Professional Documents
Culture Documents
CHM 580 Exp Aas
Uploaded by
Azreen AnisOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CHM 580 Exp Aas
Uploaded by
Azreen AnisCopyright:
Available Formats
Objective : To determine Cadmium (Cd) and Chromiun (Cr) in plant tissue using absorption spectroscopy Introductions : Cadmium is well
known to be one of the most toxic heavy elements for animals. It has recently become a serious problem that rice grains contain cadmium in some area of Japan. On the other hand, some types of plants can grow in contaminated soils and absorb a large amount of cadmium in their bodies. Such hyperaccumulator plants are expected to be used for remediation of environments. However, the accumulation mechanism has not yet been revealed, with the elemental distribution of cadmium and transportation during uptake remaining unclear. Cadmium appear to play a very pivotal role in thyroid disease, it is a very unique mineral. It is extremely toxic and has toxic biological effects at concentrations smaller than almost any commonly found mineral. An environmental poison found in water, on our food and in the air. It's found in processed grains, dairy products, meats, fish, fertilizers, auto exhaust, cigarette smoke, batteries, solder and dentures. It disrupts the absorption of other minerals and tends to settle in the heart and right kidney and affects proper functioning of several enzymes. Whereas for Chromium, taken in the right quantity, chromium has immense health benefits. It is available in extremely low quantities in animal and plant tissues which is why it is called a trace metal. Some of the sources of chromium are brewers yeast, coffee, tea, cereals, potatoes, peas, oysters, rye, thyme, processed meats, whole grains, and beer. Chromium helps metabolize carbohydrates. It monitors blood sugar levels, and helps stabilize blood sugar. It can also prevent hypertension or high blood pressure. Although trials are still being conducted, chromium compounds are considered helpful in preventing memory loss and in treating Alzheimers disease. Procedure : Day 1
1. Plant tissue was prepared ( mustard and spinach). 2. The vegetable was dried in and oven of 110C Day 2 1. The dried vegetables was cut into pieces 2. About 3 grams of vegetable was weighed and placed in 250 ml beaker 3. 10 ml of nitric acid (HNO3) was added into the beaker and was allowed to stand Overnight
Day 3 A) Sample preparation 1. The sample was heated until red fume came out 2. Then it was cooled 3. 1 ml of peroxide (H2O2) was added into the cooled sample 4.The sample was reheated till concentrate 5. It was then filtered into 250ml volumetric flask 6. The sample was diluted with distilled water till marked B) Standard preparation Cr 1 ppm 2 ppm 3 ppm 4 ppm 5 ppm Volume 0.5 ml 1.0 ml 1.5 ml 2.0 ml 2.5 ml Cd 0.2 ppm 0.4 ppm 0.6 ppm 0.8 ppm 1.0 ppm Volume 1 ml 2 ml 3 ml 4 ml 5 ml
1. The standard was prepared as in table given above in 100 ml volumetric flask 2. The stock solution given was in 1000 ppm for each sample 3. 5 ml of the stock solution was pipette into a 100 ml volumetric flask and diluted with distilled water till marked 4. For each concentration, the volume was pipette into a 50 volumetric flask as stated in the table above respectively 5. 7 ml of the sample was pipette into each volumetric flask and was diluted with distiiled water till mark 6. The sample was then analyzed using atomic absorption spectroscopy
