Professional Documents
Culture Documents
Rate of Solubiliyu
Uploaded by
Aj BascosOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Rate of Solubiliyu
Uploaded by
Aj BascosCopyright:
Available Formats
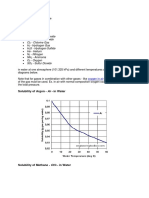
Solubility of pure gases like
O Ar - Argon
O CH4 - Methane
O C2H4 - Ethylene
O C2H6- Ethane
O CO - Carbon Monoxide
O CO2 - Carbon Dioxide
O Cl2 - Chlorine Gas
O H2 - Hydrogen Gas
O H2S - Hydrogen Sulfide
O He - Helium
O 2 - itrogen
O H3 - Ammonia
O O2 - Oxygen
O SO2 - Sulfur Dioxide
in water at one atmosphere (101.325 kPa) and different
temperatures are indicated in the diagrams below.
Note that for gases in combination with other gases -
like oxygen in air - the partial pressure of the gas must be
used. Ex. in air with normal composition oxygen counts for
aprox. 20% of the total pressure.
SoIubiIity of Argon - 7- in Water
SoIubiIity of Methane - CH4- in Water
SoIubiIity of EthyIene - C2H4- in Water
SoIubiIity of Ethane - C2H6- in Water
SoIubiIity of Carbon Monoxide - CO - in Water
SoIubiIity of Carbon Dioxide - CO2 - in Water
SoIubiIity of ChIorine Gas - Cl2 - in Water
SoIubiIity of Hydrogen Gas -H2- in Water
SoIubiIity of Hydrogen SuIfide - H2S - in Water
SoIubiIity of HeIium - He - in Water
SoIubiIity of Nitrogen - 2 - in Water
SoIubiIity of Ammonia - H3 - in Water
SoIubiIity of Oxygen - O2 - in Water
SoIubiIity of SuIfur Dioxide - SO2- in Water
Ar Argon has approximately the
same solubility in water as oxygen gas and is
2.5 times more soluble in water than
nitrogen gas
CH
4
Methane mg/L (17 C)
C
2
H
4
Ethylene mg/100 mL (17 C);
2 mg/
C
2
H
6
- Ethane very Iow
CO - Carbon Monoxide 2 mg/100 mL (20
C)
CO
2
- Carbon Dioxide 1 g/L at 2 C, 100
kPa
Cl
2
- Chlorine Gas
H
2
- Hydrogen Gas
H
2
S - Hydrogen Sulfide g dm
-
(at 20 C)
He Helium
2
- itrogen
H
3
Ammonia 7% (0 C); 1% (2 C);
28% (0 C)
O
2
- Oxygen
SO
2
- Sulfur Dioxide g dm
-
You might also like
- Dissolved Gas Concentration in Water: Computation as Functions of Temperature, Salinity and PressureFrom EverandDissolved Gas Concentration in Water: Computation as Functions of Temperature, Salinity and PressureNo ratings yet
- Solubility of Pure GasesDocument8 pagesSolubility of Pure GasesBindu joglekarNo ratings yet
- 4.5 NotesDocument30 pages4.5 NotesMaria OnisorNo ratings yet
- Ch7Summary AlcoholDocument6 pagesCh7Summary AlcoholdanielmahsaNo ratings yet
- BD Chap4Document9 pagesBD Chap4Joko SantosoNo ratings yet
- Water Chemsitry CourseDocument44 pagesWater Chemsitry CourseMalik HazaaNo ratings yet
- Determination of Co2 in Water2 PDFDocument4 pagesDetermination of Co2 in Water2 PDFWaleed EmaraNo ratings yet
- General Chemistry-Non-Metals OxygenDocument4 pagesGeneral Chemistry-Non-Metals OxygenMarvin IdigaNo ratings yet
- Patterns and Properties of Non-MetalsDocument14 pagesPatterns and Properties of Non-MetalsiluvreadingbooksNo ratings yet
- Aldehyde Ketone and AcidDocument15 pagesAldehyde Ketone and AcidAbir DuttaNo ratings yet
- Alcohols 1Document13 pagesAlcohols 1Suresh VedpathakNo ratings yet
