream we need MCPH:
1: C02 2: CO 3:02 4: N2 5: H20
TO := 298.15
R .= 1.986
T := 400 + 459.67 1.8
T == 477.594
3 11.8
1.045 0.$57 .0.~06 0.593 1,450
·1539T+y·
i := 1 .. 5 A(y):= L n(YkAj B(y):= L n(y)j-Bj D(y):= L n(y)j-Dj
T
.... ~ '.- -
TO
1 = 1.602
Cp(y) := R'[ACY) + B~Y) ·To{r + 1) + D(Y~J 1'·TO
90
4.12 (a)ro::::: 0.210
Tc:::::562.2K
Pc:;:: 48.9,8bar
Zc:::::O.271
3 Vc:= 259. em mol
Tn ;=<: 353.2K
P:= Ibar
T; = 0.628
P
Pr:= -
Pc
Pr = 0.02
Generalized Correlations to estimate volumes
Vapor Volume
0.422 Bo := 0.083 - --
r. 1.6
Bo := -·0.805
Eq. (3.61)
0.172 Bl := 0.139 - _-
Tr4.2
Bj = -1.073
Eq. (3.62)
Z = 0.967
p
3 4cm V := 2.838x 10 -
mol
Z·R-Tn V:=~--
Liquid Volume
3 em Vsat = 96.807- mol
d Combining the Clapyeron equation (4.11) ~H = T·~V·-Psat
dT
with Antoine's Equation
Psat = e
B A-T-C
gives
67
~V:"" V - Vsat
3 4cm ~V "" 2.829x 10 - mol
A i= 13.8594
B:= 2773.78
C ::=53.00
I A ~-c
(:-cr K
Answers for parts (b)-(e) kJ
(b) ~H = 36.262 - mol
kJ (e) ~H = 32.278 - mol
kJ (d) ~H = 28.948 - mol
kJ (e) ~H = 33.838 - mol
4.13 Let P represent the vapor pressure.
T :=348.I5,K
P := JOO.kPa
(guess)
ADs.
Given
( P ) 5622.7·K (T)
In kPa = 48.157543 - T - 4.70504·ln K
P .= Find(P)
dPdT := p.(5622.7.K _ 4_70504)
T2 T
P = 87.396k:Pa
.. '. . joule
Ml:=.31600·_· .'-" . . . ' .... mol
68
dPdT = 0.029 bar K
, 3
...... ····cm
VIiq.:=96A9·-. -'1
. '. 'mo
Clapeyron equation:
dPdT = L\H
T(V - YJjq)
L\H
V = vapor molar volume. V:-= Vliq + ---
T·dPdT
Eq. (3.38):
(PoY )
B :=y. --1
R·T
Ans.
4.14 (a) Methanol: Te::::: 512.6K
Pc ;=8b~97bar Tn;S: 337.9K
BL :=~51.2S·10-:3 CL:=131.13.10- 6
AV := 2.211
.. ·-3
BV := 12.216·10
-6 Cv r= ...:3.450·10
( BV Cv _2)
Cpv(T):= Av + _oT + -·r ·R
K K2
P:= 3bar
Tsat := 368.0K TI:= 300K T2:::::; SOOK
Estimate ilHv using Riedel equation (4.12) and Watson correction (4.13)
Tn Tm:=Tc
Tm = 0.659
Tsat Trsat:= - Tc
Trsat = 0.718
L092·H ~) - 1.013 J
L\Hn := ·R·Tn
0.930 - Trn
kJ L\Hn = 38.301- mol
( 1- T )0.38
rsat
L\Hy := L\Hn'
1 - T rn
kJ L\Hy = 35.645 - mol
69
fTsat fT2
,ill := CpdT) dT + L'lHv + CpV(T) dT
Tj Tsat
,6.H :::: 49.38 kJ mol
. kmol n:= 100 _ .. - .• _.
. hr
Ans.
(b) Benzene:
kJ xn, = 28.273- mol
kJ L'lH = 55.296- mol
(c) Toluene
kJ L'lHv = 30.625- mol
kJ 1lH = 65.586- mol
4.15 Benzene T c := 562 .. 2K
Pc:= 48.98bar
Tn :=353.2K
Tlsat:=ASl.7K
J
Cp::::162· ···•·.
. mol-K
Estimate L'lHv using Riedel equation (4.12) and Watson correction (4.13)
Tm = 0.628
T2sat Tr2sat:= -Te
Tr2sat = 0.638
1.092{ m( ::) - 1.013)
LV-! := -R-T
n 0.930 _ Tm n
kJ L'lHn = 30.588- mol
._ .( 1 - Tr2sat)0.38
L'lHv .- L'lHn
I -Tm
kJ L'lHv = 30.28- mol
Assume the throttling process is adiabatic and isenthalpic. Guess vapor fraction (x): x := 0.5
4.16 (a) For acetylene:
Te':= 308.3·K T;=::298.1S·K
... ~.