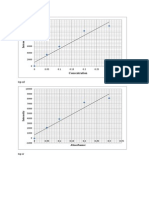
Results : CONCENTRATION CHROMIUM CADMIUM Blank Blank 1 ppm 0.2 ppm 2 ppm 0.4 ppm 3 ppm 0.6 ppm 4 ppm 0.8 ppm 5 ppm 1.0 ppm ABSORBANCE CHROMIUM CADMIUM 0.036 0.264 0.045 0.290 0.058 0.311 0.071 0.330 0.082 0.352 0.093 0.369 VOLUME CHROMIUM CADMIUM 0 0 0.5 ml 1 ml 1.0 ml 2 ml 1.5 ml 3 ml 2.0 ml 4 ml 2.5 ml 5 ml
Calculation : Least square method : CONCENTRATI ON Cr Cd Blank 1 ppm 2 ppm 3 ppm 4 ppm 5 ppm Blank 0.2 ppm 0.4 ppm 0.6 ppm 0.8 ppm 1.0 ppm ABSORBANCE (y1) Cr Cd 0.036 0.045 0.058 0.071 0.082 0.093 y= 0.385 0.264 0.290 0.311 0.330 0.352 0.369 y= 1.916 VOLUME (x1) Cr 0 0.5 ml 1.0 ml 1.5 ml 2.0 ml 2.5 ml
= 7.5
X1X1 Cr 0 0.25 1.00 2.25 4.00 6.25 xx= 13.75 Cd 0 1 4 9 16 25 xx= 55 Cr
X1Y1 Cd 0 0.290 0.622 0.990 1.408 1.845 xy= 5.155
Cd 0 1 ml 2 ml 3 ml 4 ml 5 ml
= 15
0 0.0225 0.0580 0.1065 0.1640 0.2325 xy= 0.5835
CHROMIUM
chromium
0.1 0.09 0.08 0.07 0.06 0.05 0.04 0.03 0.02 0.01 0 0 1 2 3 y = 0.0234x + 0.035 R = 0.9979
chromium Linear (chromium)
xy = ((xy) =0.5835 -
= 0.10225
xx = (xx)
= 13.75 = 4.375 m=
=
= 0.0234
= 0.035 Concentration of Chromium in plant tissue : C=
=
= 2.137 ppm
CADMIUM
cadmium
0.4 0.35 0.3 0.25 0.2 0.15 0.1 0.05 0 0 1 2 3 4 5 6 cadmium Linear (cadmium) y = 0.0209x + 0.2672 R = 0.9965
xy = ((xy) =5.155 -
= 0.365
xx = (xx)
= 55 = 17.5 m=
=
= 0.0209
= 0.2671 Concentration of cadmium in plant tissue C=
=
=12.78 ppm
Discussions : In this experiment, we used wet digestion methods for sample preparation. It is for elemental analysis that involves the chemical degradation of sample matrices in solution, usually with a combination of acids to increase solubility as it has been done on day 2 and allow to stand overnight. The various acid and ux treatments are carry out at high temperatures in specially designed vessels that help to minimize contamination of the sample with substances in the air, the local environment, and from the vessel walls. Sample may be loss due to adsorption onto the vessel walls, volatilization, and coextraction, but these can be reduced by procedural modications. The use of closed systems, where the digestion reaction is completely isolated
from the surroundings, may help to reduce both contamination and sample loss. For this experiment, the sample was covered with aluminium foil. Standard addition method are particularly useful for analyzing complex samples in which the likelihood of matrix effects is substantial. A standard addition method can take several form. One of the most common form is spiking method as we had use it in preparing standard. Each solution was diluted to marked point before measuring or analyzing it. The least square method, is a typical calibration graph where I have plotted in calculation area for both sample chromium and cadmium respectively. The sample was injected into the atomic absorption spectroscopy and the result was obtained. Absorbance versus volume graph was plotted and the linear equation and R2 was calculated in Microsoft excel and as well as manually which had been shown. Conclusion : in this experiment, I had used mustard in determination of Chromium while for determination of Cadmium, I had used spinanch. I had achieved my objective to determine Cadmium and Chromium in plant tissue. The concentration of Cadmium in spinach is 12.78 ppm. The concentration of Chromium in mustard is 2.137 ppm.
