Professional Documents
Culture Documents
Energetics of Displacement Reaction (ΔH = -219 kJ/mol
Uploaded by
newacademy234Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Energetics of Displacement Reaction (ΔH = -219 kJ/mol
Uploaded by
newacademy234Copyright:
Available Formats
Topic 10/9: Energetics
Hazard Code A
The enthalpy change of a displacement reaction
Zinc is more reactive than copper, so when zinc is added to copper(II) sulphate solution, copper is displaced. This reaction is associated with an enthalpy change and enthalpy is converted into heat energy. The heat energy warms up the surroundings (which in this case is the water in which the reaction takes place). Requirements: 25 cm measuring cylinder watch glass stop clock
2.5g of zinc powder 0-100C thermometer
3
Procedure: a) Measure out 25 cm of 1.0M copper (II) sulphate solution in a measuring cylinder and pour it into an expanded polystyrene cup. b) Stir with a thermometer, start a stop clock and measure the temperature every 30 seconds. c) Meanwhile weigh out about 2.5g of zinc powder onto a watch glass. Record the mass. d) At three minutes (when the temperature of the solution is constant) add all of the zinc powder to the cup and stir carefully with the thermometer. Continue to stir and record the temperature every 30 seconds until the temperature has passed its maximum and has been falling for two minutes. e) Note any changes to the appearance of the solution and the solid and then dispose of the contents of the cup into the container provided.
3
25cm3 of 1.0M copper (II) sulphate solution
expanded polystyrene cup
Safety: No particular hazards
Results Record your results (and any additional observations) in your rough book using a table based on the template below. When you have finished the experiment and cleared away your apparatus, copy your results and the completed table into your exercise book after this sheet. Mass of zinc = Time (mins) Temp (C) 0.0 g 0.5 1.0 1.5 2.0 2.5 3.0 Add Zn Temperature rise (T) = Maximum temp - Temp before zinc was added = C 3.5
Analysis Answer the following questions after the results in your exercise book, using full sentences and showing your working for calculations. 1. 2. 3. 4. 5. 6. 7. Calculate the number of moles of copper(II) sulphate in 25cm of 1.0M copper (II) sulphate solution. Calculate the number of moles of zinc in the mass you weighed out. [Ar(Zn) = 65] Write a balanced equation for the reaction of zinc with copper(II) sulphate (CuSO4) solution. How many moles of zinc would react with one mole of copper(II) sulphate? By considering your answers to (1), (2) and (4), state which reagent is in excess. How many moles of each substance will actually have reacted? The energy required to raise the temperature of 1g (or 1cm ) of water by 1C is 4.2J. Calculate the energy 3 required to raise the temperature of 25cm of your solution by T using the equation: 3 Energy absorbed by solution = (mass of solution) x 4.2 x T [assume that mass (in g) = volume (in cm )] Where has this energy come from? Calculate the enthalpy change (H) for this displacement reaction in kJ mol . Remember that your answer to (7) will be in J (not kJ) and that you used much less than 1 mole of reactant.
-1 -1 3 3
8. 9.
10. Is the reaction exothermic or endothermic. Will the sign of H be positive or negative? 11. The accepted value of H for this reaction is -219 kJ mol . Give two reasons for the difference between your answer and this value. 12. Suggest how you could improve the experiment to obtain a more accurate value.