'(ri;~189A'K
70
T
T -nh1.1
T
T
T = () QF.7
1n(~J - 1.013
bar
ilHn:= R·Tn·1.092·------ 0.930 - Tm
( 1 - r, )0.38 LlHv := ilHn·
1 - Trn
kJ (b) For 1,3-butadiene: LlH298 = 88.5-
mol
(c) For ethylbenzene:
kJ LlH298 = -12.3·mol
(d) For n-hexane:
kJ LlH298 = -198.6·mol
(e) For styrene:
kJ LlH298 = 103.9·mol
4.17
1st law:
dQ = dU - dW = Cv-d'T + P·dV
Ideal gas;
and
p·V = R-T
Whence
V·dP = R·dT - P·dV
kJ ilHn = 16.91-mol
kJ ~Hv = 6.638- mol
(A)
P·dV + V·dP = R·dT
(B)
Since
o _
p·V - const
then
0-1 0
r-sv .av = -V ·dP
from which
V·dP = -P·o·dV
Combines with (8) to yield:
71
P.dV = R·dT 1-0
Ans.
Combines with (A) to give:
R·dT dQ = Cv·dT + -- 1-6
R·dT
or dQ = Cp·dT-R·dT+--
1 - 6
o
which reduces to dQ = Cp-d'T + --·R·dT
1 - 6
or dQ = (CP + _6_).R.dT R 1-0
(C)
Since Cp is linear in T, the mean heat capacity is the value of
Cp at the arithmetic mean temperature. Thus Tarn:=q75
Integrate (C):
T:=AOO·K
.1 .
6.:= 1.55
Ans.
Ans.
4.18 For the combustion of methanol:
CH30H(g) + (3/2)02(g) = C02(g) + 2H20(g)
Mf298 := -393509 +2·(-241818) - (-200660) fiH298 = -676485
For 6 MeOH:
Ans.
For the combustion of 1-hexene:
C6H12(g) + 902(g) = 6C02(g) + 6H20(g)
72
~H298 :0:0 6·(-393509) + 6·(-241818) - (-41950)
~~~~~_':c~0~,~i~1~[~~gl;~tJ
Ans.
ilH298 0:0 -3770012
Comparison is on the basis of equal numbers of C atoms.
4.19 C2H4 + 302 = 2C02 + 2H20(g)
c c: c c c c,' ,""" """ " "" "' ,J"
MI298:=r2·(~241818) +2·(-'-'393509) '-"S2$10}-'
"", " ',mol
Parts (a) - (d) can be worked exactly as Example 4.7. However, with Mathcad capable of doing the iteration, it is simpler to proceed differently.
Index the product species with the numbers: 1 = oxygen
2 = carbon dioxide 3 = water (g)
4 == nitrogen
(a) For the product species, no excess air:
0 3.639 0.506
2 5.457
n:=
2 3.470
11.286 3.280 0.593
1 - 1..4 A ;= :Lni·Ai B :=:Lni·Bi A = 54.872
1 B = 0.012- K
For the products,
fT
Cp Lllip = R- R dT
To
To:=298J5K
The integral is given by Eq. (4.7). Moreover, by an energy balance,
LliI298 + i1Hp = 0
73
t .= 2
(guess)
-111129& = R[ A.TO·(' - 1) + ~ .T02(l- I) + ~O -(' ~ 1)]
Given
't := Find(r)
1: = 8.497
T := TO''t
Ans.
Parts (b), (e), and (d) are worked the same way, the only change being in the numbers of moles of products.
(b) no = 0.75 nn = 14.l07
2 2
(c) nO = l.5 nn = 16.929
2 2
(d) no = 3.0 nn = 22.571
2 2 T = 2198.6·K Ans.
T = 1950.9·K Ans.
T = 1609.2·K Ans.
(e) 50% xs air preheated to 500 degC. For this process,
L'l..Hair + L'l..H298 + L'l..Hp = 0
L'l..Hair = MCPH-(298.15 - 773.15)
For one mole of air:
MCPH(?73.15 ,298.15, 3.355 ,0.575'10~ 3,0.0,-0.016,105) = 3.65606
For 4.5/0.21 = 21.429 moles of air:
L'l..Hair = n·R·MCPH-L'l..T
L'l..Hair := 2l.429·8.314·3.65606·(298.15 - 773.15)._1_ mol
1 lI..Hair = -309399- mol
The energy balance here gives: [l.H298 + &fair + tl.Hp = 0
74
n·-
.-
A:='
3.470
3.280 .
3.639 5.457
1.5 2 2
16.929
A = 78.84
1: := 2 (guess)
0;506 1.045
. ··~1.lS?105 .K2 . 0.121.