References :
1. http://www.writescience.com/RMT%20PDFs/Elsevier/eans%20wetdig.pdf 2. http://www.newsmax.com/FastFeatures/chromium-health-benefitsnutrition/2011/01/21/id/369681 3. http://www.ipap.jp/proc/cs7/pdf/cs7_323.pdf 4. http://www.best-home-remedies.com/minerals/cadmium.htm 5. Principle of instrumental analysis 6th analysis, Douglas.A.Skoog.F.James Holler, Stanley R.Crouch
You might also like
- Chm580 Experiment 1Document9 pagesChm580 Experiment 1ohhiNo ratings yet
- Faculty of Applied Sciences: Spectrochemical Methods of Analysis (CHM 580)Document7 pagesFaculty of Applied Sciences: Spectrochemical Methods of Analysis (CHM 580)Husna Insyirah Bt SamadNo ratings yet
- Chm580 Experiment 2Document8 pagesChm580 Experiment 2ohhi100% (1)
- CHM 580 Spectrochemical Methods of Analysis Laboratory Report Experiment 2: Ultraviolet-Visible SpectrosDocument10 pagesCHM 580 Spectrochemical Methods of Analysis Laboratory Report Experiment 2: Ultraviolet-Visible SpectrosAiNo ratings yet
- CHM 580 Exp 1 & 7Document19 pagesCHM 580 Exp 1 & 7Nabilah60% (5)
- Faculty of Applied Sciences: Spectrochemical Methods of Analysis (CHM 580)Document6 pagesFaculty of Applied Sciences: Spectrochemical Methods of Analysis (CHM 580)Husna Insyirah Bt SamadNo ratings yet
- Lab Report Experiment 1 Chm624Document11 pagesLab Report Experiment 1 Chm624Hazwan HamimNo ratings yet
- Sample Exp 6 CHM 477Document11 pagesSample Exp 6 CHM 477ommy madinaNo ratings yet
- CHM557 Exp 2Document12 pagesCHM557 Exp 2syafNo ratings yet
- Experiment 1 578Document12 pagesExperiment 1 578aisyah fauzi100% (1)
- Spectrochemical Method of Analysis (CHM 580) EXPERIMENT 1:qualitative Analysis of Aspirin Phenacetin Caffeine and Sample Using FTIR and NMRDocument9 pagesSpectrochemical Method of Analysis (CHM 580) EXPERIMENT 1:qualitative Analysis of Aspirin Phenacetin Caffeine and Sample Using FTIR and NMRbatrisyia hazirahNo ratings yet
- Tutorial Chap 9 Ans-CHM580Document2 pagesTutorial Chap 9 Ans-CHM580tirahNo ratings yet
- Exp 4 chm556Document7 pagesExp 4 chm556Azli AzmanNo ratings yet
- Chm361 Lab ReportsDocument20 pagesChm361 Lab Reportswatiqah adilahNo ratings yet
- Water Distillation & Hardness AnalysisDocument9 pagesWater Distillation & Hardness Analysisaremyrah AzlanNo ratings yet
- Corrosion Rate Experiment of Steel and Stainless SteelDocument4 pagesCorrosion Rate Experiment of Steel and Stainless SteelNurul HusnaNo ratings yet
- Lab Report 7Document7 pagesLab Report 7Iena KasimNo ratings yet
- Determination of caffeine in tea bag using second derivative UV spectrometryDocument8 pagesDetermination of caffeine in tea bag using second derivative UV spectrometrySuhailyShukriNo ratings yet
- Experiment 7 CHM420Document5 pagesExperiment 7 CHM420Faziman KhalilNo ratings yet
- Experiment 7 Chlorpyrifos chm510Document6 pagesExperiment 7 Chlorpyrifos chm510Amar SafwanNo ratings yet
- Experiment 3 Determination of Fatty Acid Using Gas Chromatography (GC)Document7 pagesExperiment 3 Determination of Fatty Acid Using Gas Chromatography (GC)NUR IZZATI OTHMAN BASRI100% (1)
- Lab 1 CHM 510 Complete 2011Document20 pagesLab 1 CHM 510 Complete 2011Nor Hasliza100% (1)
- Exp 3Document6 pagesExp 3ohhiNo ratings yet
- Experiment 2 HPLC chm510Document9 pagesExperiment 2 HPLC chm510Amar Safwan75% (12)
- CHM361 Case Study PDFDocument1 pageCHM361 Case Study PDFHazwan0% (1)
- Experiment 3 CHM 432Document7 pagesExperiment 3 CHM 432Amirah NajihahNo ratings yet
- CHM 510 Experiment 2Document16 pagesCHM 510 Experiment 2NabilahNo ratings yet
- AbstractDocument5 pagesAbstractUnta Solehah67% (3)