11/01/2005
You might also like
- IA - Enthalpy Change Copper Sulfate and ZincDocument9 pagesIA - Enthalpy Change Copper Sulfate and Zincannehindenberg0% (1)
- Electrolysis of Copper SulphateDocument3 pagesElectrolysis of Copper Sulphatewscience100% (1)
- Maintenance & Use Copper-SulphateDocument3 pagesMaintenance & Use Copper-SulphateanthonyazNo ratings yet
- Chlorination of MethaneDocument11 pagesChlorination of MethaneEngr Abuzar KhanNo ratings yet
- WM Chemistry Ia Final Risma RemsudeenDocument12 pagesWM Chemistry Ia Final Risma RemsudeenPriyanshi PeelwanNo ratings yet
- Investigation The Relationship Between Hydrochloric Acid and Sodium ThiosulphateDocument9 pagesInvestigation The Relationship Between Hydrochloric Acid and Sodium Thiosulphatelena0% (1)
- Topic 6 Using Hess Law Sodium Hydrogen CarbonateDocument1 pageTopic 6 Using Hess Law Sodium Hydrogen CarbonatechuralaNo ratings yet
- Chem Lab - A Velocity Constant TitrationDocument6 pagesChem Lab - A Velocity Constant TitrationMiguel Ackah-Yensu50% (2)
- Finding The Enthalpy of The Displacement Reaction of Zinc and Copper Sulfate SolutionDocument2 pagesFinding The Enthalpy of The Displacement Reaction of Zinc and Copper Sulfate SolutionBen Chalmers100% (2)
- Enthalpy Formation CaCO3Document7 pagesEnthalpy Formation CaCO3saNo ratings yet
- Two-Phase Component Equilibrium AnalysisDocument15 pagesTwo-Phase Component Equilibrium AnalysisNur Halimah75% (4)
- LR 1 Electrolysis of Copper SulphateDocument6 pagesLR 1 Electrolysis of Copper SulphateCloud D. LuffyNo ratings yet
- Chem IA NUMERO 5Document4 pagesChem IA NUMERO 5BrittanyNo ratings yet
- UTAR Chem Lab 1 Full Report Exp12Document7 pagesUTAR Chem Lab 1 Full Report Exp12Izykiel EdwardNo ratings yet
- Titus John - Enthalpy Prac ReportDocument12 pagesTitus John - Enthalpy Prac Reportapi-295071132No ratings yet
- Adsorption IsothermDocument4 pagesAdsorption Isothermahkiujtsw0% (1)
- Joshua Haholongan - Science Rate of Reaction ReportDocument13 pagesJoshua Haholongan - Science Rate of Reaction ReportJoshua HaholonganNo ratings yet
- Rate of ReactionDocument9 pagesRate of ReactionShamshul Didarelly0% (1)
- WM Final Chemistry Ia Watermark 1Document12 pagesWM Final Chemistry Ia Watermark 1ppNo ratings yet
- Reaction RateDocument19 pagesReaction RateMuhd Hafiz NizamNo ratings yet
- Transition Temperature of Salt HydratesDocument2 pagesTransition Temperature of Salt HydratesDelin Shaji JohnNo ratings yet
- Diffusion of Solids in Gases Chemistry ProjectDocument11 pagesDiffusion of Solids in Gases Chemistry ProjectSiddharth KUMARNo ratings yet
- Lab Experimental Determination of The Molar Volume of A Gas RevisedDocument3 pagesLab Experimental Determination of The Molar Volume of A Gas RevisedQueenieTantiongcoNo ratings yet
- Yeast Fermentation Rate Peak at 45°CDocument3 pagesYeast Fermentation Rate Peak at 45°CRuifa Huang100% (1)
- Job's Method Determination of Complex StoichiometryDocument3 pagesJob's Method Determination of Complex StoichiometryVaid RahulNo ratings yet
- Kinetic Study of the Iodide-Iron ReactionDocument4 pagesKinetic Study of the Iodide-Iron ReactionStefani KavangoNo ratings yet
- IA Chemistry AssignmentDocument10 pagesIA Chemistry AssignmentAlexander ZinchenkoNo ratings yet
- Iron-Copper Single Replacement ReactionDocument9 pagesIron-Copper Single Replacement Reactionomaralvarezronaldinho100% (7)
- Elevation in Boiling Point Chemistry ProjectDocument11 pagesElevation in Boiling Point Chemistry ProjectShivam ShuklaNo ratings yet
- Experiment 5 - Rates of ReactionDocument38 pagesExperiment 5 - Rates of ReactionAmy WuNo ratings yet