0.040
1 B = 0.016- K
Given
t := Find(t)
1: =: 7.656
T :=: To·K1:
Ans.
4.20 n-C5H12 + 802 = 5C02 + 6H20(l)
By Eq. (4.15) with data from Table C.4:
AH298 := 5-(-393509) +6+,,2&5830) - (-'-146760)
4ff~~§~-_~~'~~':~-,~35;~i6~:~J Ans.
" ~ _. '.'~'.. ~ i, "-c_,,_,,,,··_ :~ ·,_·~L_:.::.-:
4.21 The following answers are found by application ofEq. (4.15) with data from Table C.4.
(a) -92,220 J
(b) -905,468 J
(c) -71,660 J (d) -61,980 J
(e) -367,582 J
(n) 180,500 J
(0) 178,321 J
(p) -132,439 J
(q) -44,370 J
(r) -68,910 J
75
Given
Cp(y)·(400 - 77}BTU = Q - Llli29S(Y)
y := Find(y)
y = 49.782
Whence
(lbmol H2O evaporated)
(lb H20 evap. per lb oil burned) Ans.
4.37 BASIS: One mole of product gas containing 0.242 mol HCN, and (1-0.242)/2 = 0.379 mol each ofN2 and C2H2. The energy balance is
Q = Llli = ~H298 + ~Hp
. .
.'. .. .... . .... ..... . .c: .0.242
AH298;= (2-135100 ~227480):-· .' -.-'J
. 2
Products:
[0242J
n:=' 0.379.'.
0.379
(4.736J
A:= 3280
6.132
i := 1 .. 3
R := 8.314
A = 4.7133
3 ~H298 = 5.169 x 10 J
"[1.359J' ..
B := 0.593 .\0- J
1.952
. ... ( . ..;;.0.725J.
D :=0.040.105
-1.299
B = 1.2934 x 10- 3
4 D = -6.526x 10
( -3 4)
MCPH 298.15,873.15,4.7133,1.2934·10 ,0.0,-6.526·10 = 5.22010
MCPH := 5.22010
AHp := R·MCPH·(873. 15 - 298.15)-J Q .= ~H298 + ~Hp
4 Lllip = 2.495 x 10 J
&~o:~B~.Q~'g~~~ Ans,
4.38 BASIS: 1 mole gas entering reactor, containing 0.6 mol HCI, 0.36 mol 02, and 0.04 mol N2.
Hel reacted = (0.6)(0.75) = 0.45 mol 4HCI(g) + 02(g) = 2H20(g) + 2C12(g)
For this reaction,
91
Llli298:= 2·{-241818)-4,{-92307)
Evaluate
TO:=:: 298;15
n i= .
-4
-I
i := 1.. 4
L\HS23 by Eq. (4.21) with
T:=823.15
R := 8.314
5 Llli298 = -1.144x 10
4 L\D = -8.23 x 10
( - 5 4)
1vIDCPH 298.15,823.15,-0.439,8.0'10 ,0.0,-8.23·10 = -0.72949
1vIDCPH := -0.72949
L\HS23 := Mf298 + 1vIDCPH.R.(T - To)
1: H20 2: CI2 3: HCl 4=02 2 2
L\A = -0.439
-5 Lill=8xlO
Heat transferred per mol of entering gas mixture:
4.39 C02 + C = 2CO
2C+ 02=2CO
~H823·J
Q := ·0.45
4
Mf823 = -117592
AH298a := 172459 (a)
AH298b.:;O; ...,221050 (b)
Eq. (4.21) applies to each reaction:
For (a):
i := 1.. 3
. (3376J.
A'"' .. · .. 1.17i .• • ··.5,457
92
.------~.- --- ---
1M = -0.476
-4 ,:ill = -7.02 x 10
5 Lill = 1.962 x 10
( - 4 5)
MDCPH 298.15,1148.15,-0.476,-7.02·10 ,0.0,1.962·10 = -0.410505
MDCPHa := -0.410505
. 5 ,6.H1148a := ,6.H298a + R·MDCPHa·(l 148.15 - 298. IS) AH1148a = 1.696 x 10
For (b):
.: (3.376J
A :=~:~;~
i := 1 .. 3
M = -0.429
-4 ,:ill = -9.34 x 10
5 Lill = 1.899 x 10
( - 3 5)
MDCPH 298.15,1148.15,-0.429,-0.934·10 ,0.0,1.899·10 = -0.549680
MDCPHb := -0.549680
Llli1148b:= AH298b + R·MDCPHb·(l148.15 -298.15) 5
L1H 1148b = -2.249 x 10
The combined heats of reaction must be zero:
nco ·AHl148a + no ·L1H1148b = 0
2 2
Define:
nco 2 r=--
nO
2
-Mi1148b
r:=----
AHl148a
Flue gas
Air
For 100 mol flue gas and x mol air, moles are:
Feed mix
C02 12.8
o
CO 3.7
o
12.8
3.7
93
r = 1.327
02
5.4
O.21x
5.4 + O.2lx
N2
78.1
0.79x
78.1 + 0.79x
Whence in the feed mix:
12.8
r=----
5.4+0.21·x
12.5 _ 5.4
r
x := ----·mol
X= 19.155mol
0.21
Flue gas to air ratio =
ADs.