- 1 Step PolymerizationDocument8 pages1 Step Polymerizationzatty kimNo ratings yet
- CHM457 - Exp 4-Preparation of Aspirin - N.syamimi BT Zainal - As2452s1 PDFDocument5 pagesCHM457 - Exp 4-Preparation of Aspirin - N.syamimi BT Zainal - As2452s1 PDFsyamimi zainalNo ratings yet
- Sodium Borohydride Reduction of CyclohexanoneDocument6 pagesSodium Borohydride Reduction of CyclohexanoneIqmal HakimiNo ratings yet
- Lab Report Experiment 3 PDFDocument20 pagesLab Report Experiment 3 PDFAsla MarleenaNo ratings yet
- EXPERIMENT 8: DETERMINATION OF Fe IN A FERUM SOLUTION BY GRAVIMETRIC ANALYSIS CHM421Document4 pagesEXPERIMENT 8: DETERMINATION OF Fe IN A FERUM SOLUTION BY GRAVIMETRIC ANALYSIS CHM421Naz Helmi75% (8)
- CHM557 Experiment 5 - The Robinson Annulation ReactionDocument9 pagesCHM557 Experiment 5 - The Robinson Annulation ReactionMamamia0% (1)
- Suggested Answer - Tutorial 2 Chm510Document6 pagesSuggested Answer - Tutorial 2 Chm510Mark SullivanNo ratings yet
- Lab Report CHM 510Document30 pagesLab Report CHM 510Amirah AzlanNo ratings yet
- Experiment 3 CHM421Document8 pagesExperiment 3 CHM421pufff witches100% (1)
- Chm580 Experiment 3Document9 pagesChm580 Experiment 3ohhiNo ratings yet
- Exp 1 Chm510Document11 pagesExp 1 Chm510Nur Nadia Syahira100% (1)
- UV-Vis Determination of KMnO4 ConcentrationDocument5 pagesUV-Vis Determination of KMnO4 ConcentrationMustafidzul Mustapha56% (9)
- Experiment 4 CHM 557 PDFDocument19 pagesExperiment 4 CHM 557 PDFinsyirah shazrinNo ratings yet
- Neutralisation Capacity of Commercial Antacid TabletDocument6 pagesNeutralisation Capacity of Commercial Antacid TabletEmmilia25% (4)
- Exp5 Baru..Document13 pagesExp5 Baru..ibrahimkhadijah100% (41)
- chm421 Exp 3Document8 pageschm421 Exp 3Irfan Azahar100% (1)
- Lab Report Experiment 2 CHM457Document9 pagesLab Report Experiment 2 CHM457lala lalaNo ratings yet
- Experiment 7: ECM TissuesDocument5 pagesExperiment 7: ECM TissuesnajwaNo ratings yet
- Exp 2Document11 pagesExp 2ohhiNo ratings yet
- Experiment 5 Analysis of Chlorpyrifos in Water Using Solid-Phase Extraction (SPE) and Gas Chromatography-Electron Capture Detector (GC-ECD)Document8 pagesExperiment 5 Analysis of Chlorpyrifos in Water Using Solid-Phase Extraction (SPE) and Gas Chromatography-Electron Capture Detector (GC-ECD)NUR IZZATI OTHMAN BASRINo ratings yet
- CHM557 Experiment 3 - Esterification Reaction of Vanillin - The Use of NMR To Determine A StructureDocument9 pagesCHM557 Experiment 3 - Esterification Reaction of Vanillin - The Use of NMR To Determine A StructureMamamiaNo ratings yet
- CHM 421 Exp 3Document6 pagesCHM 421 Exp 3EmmiliaNo ratings yet
- Chm557 Laboratory Report: Experiment 5 The Robinson Annulation ReactionDocument14 pagesChm557 Laboratory Report: Experiment 5 The Robinson Annulation ReactionsyafNo ratings yet
- Experiment 2 Electrolytic Cell Nurul Husna Binti IbrahimDocument4 pagesExperiment 2 Electrolytic Cell Nurul Husna Binti IbrahimNurul HusnaNo ratings yet
- chm510 Experiment 3Document7 pageschm510 Experiment 3Naz Helmi100% (1)
- CHM 477 Synthesis of Potassium Tris(oxalate) chromiumDocument5 pagesCHM 477 Synthesis of Potassium Tris(oxalate) chromiumSarah HannisNo ratings yet
- Fatty Acid Determination Using Gas Chromatography (GC)Document5 pagesFatty Acid Determination Using Gas Chromatography (GC)Amirul Azhar100% (10)
- Exp5 520Document11 pagesExp5 520syamsaufi33% (3)
- Lab Report Experiment 3 4 and 5Document13 pagesLab Report Experiment 3 4 and 5Nurul Iman Che Awang90% (40)
- Atomic Absorption SpectrosDocument9 pagesAtomic Absorption Spectrosamirul azhar80% (10)
- Complete AAS N ICPDocument14 pagesComplete AAS N ICPMaxvicklye Rayner100% (1)