- Experiment 7: IN IN IN INDocument5 pagesExperiment 7: IN IN IN INLimYuEnNo ratings yet
- Lab, Solubility and ThermodynamicsDocument8 pagesLab, Solubility and ThermodynamicsAna PaulaNo ratings yet
- Calcium Carbonate Formation EnthalpyDocument11 pagesCalcium Carbonate Formation Enthalpystephenliyuting_1992100% (2)
- Applications of Redox ReactionsDocument50 pagesApplications of Redox ReactionsMlamuli MlarhNo ratings yet
- Exp 1Document4 pagesExp 1Varshni VsNo ratings yet
- Rate ReactionDocument10 pagesRate ReactionTsabit AlbananiNo ratings yet
- Enthalpy of VaporizationDocument6 pagesEnthalpy of VaporizationseirieNo ratings yet
- 6 Good Tritration PDFDocument5 pages6 Good Tritration PDFUjak KimiaNo ratings yet
- Acid Base TitrationDocument4 pagesAcid Base TitrationNeeta PandeyNo ratings yet
- Bromine ClockDocument5 pagesBromine ClockOCRChemistrySalters0% (1)
- Iodination of Acetone Rate DeterminationDocument4 pagesIodination of Acetone Rate DeterminationLevy Medina TrayaNo ratings yet
- IB Chem IA Bleach PDFDocument17 pagesIB Chem IA Bleach PDFsushma111No ratings yet
- Practical Guide Edexcel2Document42 pagesPractical Guide Edexcel2Hady JawadNo ratings yet
- Practical Guide EdexcelDocument43 pagesPractical Guide EdexcelUsman BokhariNo ratings yet
- Benzaldehyde Cannizzaro Reaction ProductsDocument5 pagesBenzaldehyde Cannizzaro Reaction ProductsUsman GhaniNo ratings yet
- Some Basic Concepts of ChemistryDocument12 pagesSome Basic Concepts of ChemistryNikhil BhattNo ratings yet
- Voltaic Cell Design Lab - How Temperature Affects VoltageDocument2 pagesVoltaic Cell Design Lab - How Temperature Affects VoltageTheVioletFrost83% (6)
- Chem Practice IADocument9 pagesChem Practice IAnarakhantiNo ratings yet
- The Heat of Solution LabDocument4 pagesThe Heat of Solution Labapi-310957734No ratings yet
- To Synthesize Potassium Tri Oxalato Ferr PDFDocument5 pagesTo Synthesize Potassium Tri Oxalato Ferr PDFApheleleNo ratings yet
- Cement AnalysisDocument4 pagesCement AnalysisDaryl McCollNo ratings yet
- VLE of Methanol-Water MixtureDocument14 pagesVLE of Methanol-Water MixtureHafiniHambaliNo ratings yet
- Magnesium and hydrochloric acid (model) - Key factors affecting reaction ratesDocument3 pagesMagnesium and hydrochloric acid (model) - Key factors affecting reaction ratesEduar E Perez RojasNo ratings yet
- Pchem ExamDocument8 pagesPchem ExamDanielson CulanibanNo ratings yet
- Rate of Reaction Between Calcium Carbonate and Hydrochloric AcidDocument6 pagesRate of Reaction Between Calcium Carbonate and Hydrochloric AcidSimon WayneNo ratings yet
- Lab Report (Final Editied)Document8 pagesLab Report (Final Editied)Alexia Channer100% (4)
- IA Chemistry 2 Rate of ReactionDocument2 pagesIA Chemistry 2 Rate of ReactionAldo Hamka0% (1)
- 6BEnergeticsZnCuSO4 Christopher Golding Lab 4Document6 pages6BEnergeticsZnCuSO4 Christopher Golding Lab 4Mr. CrustNo ratings yet
- EnergeticsDocument9 pagesEnergeticsrichardNo ratings yet
- CHEMCAD TutorialDocument33 pagesCHEMCAD TutorialIgnatius Eko HarwinantoNo ratings yet
- Solutions Manual For Thermodynamics and Chemistry: Howard DevoeDocument110 pagesSolutions Manual For Thermodynamics and Chemistry: Howard DevoeAshna GautamNo ratings yet
- Chapter 34Document66 pagesChapter 34Nina FairuzNo ratings yet
- Chem Thermo 2Document23 pagesChem Thermo 2rohan rajNo ratings yet
- Engineering Data Book-Spirax Sarco (2009) PDFDocument187 pagesEngineering Data Book-Spirax Sarco (2009) PDFVILLANUEVA_DANIEL2064100% (1)
- Open Rack Vaporizer Mass and Energy BalanceDocument12 pagesOpen Rack Vaporizer Mass and Energy BalanceMuhammad Nanda100% (1)