Product composition:
nco := 3.7 + 2·(12.8 + 5.4 + 0.21·19.155)
nco > 48.145
nN := 78.1 +0.79·19.155 2
nN = 93.232 2
Mole % co=
ADs.
4.40 CH4 + 202 = C02 + 2H20(g) MI298a:::-:- -802625
CH4 + (3/2)02 = CO + 2H20(g)~298b::::: -519641 BASIS: 1 mole of fuel gas consisting of 0.94 mol CH4 and 0.06 mol N2 Air entering contains:
1.35·2·0.94 ;:;:; 2.538 mol 02
79
2.538·- = 9.548 mol N2
21
Moles C02 formed by reaction = 0.94·0.7 = 0.658
Moles CO formed by reaction = 0.94·0.3 = 0.282
llH298 := (O.658.Lill298a + O.282.llH298b}-J_ mol
5 J AHZ98 = -6.747 x 10 -
mol
94
... _-----
Moles H20 formed by reaction ;;;;
0.94· 2.0 = 1.88 3
2·0.658 + -·0.282 = 1.739 2
Moles 02 consumed by reaction =
Product gases contain the following numbers of moles:
(1) C02: 0.658 (2) CO: 0.282 (3) H20: 1.880
(4) 02: 2.538 - 1.739;;;; 0.799 (5) N2: 9.548 + 0.060 ;;;; 9.608
0.658 0.282 1.880 0.799· 9.608
i := 1 .. 5
0.040
R := 8.314._1- A = 45.4881 B = 9.6725 x 10- 3 D = -3.396 x 104
mol-K
B:=
( -3 4)
MCPH 298.15,483.15,45.4881,9.6725·10 ,0.0,-3.396·10 = 49.03091
MCPH := 49.03091
4 1 AHp = 7.541 x 10 -
mol
AHrx = -599.252 k1 mol
kg mdotH20 := 34.0·sec
AHp := R·MCPH·(483.15 - 298.15)·K
Energy balance:
From Table C.l:
.. . .. ..• kJ MIH20:=(398.0 -'- 104.8}-· .. .. .. ..kg
-AHH20' mdotH2o ndotfueI := -----AHrx
mol ndotfuel = 16.635- sec
95
Volumetric flow rate of fuel, assuming ideal gas:
ndotfue]·R·298.l5·K V:=------- 101325'Pa
Ans.
4.41 C4H8(g) = C4H6(g) + H2(g)
BASIS: 1 mole C4H8 entering, of which 33% reacts.
The unreacted C4H8 and the diluent H20 pass throught the reactor unchanged, and need not be included in the energy balance. Thus
Evaluate
1'.H798 by Eq. (4.21):
1:C4H6 2:H2 3:C4H8
' ..••. (·2.134J.
A:== •. 3.· .. 2 .. 4.9.' • -'1.967 '.
(26.786J· '.' .
. ." :3
it:~:;:t .: ·10-
"("0.0 J
D :=. 0°.8.3.'105
0.0 '.'
i:= 1.. 3
M:= L ni'A~B:= L nj·Bi i
M = 4.016 <ill = -4.422 x 10- 3 LIe = 9.91 x 10- 7 r = 8.3 x 103
MDCPH(298.15, 798.15 ,4.016, -4.422·10- 3,9.91.10-7, 0.08f·l 05) = 1.94537 MDCPH ::::: 1.94537
i'.H798 := 1'.H298 + MDCPH-R.(T - To) llH798 = 1.179 x 105_1_
mol
Q := O.33·mol·t.H798 'i
'"'\' .-
ADS.
96
Chapter 5 - Section A - Mathcad Solutions
5.2 Let the symbols Q and Work represent rates in kJ/s. Then by Eq. (5.8)
IWorkl rc
11= =1--
IQHI TH
Te :=:.323.15·K
Work:= QH{1 - ~~)
TR.:= 798.1S·K
k1 IWorkl = 148.78- s
.... . .kJ QH:==2~O·~
.. . ···s
By Eq. (5.1),
Qc := IQH] - IWorkl
Ans.