- Aldehydes, Ketones, Carboxylic Acids: R-COH Aldehyde R-CO-R Ketone R-CoohDocument17 pagesAldehydes, Ketones, Carboxylic Acids: R-COH Aldehyde R-CO-R Ketone R-CoohMoshe Cohen'sNo ratings yet
- N, Co, O and Inert/ Noble Gases. Air Also Contains Very Small Traces of Methane Gas, S, So, CO and Oxides of Nitrogen E.G. NO, N O, NoDocument16 pagesN, Co, O and Inert/ Noble Gases. Air Also Contains Very Small Traces of Methane Gas, S, So, CO and Oxides of Nitrogen E.G. NO, N O, NotavongaNo ratings yet
- 5 Hydrocarbon Derivatives 2Document28 pages5 Hydrocarbon Derivatives 2Marivic TayabanNo ratings yet
- Organic Reactions Summary SheetDocument2 pagesOrganic Reactions Summary Sheetthacheee64% (11)
- Aldehydes & KetonesDocument104 pagesAldehydes & KetonesCharin Kadian75% (4)
- Carbon Compounds DiagramDocument1 pageCarbon Compounds Diagramamin_zamanNo ratings yet
- Carbonate SystemDocument64 pagesCarbonate Systemmohan kumarNo ratings yet
- COD Practical 6Document11 pagesCOD Practical 6Gilbert NdibeNo ratings yet
- I. Tittle: Aldehyde and Ketone II. Date of Experiment: 13th Mart 2013 Iii. PurposeDocument27 pagesI. Tittle: Aldehyde and Ketone II. Date of Experiment: 13th Mart 2013 Iii. PurposeNurel HidayahNo ratings yet
- Alcohols From Carbonyl Compounds: Oxidation-Reduction: Central Linking Role of Alcohols and CarbonylsDocument12 pagesAlcohols From Carbonyl Compounds: Oxidation-Reduction: Central Linking Role of Alcohols and CarbonylsAmihanNo ratings yet
- 1B Carbonyl CompoundsDocument14 pages1B Carbonyl CompoundsRida Nadeem SheikhNo ratings yet
- Natural Gas ProcessingDocument35 pagesNatural Gas ProcessingAleem AhmedNo ratings yet
- Carboxylic AcidDocument6 pagesCarboxylic AcidVishu AgrawalNo ratings yet
- 5) Question and AnswerDocument4 pages5) Question and Answeramanxboat18No ratings yet
- Chem Class12 Chapter 8Document16 pagesChem Class12 Chapter 8rohithardy45No ratings yet
- Role of Acids in The Degradation of Alkanolamine During Co2Document7 pagesRole of Acids in The Degradation of Alkanolamine During Co2cargscribNo ratings yet
- Gases and Vapours@rautbholaDocument12 pagesGases and Vapours@rautbholaBholakant RautNo ratings yet
- DR Mike Thompson: Winchester College, UKDocument4 pagesDR Mike Thompson: Winchester College, UKazilatulNo ratings yet
- ALKANOLSDocument25 pagesALKANOLSKoki KingNo ratings yet
- Gaseous FuelDocument20 pagesGaseous FuelCaguioa Mark Anthony G.100% (3)
- Class 12 Aldehydes, Ketones and Carboxylic AcidsDocument20 pagesClass 12 Aldehydes, Ketones and Carboxylic Acidsst06082005No ratings yet
- Acids Bases and SaltsDocument19 pagesAcids Bases and SaltsCaron Asgarali100% (1)
- Carbonyl Compounds Xi Xii Study MaterialsDocument171 pagesCarbonyl Compounds Xi Xii Study MaterialsCristiano Hamdiansyah SempadianNo ratings yet
- Organic Chemistry 2Document2 pagesOrganic Chemistry 2nila95No ratings yet
- AcetaldehydeDocument98 pagesAcetaldehydeEr Bali Pandhare89% (9)
- Research ArticleDocument6 pagesResearch ArticleJoão Pedro GomesNo ratings yet