- Analysis of Chromic Oxide in Feed and FecesDocument4 pagesAnalysis of Chromic Oxide in Feed and FecesDiu NgoNo ratings yet
- English Year 3 Paper 2 140204012751 Phpapp01Document6 pagesEnglish Year 3 Paper 2 140204012751 Phpapp01Zatul Izawati Ahmad IbrahimNo ratings yet
- Indices and Logarithm - ScribdDocument2 pagesIndices and Logarithm - ScribdAzreen AnisNo ratings yet
- SPM Form 4Document1 pageSPM Form 4Azreen AnisNo ratings yet
- Types of MicroorganismsDocument1 pageTypes of MicroorganismsAzreen AnisNo ratings yet
- Vision Note For Malaysia SPM Form 4Document1 pageVision Note For Malaysia SPM Form 4Azreen AnisNo ratings yet
- Nutrition Absorption ProcessDocument1 pageNutrition Absorption ProcessAzreen AnisNo ratings yet
- Double Celebration Meal VoucherDocument1 pageDouble Celebration Meal VoucherAzreen AnisNo ratings yet
- Fs 11900Document7 pagesFs 11900Azreen AnisNo ratings yet
- Kedah Module Peningkatan Prestasi Tingkatan 5 SPM 2014 English (D1FA424A)Document31 pagesKedah Module Peningkatan Prestasi Tingkatan 5 SPM 2014 English (D1FA424A)Azreen Anis100% (1)
- Time Table Test Session Mac - JUN 2012 Date Time Course Code Test PlaceDocument1 pageTime Table Test Session Mac - JUN 2012 Date Time Course Code Test PlaceAzreen AnisNo ratings yet
- BOD ProcedureDocument14 pagesBOD ProcedureSajith Ranatunga100% (1)
- BOD ProcedureDocument14 pagesBOD ProcedureSajith Ranatunga100% (1)
- Tution NsDocument2 pagesTution NsAzreen AnisNo ratings yet
- Shafiq Add Math Test 1Document5 pagesShafiq Add Math Test 1Azreen AnisNo ratings yet
- Rate Laws and StoichiometryDocument20 pagesRate Laws and StoichiometryAzreen AnisNo ratings yet
- + y 9. Give Your Answer To Two DecimalDocument4 pages+ y 9. Give Your Answer To Two DecimalAzreen AnisNo ratings yet
- Tution NsDocument2 pagesTution NsAzreen AnisNo ratings yet
- CHM580Document8 pagesCHM580Azreen AnisNo ratings yet
- Graph showing intensity vs concentration for ICP-CD and ICP-CR analysisDocument1 pageGraph showing intensity vs concentration for ICP-CD and ICP-CR analysisAzreen AnisNo ratings yet
- Tution NsDocument2 pagesTution NsAzreen AnisNo ratings yet
- Trial Kedah English SPM 2013 k2 Dan JawapanDocument25 pagesTrial Kedah English SPM 2013 k2 Dan Jawapancmfoo2988No ratings yet
- AtrDocument4 pagesAtrAzreen AnisNo ratings yet
- Universiti Teknologi Mara Final Examination: Confidential AS/APR 2006/CHWI561Document3 pagesUniversiti Teknologi Mara Final Examination: Confidential AS/APR 2006/CHWI561Azreen AnisNo ratings yet
- April 2011Document4 pagesApril 2011Azreen AnisNo ratings yet
- Analytical Quality Assurance - Who PDFDocument17 pagesAnalytical Quality Assurance - Who PDFvabimhahNo ratings yet
- CHM580Document7 pagesCHM580Azreen AnisNo ratings yet
- Oct 2007Document4 pagesOct 2007Azreen AnisNo ratings yet
- CHM580Document8 pagesCHM580Azreen AnisNo ratings yet
- Paper 2 There Are Three Questions in This Paper. Answer All The Questions. Section ADocument7 pagesPaper 2 There Are Three Questions in This Paper. Answer All The Questions. Section AAzreen AnisNo ratings yet
- Nota PendekDocument9 pagesNota PendekBeevy GB71% (7)
- Andrews Et Al-1999-J Marine SystDocument11 pagesAndrews Et Al-1999-J Marine SystKadane CoatesNo ratings yet
- Comparing characteristics of transition metal seriesDocument20 pagesComparing characteristics of transition metal seriesAlaa KareemNo ratings yet
- 13CR 4v 1210Document8 pages13CR 4v 1210Luis David Concha CastilloNo ratings yet
- Chromium Metal: Standard Specification ForDocument2 pagesChromium Metal: Standard Specification ForJerry Bean100% (1)