TH:= 750'K
5.3 (a) Let symbols Q and Work represent rates in kJ/s
Work := -95000· kW
By Eq. (5.8):
TC ::=: 30Q·K TC 11 :"" 1 -TH
But
(b) 11:= 0.35
Qc:= IQHI-Iworkl lWorkl
QH := -'----'-
11
QC:= IQHI - IWorkl
5.4 (a) TC:= 303.15·K TC 11 Carnot := I - - TH
TH:= 623.15·K
11 := 0.55'11 Carnot
97
11 = 0.6
IWorkl
QH : = -'-----'-
11
Ans.
Ans.
7{i;~~¥t~j~~:T9}~~ :~6\~':i~:lti~~:{i6:~~W
Ans.
Ans.
Ans.
You might also like
- Chemical Engineering Thermodynamics Solution Manual PDFDocument725 pagesChemical Engineering Thermodynamics Solution Manual PDFNaveen100% (1)
- Kinetics (Gjjkkkgty)Document5 pagesKinetics (Gjjkkkgty)Chrysler Kane DepnagNo ratings yet
- Solution Manual LevenSpiel by 13BCH@ITNUDocument370 pagesSolution Manual LevenSpiel by 13BCH@ITNUManishMakwana87% (156)
- Solution Manual For Chemical Engineering ThermodynamicsDocument4 pagesSolution Manual For Chemical Engineering ThermodynamicsBayu Prayudi Wibowo8% (36)
- Solution - Van Ness 8th Ed (Sample)Document40 pagesSolution - Van Ness 8th Ed (Sample)Demétrio Cavalcante100% (1)
- Transport Phenomena and Unit Operations - GriskeyDocument458 pagesTransport Phenomena and Unit Operations - Griskeymls333100% (12)
- Reaction Kinetics for Chemical Engineers: Butterworths Series in Chemical EngineeringFrom EverandReaction Kinetics for Chemical Engineers: Butterworths Series in Chemical EngineeringRating: 4 out of 5 stars4/5 (3)
- Coulson and Richardson’s Chemical Engineering: Volume 2A: Particulate Systems and Particle TechnologyFrom EverandCoulson and Richardson’s Chemical Engineering: Volume 2A: Particulate Systems and Particle TechnologyNo ratings yet
- Modeling in Transport Phenomena: A Conceptual ApproachFrom EverandModeling in Transport Phenomena: A Conceptual ApproachRating: 3 out of 5 stars3/5 (2)
- Thermodynamic Models for Chemical Engineering: Design, Develop, Analyse and OptimizeFrom EverandThermodynamic Models for Chemical Engineering: Design, Develop, Analyse and OptimizeNo ratings yet
- Fundamentals of Chemical Reaction EngineeringFrom EverandFundamentals of Chemical Reaction EngineeringRating: 2.5 out of 5 stars2.5/5 (3)
- Phase Equilibria in Chemical EngineeringFrom EverandPhase Equilibria in Chemical EngineeringRating: 4 out of 5 stars4/5 (11)
- Mass Transfer and Absorbers: International Series of Monographs in Chemical EngineeringFrom EverandMass Transfer and Absorbers: International Series of Monographs in Chemical EngineeringRating: 4.5 out of 5 stars4.5/5 (3)
- Fixed-Bed Reactor Design and Diagnostics: Gas-Phase ReactionsFrom EverandFixed-Bed Reactor Design and Diagnostics: Gas-Phase ReactionsRating: 4 out of 5 stars4/5 (5)
- Coulson and Richardson’s Chemical Engineering: Volume 1B: Heat and Mass Transfer: Fundamentals and ApplicationsFrom EverandCoulson and Richardson’s Chemical Engineering: Volume 1B: Heat and Mass Transfer: Fundamentals and ApplicationsRating: 3.5 out of 5 stars3.5/5 (3)
- Process Heat Transfer: Principles, Applications and Rules of ThumbFrom EverandProcess Heat Transfer: Principles, Applications and Rules of ThumbRating: 4.5 out of 5 stars4.5/5 (11)
- Coulson and Richardson’s Chemical Engineering: Volume 3B: Process ControlFrom EverandCoulson and Richardson’s Chemical Engineering: Volume 3B: Process ControlRating: 2 out of 5 stars2/5 (1)
- Computational Flow Modeling for Chemical Reactor EngineeringFrom EverandComputational Flow Modeling for Chemical Reactor EngineeringRating: 3 out of 5 stars3/5 (3)
- Coulson and Richardson’s Chemical Engineering: Volume 1A: Fluid Flow: Fundamentals and ApplicationsFrom EverandCoulson and Richardson’s Chemical Engineering: Volume 1A: Fluid Flow: Fundamentals and ApplicationsRating: 2 out of 5 stars2/5 (2)
