Professional Documents
Culture Documents
Magnetic Properties of Materials
Uploaded by
Ayesh Nayana GunawardanaCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Magnetic Properties of Materials
Uploaded by
Ayesh Nayana GunawardanaCopyright:
Available Formats
0
PHYS 44064 SOLID STATE PHYSICS
BRIEF REPORT OF
MAGNETIC PROPERTIES OF SOLIDS
Student name: M.A.A.N Gunawardana Student no: PS/2007/086
MAGNETIC PROPERTIES OF SOLIDS Index Introduction..2 Diamagnetism, Paramagnetism and ferromagnetism ..3 Langevins theory of diamagnetism.4 Langevins theory of Paramagnetism.6 Failure of Langevins theory .8 Weiss Modification8 Ferromagnetism.8 Antiferromagnetism and Ferrimagnetism..9 Weiss theory of Ferromagnetism ..9 Applications12 Nuclear Magnetic Resonance.12 NMR Spectrometer.13
Introduction According to their magnetic properties ,magnetic materials are divided into 3 groups they are Diamagnetic Paramagnetic Ferromagnetic
The vector quantity M defined as the intensity of magnetization or more often magnetization ,which is used to characterization of substance, it is the vector sum of the magnetic moments of atoms (or molecules) contained in unit volume
Where N=the number of particles in volume V of magnetic material =magnetic moment of I th atom (or molecule) For large number of magnetic materials it is found out that the intensity of magnetization is directly proportional to the magnetic field intensity i.e. Where is known as the magnetic susceptibility of the substance
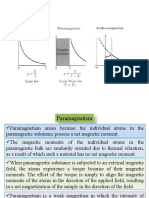
Diamagnetism In 1846 Michael Faraday discovered that a specimen of bismuth brought near to the pole of strong magnet is repelled. He called such substance diamagnetic. Antimony, bismuth, mercury, gold and copper are some example of diamagnetic substance. For diamagnetic substance and is independent of temperature. Unlike paramagnetic materials, whose atoms or molecules have a net magnetic moment, the atoms or molecules of a diamagnetic material have zero magnetic moment in absence of an external magnetic field. With external magnetic field a net dipole moment, opposing the field is induced in the atoms or molecules. In paramagnetic materials there is also diamagnetic effect but it is much weaker than that of the paramagnetism Paramagnetism Paramagnetic substances are attracted towards the region of stronger magnetic field. Aluminum oxygen and platinum are some examples of paramagnetic materials. Their magnetic susceptibility and depends on temperature according to the Curie Law i.e. Where C is Curie constant Paramagnetism occurs on substances where the individual atoms, ions, or molecules possess a permanent magnetic dipole moment. In the absence of external magnetic field, the atomic dipoles point in random directions. So there is no resultant magnetization of substance as a whole in any direction. This random orientation is the result of thermal agitation within the substance. When external field is applied, the atomic dipoles tend to orient themselves parallel to the field, since this is a state of lower energy than the anti parallel position. This gives net magnetization parallel to field and a positive contribution to susceptibility. At lower temperatures, the thermal agitation, which tends to give a random orientation to the atomic dipoles, is less. Hence bigger proportions of dipoles are able to align themselves parallel to field, and the magnetization is greater for a given field. For ordinary field temperatures . For large fields at low temperatures the magnetization produced is no longer proportional to the applied field, and tends to a constant value. This saturation effect is produced when all atomic dipoles are aligned parallel to the field, so that magnetization reaches to limiting maximum value. Ferromagnetism Ferromagnetic materials are very strongly magnetic. For a given field, magnetization in ferromagnetic substance is about times stronger than dia or Para magnetic substances. Irons, Nickel, cobalt are some examples of ferromagnetic substances. As temperature increases, the value of decreases. Above a certain temperature, Known as the Curie temperature, ferromagnets becomes paramagnets
Langevins theory of Diamagnetism Consider an electron (mass =m, charge = e) rotating about the nucleus (charge=Ze) in a circular orbital of radius r. let be angular velocity of electron. Then
Or
.(1)
The magnetic moment of electron is =current area=
(2)
Let a magnetic field of induction be now applied. is normal to and into the page
r r
Ze
Electron
Ze
Electron
Figure 1 An additional force called Lorentz force acts on the electron ( )
The condition of stable motion is now given by .(3) Or Solving the quadratic equation in ,
( )
Thus the angular frequency is now different from . The result of establishing a field of flux density is to set up a precessional motion of the electronic orbits with angular velocity , this is called the Larmor theorem. Then
Change in frequency of Revolution of electron= The corresponding change in the magnetic momentum of the electron is { ( )}
On summing over all electrons in the atom, the induced moment per atom becomes Let N be the number of atoms per unit volume. Then the magnetization M is given by All the electron orbits are not oriented normal to the magnetic field. Hence in Eq. (6) should be replaced by the average of square of the projection of orbit radii for various electrons in a plane perpendicular to . Hence we should replace in Eq.(6) by therefore Volume susceptibility of material
Eq.(7) shows that is independent of the field strength and temperature. This is in accordance with Curies experimental results. Langevins theory of Paramagnetism Langevin assumes that each atom has a permanent magnetic momentum m. The only force acting on atom is that due to the external field .Let be the angle of inclination of the axis of the atomic dipole with the direction of the applied field . Then magnetic potential energy of the atomic dipole is
Now, on classical statistics, the number of atoms making an angle between is
Where K is Boltzmanns constant and T is the absolute temperature Put
. Then
Hence the total number of atomic magnets in unit volume of paramagnetic material Put then
The component of each dipole moment parallel to B in . The total magnetic moment of all the n atoms contained in unit volume of the gas is the magnetization M. it is given by Put therefore, we get
Evaluating integral and substituting the value of C from (3), we get [ ]
Where
+ is called the langevins function
is shown in figure 2.