- Breeze 2015 Full LineDocument44 pagesBreeze 2015 Full Linemohan raoNo ratings yet
- High Temperature Oxidation Behavior of P91, P92 and E911 Alloy Steels in Dry and Wet AtmospheresDocument9 pagesHigh Temperature Oxidation Behavior of P91, P92 and E911 Alloy Steels in Dry and Wet AtmospheresPaco100% (1)
- Block 1Document68 pagesBlock 1Prasad GutteNo ratings yet
- 24.12.22 - SR - STAR CO-SC (MODEL-A & B) - Jee - Main - PTM-14 - QPDocument20 pages24.12.22 - SR - STAR CO-SC (MODEL-A & B) - Jee - Main - PTM-14 - QPONLY SPIDEYNo ratings yet
- Synova ChemicalsDocument201 pagesSynova ChemicalsInnovation InnovationNo ratings yet
- Zimbabwe mine dumps impact on river water qualityDocument7 pagesZimbabwe mine dumps impact on river water qualityAngelica Ma ZapataNo ratings yet
- Doga Rulman Dana Gözü S.B.gennDocument31 pagesDoga Rulman Dana Gözü S.B.gennemrah nalbantNo ratings yet
- Corrosion and Corrosion ControlDocument14 pagesCorrosion and Corrosion ControlalguNo ratings yet
- Transition Metal Isotope Patterns in Mass SpectraDocument3 pagesTransition Metal Isotope Patterns in Mass SpectraNia LisnaNo ratings yet
- Section 3 - Corrosion - ProtectionDocument44 pagesSection 3 - Corrosion - ProtectionChris MedeirosNo ratings yet
- Poly (Acrylamide-Co-Maleic Acid) Hydrogels For Removal of CR (VI) From Aqueous Solutions, Synthesis and SwellingDocument11 pagesPoly (Acrylamide-Co-Maleic Acid) Hydrogels For Removal of CR (VI) From Aqueous Solutions, Synthesis and Swellingnadher damanNo ratings yet
- Chemistry Practice Olympiad Stage 1Document27 pagesChemistry Practice Olympiad Stage 1DevYShethNo ratings yet
- AWS A5.12: Material Safety Data Sheet (MSDS)Document3 pagesAWS A5.12: Material Safety Data Sheet (MSDS)Mani VannanNo ratings yet
- Mark Scheme (Results) June 2010: GCE Chemistry (6CH08/01)Document14 pagesMark Scheme (Results) June 2010: GCE Chemistry (6CH08/01)sedara samarasingheNo ratings yet
- SP-2041 2014Document19 pagesSP-2041 2014wawanNo ratings yet
- CL3 Exploded View PDFDocument10 pagesCL3 Exploded View PDFNOEL RODRIGUEZNo ratings yet
- Materials Guideline Update: Preliminary Survey ResultsDocument44 pagesMaterials Guideline Update: Preliminary Survey ResultsmrinalkantibhaduriNo ratings yet
- Minfc930 Astm A322 Grade 4140Document3 pagesMinfc930 Astm A322 Grade 4140Muthazhagan SaravananNo ratings yet
- Cape Unit 1 Past Papers Chem PDFDocument15 pagesCape Unit 1 Past Papers Chem PDFSherlan TheodoreNo ratings yet
- UTP Sugar EN 2019 GL 186 Preview PDFDocument8 pagesUTP Sugar EN 2019 GL 186 Preview PDFMike CheNo ratings yet
- Clear Chromate Coating Technical DataDocument3 pagesClear Chromate Coating Technical Datahumbertotorresr100% (1)
- Pourbaix Diagrams For Copper at 25 To 300°CDocument8 pagesPourbaix Diagrams For Copper at 25 To 300°CfesooNo ratings yet
- Interactive CatalogDocument76 pagesInteractive CatalogahmedelhajNo ratings yet
- 4 Material StandardsDocument29 pages4 Material Standardsloqueluq100% (3)
- ExtranDocument12 pagesExtranEmily AgostiniNo ratings yet
- BrochureDocument3 pagesBrochurejoyyleeeeNo ratings yet
- Wayfinding: The Science and Mystery of How Humans Navigate the WorldFrom EverandWayfinding: The Science and Mystery of How Humans Navigate the WorldRating: 4.5 out of 5 stars4.5/5 (18)
- Roxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingFrom EverandRoxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingRating: 3.5 out of 5 stars3.5/5 (33)
- Roxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingFrom EverandRoxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingRating: 5 out of 5 stars5/5 (5)