- Coulson and Richardson’s Chemical Engineering: Volume 3A: Chemical and Biochemical Reactors and Reaction EngineeringFrom EverandCoulson and Richardson’s Chemical Engineering: Volume 3A: Chemical and Biochemical Reactors and Reaction EngineeringR. RaviRating: 5 out of 5 stars5/5 (1)
- Elementary Chemical Reactor Analysis: Butterworths Series in Chemical EngineeringFrom EverandElementary Chemical Reactor Analysis: Butterworths Series in Chemical EngineeringRating: 5 out of 5 stars5/5 (2)
- Mathcad Solutions Chapter 2 Section ADocument11 pagesMathcad Solutions Chapter 2 Section AFaris Naufal100% (1)
- Prob 4.44 Table Lookups Given Values: R and V Are Constant So..Document5 pagesProb 4.44 Table Lookups Given Values: R and V Are Constant So..tkshoeNo ratings yet
- Chapter5 ADocument21 pagesChapter5 ANic BlandoNo ratings yet
- CH 10Document34 pagesCH 10hirenpatel_universalNo ratings yet
- Chapter 1 - Section A - Mathcad SolutionsDocument4 pagesChapter 1 - Section A - Mathcad SolutionsdjeofitraNo ratings yet
- Introduction To Statistical Physics Solution Manual: Kerson HuangDocument105 pagesIntroduction To Statistical Physics Solution Manual: Kerson Huangsumivrindavan100% (7)
- Statistical Physics Solution ManualDocument105 pagesStatistical Physics Solution Manualgeorgeattack100% (4)
- Self Assessment Solutions Tutorial 7 Self Assessment Exercise No. 1Document5 pagesSelf Assessment Solutions Tutorial 7 Self Assessment Exercise No. 1Alexander MugabeNo ratings yet
- Zeroth Law and Introductory ConceptsDocument13 pagesZeroth Law and Introductory ConceptsMohd Danial Muhd AliNo ratings yet
- Mathcad - Pond Hex - FinalDocument6 pagesMathcad - Pond Hex - FinalMohammed A IsaNo ratings yet
- Thermo Homework 6Document7 pagesThermo Homework 6Danny BoyleNo ratings yet
- Casa de Dos Pisos EtabsDocument19 pagesCasa de Dos Pisos EtabsYuber Tacuri CristobalNo ratings yet
- Herrera Alisson Deber3 EjerciciosBraytonEESDocument11 pagesHerrera Alisson Deber3 EjerciciosBraytonEESAly HerreraNo ratings yet
- Paper Thermo Mechanical EngineeringDocument14 pagesPaper Thermo Mechanical EngineeringAdif HerawanNo ratings yet
- Calculating thermodynamic properties of an ideal gas undergoing cyclic processesDocument8 pagesCalculating thermodynamic properties of an ideal gas undergoing cyclic processesBoddupalli Lohith KumarNo ratings yet
- Chapter 5 - Section A - Mathcad Solutions: 5.2 Let The Symbols Q and Work Represent Rates in Kj/s. Then by Eq. (5.8)Document21 pagesChapter 5 - Section A - Mathcad Solutions: 5.2 Let The Symbols Q and Work Represent Rates in Kj/s. Then by Eq. (5.8)light2618No ratings yet
- School of Chemical Engineering: Chem 251 Assignment 1 and 2Document16 pagesSchool of Chemical Engineering: Chem 251 Assignment 1 and 2Keevani NaidooNo ratings yet
- Tutorial 3 - Question 8Document2 pagesTutorial 3 - Question 8DiablofireZANo ratings yet
- Solution To Exam No. 2Document5 pagesSolution To Exam No. 2mozam haqNo ratings yet
- Engineering Mechanics: Second PartDocument9 pagesEngineering Mechanics: Second Partاحمد سلمان عزيز , مسائيCNo ratings yet
- Introduction To Convection: Flow and Thermal ConsiderationsDocument29 pagesIntroduction To Convection: Flow and Thermal ConsiderationsChris MustacchioNo ratings yet
- Bio Process Engineering Principles (Solutions Manual) - P. Doran (1997) WWDocument170 pagesBio Process Engineering Principles (Solutions Manual) - P. Doran (1997) WWAmm Roeh78% (23)
- CH 11Document22 pagesCH 11Ingenio MetalurgiaNo ratings yet
- Solution Manual For Fluid Mechanics and Thermodynamics of Turbomachinery 7th Ed Sydney Lawrence Dixon Cesare Hall PDF FreeDocument10 pagesSolution Manual For Fluid Mechanics and Thermodynamics of Turbomachinery 7th Ed Sydney Lawrence Dixon Cesare Hall PDF FreePIYUSH PORWALNo ratings yet