- Me 303 CH10Document24 pagesMe 303 CH10Osman KutluNo ratings yet
- Thermochemistry: Lecturer's Name E-Mail AddressDocument33 pagesThermochemistry: Lecturer's Name E-Mail AddressErika NatasyaNo ratings yet
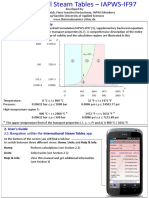
- IAPWS IF97 Range of Validity: P T DiagramDocument5 pagesIAPWS IF97 Range of Validity: P T DiagramRoyaltyGuNNo ratings yet
- Knovel Charts For Water & Steam, SI & English Units, 2006, Norwich, 26pgDocument26 pagesKnovel Charts For Water & Steam, SI & English Units, 2006, Norwich, 26pgVlad ElenaNo ratings yet
- Properties of Pure SubstancesDocument36 pagesProperties of Pure SubstancesAhmadFaisalNo ratings yet
- Prs Pressure Reducing Control ValvesDocument4 pagesPrs Pressure Reducing Control ValvesAnkit PalNo ratings yet
- EdExcel A Level Chemistry Unit 2 Paper 1 Jan 2009Document16 pagesEdExcel A Level Chemistry Unit 2 Paper 1 Jan 2009chuasioklengNo ratings yet
- Set 1Document3 pagesSet 1carlos calibara0% (1)
- Engineering ThermodynamicsDocument21 pagesEngineering Thermodynamicsrkrajesh86No ratings yet
- Predictiona ND Control of Steam AccumulationDocument14 pagesPredictiona ND Control of Steam AccumulationArvin SlayerNo ratings yet
- Process Design of TurboexpanderDocument55 pagesProcess Design of TurboexpanderSaidFerdjallahNo ratings yet
- Chemical ThermodynamicsDocument58 pagesChemical Thermodynamicsyassine ouissiNo ratings yet
- Ak47 TepmeDocument9 pagesAk47 TepmeBatuhan ParlakNo ratings yet
- XSteam Excel v2.6Document9 pagesXSteam Excel v2.6Cătălina StoicaNo ratings yet
- Energetics Test UpdatedDocument20 pagesEnergetics Test UpdatedYu Seung Kim100% (1)
- Example 7.5 Application of Pressure-Dependent Formulas in Compression of MethaneDocument2 pagesExample 7.5 Application of Pressure-Dependent Formulas in Compression of MethaneJonnah Faye MojaresNo ratings yet
- Chemical Bio Refinery PerspectivesDocument473 pagesChemical Bio Refinery PerspectivesonooruddinNo ratings yet
- Simulation of A Single-Stage Evaporator System Integrated With A Mechanical Vapor Compressor For Concentrating The Electrolytic System KNO - H ODocument7 pagesSimulation of A Single-Stage Evaporator System Integrated With A Mechanical Vapor Compressor For Concentrating The Electrolytic System KNO - H OMilanVukicNo ratings yet
- Chemistry Notes Class 11 Chapter 6 ThermodynamicsDocument16 pagesChemistry Notes Class 11 Chapter 6 ThermodynamicsFazrin Fadzili43% (7)
- Engineering Thermodynamics E-Note 04112015 044130AMDocument189 pagesEngineering Thermodynamics E-Note 04112015 044130AMDipesh Patel100% (3)
- Mathematical Modeling of Sulfide Flash Smelting ProcesDocument14 pagesMathematical Modeling of Sulfide Flash Smelting ProcesChristian Aguilar DiazNo ratings yet
- Iso 2314 1989Document11 pagesIso 2314 1989Midhun ThevalakkattumaliNo ratings yet
- Enthalpy PDFDocument119 pagesEnthalpy PDFEstuardo Javier Gan RodríguezNo ratings yet
- ThermoDocument12 pagesThermokesiled309No ratings yet
- Science Goes Viral: Captivating Accounts of Science in Everyday LifeFrom EverandScience Goes Viral: Captivating Accounts of Science in Everyday LifeRating: 5 out of 5 stars5/5 (1)
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (90)
- Is That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeFrom EverandIs That a Fact?: Frauds, Quacks, and the Real Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (3)
- It's Elemental: The Hidden Chemistry in EverythingFrom EverandIt's Elemental: The Hidden Chemistry in EverythingRating: 4 out of 5 stars4/5 (10)
- Coating and Drying Defects: Troubleshooting Operating ProblemsFrom EverandCoating and Drying Defects: Troubleshooting Operating ProblemsRating: 5 out of 5 stars5/5 (1)