- Dark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseFrom EverandDark Matter and the Dinosaurs: The Astounding Interconnectedness of the UniverseRating: 3.5 out of 5 stars3.5/5 (69)
- The Secret Life of Lobsters: How Fishermen and Scientists Are Unraveling the Mysteries of Our Favorite CrustaceanFrom EverandThe Secret Life of Lobsters: How Fishermen and Scientists Are Unraveling the Mysteries of Our Favorite CrustaceanNo ratings yet
- The Lives of Bees: The Untold Story of the Honey Bee in the WildFrom EverandThe Lives of Bees: The Untold Story of the Honey Bee in the WildRating: 4.5 out of 5 stars4.5/5 (44)
- The Revolutionary Genius of Plants: A New Understanding of Plant Intelligence and BehaviorFrom EverandThe Revolutionary Genius of Plants: A New Understanding of Plant Intelligence and BehaviorRating: 4.5 out of 5 stars4.5/5 (137)
- When You Find Out the World Is Against You: And Other Funny Memories About Awful MomentsFrom EverandWhen You Find Out the World Is Against You: And Other Funny Memories About Awful MomentsRating: 3.5 out of 5 stars3.5/5 (13)
- The Ancestor's Tale: A Pilgrimage to the Dawn of EvolutionFrom EverandThe Ancestor's Tale: A Pilgrimage to the Dawn of EvolutionRating: 4 out of 5 stars4/5 (811)
- The Mind of Plants: Narratives of Vegetal IntelligenceFrom EverandThe Mind of Plants: Narratives of Vegetal IntelligenceRating: 4.5 out of 5 stars4.5/5 (11)
- World of Wonders: In Praise of Fireflies, Whale Sharks, and Other AstonishmentsFrom EverandWorld of Wonders: In Praise of Fireflies, Whale Sharks, and Other AstonishmentsRating: 4 out of 5 stars4/5 (221)
- Eels: An Exploration, from New Zealand to the Sargasso, of the World's Most Mysterious FishFrom EverandEels: An Exploration, from New Zealand to the Sargasso, of the World's Most Mysterious FishRating: 4 out of 5 stars4/5 (30)
- The Hidden Life of Trees: What They Feel, How They CommunicateFrom EverandThe Hidden Life of Trees: What They Feel, How They CommunicateRating: 4 out of 5 stars4/5 (1002)
- Why Fish Don't Exist: A Story of Loss, Love, and the Hidden Order of LifeFrom EverandWhy Fish Don't Exist: A Story of Loss, Love, and the Hidden Order of LifeRating: 4.5 out of 5 stars4.5/5 (699)
- Last Child in the Woods: Saving Our Children From Nature-Deficit DisorderFrom EverandLast Child in the Woods: Saving Our Children From Nature-Deficit DisorderRating: 4 out of 5 stars4/5 (283)
- Spoiled Rotten America: Outrages of Everyday LifeFrom EverandSpoiled Rotten America: Outrages of Everyday LifeRating: 3 out of 5 stars3/5 (19)
- Gathering Moss: A Natural and Cultural History of MossesFrom EverandGathering Moss: A Natural and Cultural History of MossesRating: 4.5 out of 5 stars4.5/5 (347)
- The Other End of the Leash: Why We Do What We Do Around DogsFrom EverandThe Other End of the Leash: Why We Do What We Do Around DogsRating: 5 out of 5 stars5/5 (63)
- A-level Biology Revision: Cheeky Revision ShortcutsFrom EverandA-level Biology Revision: Cheeky Revision ShortcutsRating: 5 out of 5 stars5/5 (5)
- Come Back, Como: Winning the Heart of a Reluctant DogFrom EverandCome Back, Como: Winning the Heart of a Reluctant DogRating: 3.5 out of 5 stars3.5/5 (10)
- The Big, Bad Book of Botany: The World's Most Fascinating FloraFrom EverandThe Big, Bad Book of Botany: The World's Most Fascinating FloraRating: 3 out of 5 stars3/5 (10)
- The Optimist: A Case for the Fly Fishing LifeFrom EverandThe Optimist: A Case for the Fly Fishing LifeRating: 4.5 out of 5 stars4.5/5 (17)
- The Hummingbirds' Gift: Wonder, Beauty, and Renewal on WingsFrom EverandThe Hummingbirds' Gift: Wonder, Beauty, and Renewal on WingsRating: 4.5 out of 5 stars4.5/5 (60)