- Repaso de QI MartesDocument8 pagesRepaso de QI MartesCriss TorresNo ratings yet
- Porter Easter Ling SolutionsDocument74 pagesPorter Easter Ling SolutionsKrishna Mahajan60% (10)
- Solution Manual for an Introduction to Equilibrium ThermodynamicsFrom EverandSolution Manual for an Introduction to Equilibrium ThermodynamicsNo ratings yet
- Ten-Decimal Tables of the Logarithms of Complex Numbers and for the Transformation from Cartesian to Polar Coordinates: Volume 33 in Mathematical Tables SeriesFrom EverandTen-Decimal Tables of the Logarithms of Complex Numbers and for the Transformation from Cartesian to Polar Coordinates: Volume 33 in Mathematical Tables SeriesNo ratings yet
- Tables of Coefficients for the Analysis of Triple Angular Correlations of Gamma-Rays from Aligned NucleiFrom EverandTables of Coefficients for the Analysis of Triple Angular Correlations of Gamma-Rays from Aligned NucleiNo ratings yet
- AIGA 060 - 11 PSA Membrane O2 N2 Generators - Reformated Jan 12Document35 pagesAIGA 060 - 11 PSA Membrane O2 N2 Generators - Reformated Jan 12Surya Budi WidagdoNo ratings yet
- Surface Material Roughness Coefficients - ε Values in mmDocument1 pageSurface Material Roughness Coefficients - ε Values in mmSurya Budi WidagdoNo ratings yet
- CO2 By-Product Recovery SystemsDocument2 pagesCO2 By-Product Recovery SystemsSurya Budi WidagdoNo ratings yet
- 14 1 Fritz MunderDocument23 pages14 1 Fritz MunderSurya Budi WidagdoNo ratings yet
- Natural Gas Storage TankDocument10 pagesNatural Gas Storage TankSurya Budi WidagdoNo ratings yet
- PTDocument6 pagesPTSurya Budi WidagdoNo ratings yet
- Convert Between Energy Units Like Btu, Therm and Cubic FeetDocument3 pagesConvert Between Energy Units Like Btu, Therm and Cubic FeetSurya Budi WidagdoNo ratings yet
- VaporizerDocument13 pagesVaporizerSurya Budi Widagdo100% (1)
- A-S-P-E-N-O-N-E V7.1Document14 pagesA-S-P-E-N-O-N-E V7.1Surya Budi WidagdoNo ratings yet
- MPCDocument250 pagesMPCPrathak JienkulsawadNo ratings yet
- Aspen MUSE GettingstartedDocument27 pagesAspen MUSE GettingstartedSurya Budi WidagdoNo ratings yet
- MPC Gui ManualDocument109 pagesMPC Gui ManualSurya Budi WidagdoNo ratings yet
- Customizing RADFRAC ConvergenceDocument22 pagesCustomizing RADFRAC ConvergenceSurya Budi Widagdo75% (4)
- AbsorberDocument15 pagesAbsorberShri VardhanNo ratings yet
- Air Separation JurnalDocument1 pageAir Separation JurnalSurya Budi WidagdoNo ratings yet
- MPC 5Document63 pagesMPC 5Surya Budi WidagdoNo ratings yet
- ACM Examples GuideDocument240 pagesACM Examples GuideSurya Budi Widagdo100% (8)
- ACM Aspen Modeler ReferenceDocument410 pagesACM Aspen Modeler ReferenceSurya Budi Widagdo100% (3)
- MPC 3Document165 pagesMPC 3Surya Budi WidagdoNo ratings yet
- MPC 2Document141 pagesMPC 2Surya Budi WidagdoNo ratings yet
- An Introduction To Nonlinear Model Predictive ControlDocument23 pagesAn Introduction To Nonlinear Model Predictive ControlSohibul HajahNo ratings yet
- ACM Polymer Simulations With Polymers PlusDocument64 pagesACM Polymer Simulations With Polymers PlusSurya Budi Widagdo100% (1)
- ACM Modeling Language ReferenceDocument193 pagesACM Modeling Language ReferenceSurya Budi Widagdo100% (1)
- MPC 4Document36 pagesMPC 4Surya Budi WidagdoNo ratings yet
- OVERVIEW OF PROCESS CONTROL Part 1Document80 pagesOVERVIEW OF PROCESS CONTROL Part 1NOUREDINE MorsliNo ratings yet
- ACM Library Reference GuideDocument181 pagesACM Library Reference GuideSurya Budi WidagdoNo ratings yet
- Linear Control of Nonlinear ProcessesDocument23 pagesLinear Control of Nonlinear ProcessesSurya Budi WidagdoNo ratings yet
- Transport Phenomena 2nd Ed by Bird Stewart Lightfoot (Solution Manual)Document761 pagesTransport Phenomena 2nd Ed by Bird Stewart Lightfoot (Solution Manual)Adibah Hani Azit90% (42)