- Nucleophilic Subs: AlcoholDocument11 pagesNucleophilic Subs: AlcoholZhiro Ming HanNo ratings yet
- Jaglerod Mono-Di OksidDocument10 pagesJaglerod Mono-Di OksidLiljana DimeskaNo ratings yet
- Natural Gas TechnologyDocument16 pagesNatural Gas TechnologyNabaa M. Al-KhazrajiNo ratings yet
- Module 5 Aldehydes, Ketones, and Carboxylic Acids PDFDocument19 pagesModule 5 Aldehydes, Ketones, and Carboxylic Acids PDFRica Pearl ZorillaNo ratings yet
- Aldehid Keton 08Document49 pagesAldehid Keton 08Mochamad Herdi NurzamanNo ratings yet
- Aldehydes and Ketones FinalDocument67 pagesAldehydes and Ketones FinalAnil Kumar VermaNo ratings yet
- Part 3 - LNG ProcessDocument33 pagesPart 3 - LNG ProcessPuspita Oktaviani MarthaNo ratings yet
- Organic Chem CoreDocument19 pagesOrganic Chem CoreanorchiespendiloveNo ratings yet
- Lecture-11 F&C (Gaseous Fuels)Document44 pagesLecture-11 F&C (Gaseous Fuels)Mritunjay SinghNo ratings yet
- O C SPA S A S: Rganic Hemistry Kill UmmaryDocument6 pagesO C SPA S A S: Rganic Hemistry Kill Ummaryhasan_j688675No ratings yet
- Alcohols & Phenols:: GeneralizationsDocument27 pagesAlcohols & Phenols:: GeneralizationsdoudoudoudouNo ratings yet
- Chapter 7 Carbonyl CompoundsDocument7 pagesChapter 7 Carbonyl CompoundsJacqueen0330No ratings yet
- Organic Chemistry 2 PDFDocument15 pagesOrganic Chemistry 2 PDFEnica RichardNo ratings yet
- Classification Tests For Hydroxyl - and Carbonyl - Containing CompoundsDocument6 pagesClassification Tests For Hydroxyl - and Carbonyl - Containing CompoundsShaira Jhann L. Rosales50% (2)
- Alcohols, Phenols and EpoxidesDocument134 pagesAlcohols, Phenols and EpoxidesStudent 365100% (1)
- Gcse Chemistry: UNIT 1.8: FactfileDocument14 pagesGcse Chemistry: UNIT 1.8: FactfileCrystalNo ratings yet
- Oxygen04Document42 pagesOxygen04Enzo TapingNo ratings yet
- Carboxylic AcidsDocument19 pagesCarboxylic AcidsElizabeth VivarNo ratings yet
- 11 Biology Respiration in Plants Test 01 Answer 0k2aDocument5 pages11 Biology Respiration in Plants Test 01 Answer 0k2aJaskirat SinghNo ratings yet
- Alkalinity PDFDocument38 pagesAlkalinity PDFK PushyanthNo ratings yet
- Alcohols: Which of The Structures Is/are Classified As Phenols?Document7 pagesAlcohols: Which of The Structures Is/are Classified As Phenols?Kaviraj SinghNo ratings yet
- Scientific InquiryDocument10 pagesScientific InquiryAj BascosNo ratings yet
- By The Decarboxylation of Sodium BenzoateDocument2 pagesBy The Decarboxylation of Sodium BenzoateAj BascosNo ratings yet
- Work Energy and PowerDocument6 pagesWork Energy and Powerashrafrazi100% (1)
- 1ST Summative ExercisesDocument3 pages1ST Summative ExercisesAj BascosNo ratings yet
- Hydrogen 1Document26 pagesHydrogen 1Aj BascosNo ratings yet
- Javascript Book For DummiesDocument1 pageJavascript Book For DummiesAj BascosNo ratings yet
- A Mandate To SatanDocument1 pageA Mandate To SatanAj BascosNo ratings yet
- Table ContentDocument2 pagesTable ContentAj BascosNo ratings yet
- WakaDocument2 pagesWakaAj BascosNo ratings yet
- MAPEHDocument10 pagesMAPEHAj BascosNo ratings yet