- Functional Safety from Scratch: A Practical Guide to Process Industry ApplicationsFrom EverandFunctional Safety from Scratch: A Practical Guide to Process Industry ApplicationsNo ratings yet
- Piping and Pipeline Calculations Manual: Construction, Design Fabrication and ExaminationFrom EverandPiping and Pipeline Calculations Manual: Construction, Design Fabrication and ExaminationRating: 4 out of 5 stars4/5 (18)
- Monkeys, Myths, and Molecules: Separating Fact from Fiction in the Science of Everyday LifeFrom EverandMonkeys, Myths, and Molecules: Separating Fact from Fiction in the Science of Everyday LifeRating: 4 out of 5 stars4/5 (9)
- Trevor Kletz Compendium: His Process Safety Wisdom Updated for a New GenerationFrom EverandTrevor Kletz Compendium: His Process Safety Wisdom Updated for a New GenerationNo ratings yet
- The Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsFrom EverandThe Disappearing Spoon: And Other True Tales of Madness, Love, and the History of the World from the Periodic Table of the ElementsRating: 4 out of 5 stars4/5 (146)
- The Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableFrom EverandThe Elements We Live By: How Iron Helps Us Breathe, Potassium Lets Us See, and Other Surprising Superpowers of the Periodic TableRating: 3.5 out of 5 stars3.5/5 (22)
- The Periodic Table: A Very Short IntroductionFrom EverandThe Periodic Table: A Very Short IntroductionRating: 4.5 out of 5 stars4.5/5 (3)
- Organic Chemistry for Schools: Advanced Level and Senior High SchoolFrom EverandOrganic Chemistry for Schools: Advanced Level and Senior High SchoolNo ratings yet
- Nuclear Energy in the 21st Century: World Nuclear University PressFrom EverandNuclear Energy in the 21st Century: World Nuclear University PressRating: 4.5 out of 5 stars4.5/5 (3)
- The Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsFrom EverandThe Regenerative Grower's Guide to Garden Amendments: Using Locally Sourced Materials to Make Mineral and Biological Extracts and FermentsRating: 5 out of 5 stars5/5 (3)
- Guidelines for Asset Integrity ManagementFrom EverandGuidelines for Asset Integrity ManagementRating: 5 out of 5 stars5/5 (1)
- Essential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilFrom EverandEssential Oil Chemistry Formulating Essential Oil Blends that Heal - Aldehyde - Ketone - Lactone: Healing with Essential OilRating: 5 out of 5 stars5/5 (1)
- Napoleon's Buttons: 17 Molecules That Changed HistoryFrom EverandNapoleon's Buttons: 17 Molecules That Changed HistoryRating: 4 out of 5 stars4/5 (25)
- Chemical Process Safety: Learning from Case HistoriesFrom EverandChemical Process Safety: Learning from Case HistoriesRating: 4 out of 5 stars4/5 (14)
- An Introduction to the Periodic Table of Elements : Chemistry Textbook Grade 8 | Children's Chemistry BooksFrom EverandAn Introduction to the Periodic Table of Elements : Chemistry Textbook Grade 8 | Children's Chemistry BooksRating: 5 out of 5 stars5/5 (1)
- Process Engineering for a Small Planet: How to Reuse, Re-Purpose, and Retrofit Existing Process EquipmentFrom EverandProcess Engineering for a Small Planet: How to Reuse, Re-Purpose, and Retrofit Existing Process EquipmentNo ratings yet
- Chemistry for Breakfast: The Amazing Science of Everyday LifeFrom EverandChemistry for Breakfast: The Amazing Science of Everyday LifeRating: 4.5 out of 5 stars4.5/5 (14)
- Chemical Elements Pocket Guide: Detailed Summary of the Periodic TableFrom EverandChemical Elements Pocket Guide: Detailed Summary of the Periodic TableNo ratings yet