- Aspen Custom Modeler 2004.1: Dmcplus Controllers Interface GuideDocument42 pagesAspen Custom Modeler 2004.1: Dmcplus Controllers Interface GuideSurya Budi WidagdoNo ratings yet
- MCB Specification Bsen60898Document6 pagesMCB Specification Bsen60898jayswamiiNo ratings yet
- Product Information: X-Ray Tube DG-073B-DC DG-073B-AC Stationary Anode X-Ray TubeDocument6 pagesProduct Information: X-Ray Tube DG-073B-DC DG-073B-AC Stationary Anode X-Ray TubeMohamedKecibaNo ratings yet
- Bladelesswindturbine 161123184636 PDFDocument25 pagesBladelesswindturbine 161123184636 PDFmansadaboobackerNo ratings yet
- Ex Plakat enDocument1 pageEx Plakat enFarshid Ahmadian YazdiNo ratings yet
- ALSTOM Gas Insulated Switchgear Solutions Up to 800kVDocument12 pagesALSTOM Gas Insulated Switchgear Solutions Up to 800kVgovindarulNo ratings yet
- ME 188 - Combined Brayton & Rankine CyclesDocument44 pagesME 188 - Combined Brayton & Rankine CyclesAzherRoiFerrerNo ratings yet
- Bus Engines - YC-Europe GMBHDocument9 pagesBus Engines - YC-Europe GMBHsameh aboulsoudNo ratings yet
- Cheema Boiler Limited: Presented To: MR - Deepak Bhandari (Document32 pagesCheema Boiler Limited: Presented To: MR - Deepak Bhandari (angenious100% (1)
- Estimating Design Parameters in Propane Refrigerant Systems - Pumps & SystemsDocument6 pagesEstimating Design Parameters in Propane Refrigerant Systems - Pumps & Systemsm.shehreyar.khanNo ratings yet
- PTC B1.1 Notes - Sub Module 15.12 (Air Systems)Document27 pagesPTC B1.1 Notes - Sub Module 15.12 (Air Systems)sohail aviatorNo ratings yet
- Excess Air CalculationDocument11 pagesExcess Air CalculationKarthic Keyan50% (2)
- Testing of high-voltage equipmentDocument56 pagesTesting of high-voltage equipmentYayan RnsNo ratings yet
- Eaton EX 7LEX 7li Capacitor Brochure en US 2Document4 pagesEaton EX 7LEX 7li Capacitor Brochure en US 2Huỳnh Hải KhánhNo ratings yet
- Cover Type B Exam for Electrical MachinesDocument4 pagesCover Type B Exam for Electrical MachinesT MNo ratings yet
- Diesel Fuel InjectionDocument5 pagesDiesel Fuel Injectiondamith21No ratings yet
- Stretch Wrap PackerDocument139 pagesStretch Wrap Packerthanh_cdt01100% (1)
- ATA 24 Electrical Power L1Document40 pagesATA 24 Electrical Power L1jimbokhepelNo ratings yet
- Manpower Details For O&M of Power PlantDocument3 pagesManpower Details For O&M of Power PlantNaresh Pattanaik100% (1)
- DSP 1 (Monofasico)Document2 pagesDSP 1 (Monofasico)alperda100% (2)
- Boiler Efficiency CalculationDocument5 pagesBoiler Efficiency CalculationSuparna Bhose100% (3)
- Design of Brakes: Energy Equations in BrakesDocument27 pagesDesign of Brakes: Energy Equations in BrakesManoj SP100% (1)
- 2007 iGCSE Physics Written Paper Question PaperDocument28 pages2007 iGCSE Physics Written Paper Question Paperoscar wildeNo ratings yet
- SCI19 - Q4 - M4 - Heat and WorkDocument10 pagesSCI19 - Q4 - M4 - Heat and WorklyzaNo ratings yet
- Bender VMD461 Anleitung EN PDFDocument10 pagesBender VMD461 Anleitung EN PDFAlexandru IonNo ratings yet
- GFMJ-300 VRLA Gel Battery: Main Applications DimensionsDocument2 pagesGFMJ-300 VRLA Gel Battery: Main Applications Dimensionsrcam37170No ratings yet
- 2015 JC Physics H2 Anderson Junior College PDFDocument79 pages2015 JC Physics H2 Anderson Junior College PDFeternal vorceNo ratings yet
- Ac Servo MotorDocument10 pagesAc Servo MotorJoshua AluroNo ratings yet
- By: Pravin Kumar JaisawalDocument22 pagesBy: Pravin Kumar JaisawalJaisawal PravinNo ratings yet
- Edward k12 Mechdesign DataDocument6 pagesEdward k12 Mechdesign Dataapi-254586275No ratings yet
- The Role of Solar Home Systems in Rural DevelopmentDocument101 pagesThe Role of Solar Home Systems in Rural DevelopmentSPD SOCOTECO 2No ratings yet