The variation of M with
M mn
Initial slope
Figure 2 Case(i):At low temperatures or large applied field,
Hence, the magnetization M in this case will be
So saturation is reached when all the atomic dipoles are parallel to B Case (ii): Under normal conditions is very small. Then,
Where
is called the Curie constant
Failure of langevins theory (i) Langevins theory was unable to explain a more complicated dependence of susceptibility upon temperature exhibited by several paramagnetics such as highly compressed and cooled gasses, very concentrated solutions of salts etc... Langevins theory could not account for the intimate relation between Para-and ferromagnetism.
(ii)
Weiss Modification Langevins theory applies only to gasses, where the molecules are sufficiently far apart for their mutual interactions to be negligible. In liquids and solids such interactions may be large, and many substances obey the modification Curie-Weiss law
is called the curie temperature and is characteristic of the substance. Eq.(10) holds only at | |Eq.(10) is of the same form as Eq.(9), except that the origin of temperatures where temperature is shifted from 0 to Ferromagnetism Ferromagnetic substances are very strongly magnetic, the best known examples of ferromagnets are the transition metals Fe, Co, and Ni. A ferromagnet has a spontaneous magnetic moment a magnetic moment even in zero applied fields. The atoms (or molecules)of ferromagnetic materials have a net intrinsic magnetic dipole moment which is primarily due to the spin of electrons. The interaction between the neighbouring atomic magnetic dipoles is very strong. It is called spin exchange interaction and is present even in the absence of an external magnetic field. It turns out that the energy of two neighbouring atomic magnets due to interaction is the least when their magnetic moments are parallel. The neighbouring magnetic moments are therefore strongly constrained to take parallel orientation (see figure 3,a). This effect of exchange interaction to align the neighbouring magnetic moments parallel to one another spreads over a small finite volume of the bulk a. b. c. Figure 3
This small (1-0.1 mm across) volume of the bulk is called a domain. All magnetic moments within a domain will point in the same direction, resulting in a large magnetic moment. Thus the bulk material consists of many domains. The domains are oriented in different directions. The total magnetic moment of a sample of substance is the vector sum of the magnetic moments of the component domain In unmagnetised piece of ferromagnetic material, the magnetic moments of the domains themselves are not aligned. When an external field is applied, those domains that are aligned with the field increasing size at the expense of the others. In a very strong field all the domains are lined up in the direction of the field and provide the high observed magnetization. Antiferromagnetism and Ferrimagnetism The only type of magnetic order which has been considered thus far is ferromagnetism, in which , in fully magnetized state, all the dipoles are aligned in exactly the same direction (figure 3,a). There are, however, substances which show different types of magnetic order. In antiferromagnetic materials such as Cr and MnO, the dipoles have equal moments, but adjacent dipoles joint in opposite direction (see figure 3, b). Thus the moments balance each other, resulting in a zero net magnetization. In ferrimagnetic materials (also called ferrites) such as , the magnetic moments of adjacent ions are anti parallel and of unequal strength (see figure 3, c) so there is a finite net magnetization. By suitable choice of rare earth ions in the ferrite lattices it is possible to design ferrimagnetic substances with specific magnetizations for use in electronic components. Weiss theory of Ferromagnetism According to Weiss, the atomic magnets of a ferromagnetic substance are grouped into certain regions or domains. When the substance is in the unmagnetised condition, the domains form closed chains with no free poles. When the substance is magnetized, the chains break up and the domains gradually set themselves with their magnetic axes all pointing in he field direction. Thus ferromagnetism is a crystal phenomenon Weiss assumed that a molecular magnetic field exists at the position of every atom or molecule this field arises due to the interaction of all neighbouring molecules. The molecular field is proportional to the magnetization vector
Here
=molecular field coefficient
The effective field strength may be regarded as the vector sum of external applied field strength B and the internal molecular field strength Hence
10
Consider 1 gram mole of the substance. Let
Then ( ) Then ( )
The domains will obey the general theory of paramagnetism by langevin ( Here, )
When external field is zero,
Now,
Here N=Avogadros number
11
Or
Eqs.(3) and (4) simultaneously determine the condition of spontaneous magnetization figure 4 shows a graph drawn between and corresponding to Es. (3) and (4)
curve 1 is langevin curve corresponding to Eq.(3) while straight line 2 is corresponding to Eq.(4) . the two curves meet at 0 and A. the solution true solution A B is not true. Hence is the
C (1) 0.5 (2)
2 Figure 4
It is obvious from the graph that A represents a stable state of spontaneous magnetization. If the molecules in a domain assume sate C, then the local magnetization equilibrium sate A, now the magnetization and the value of will in consequence increase until the state AD is reached . on the other hand , if the molecules in domain assume state B, then the local magnetization is more than the equilibrium value. Now the magnetization and the value of will tend to decrease until the state AD is reached We know that the slope of the tangent at the origin of the langevin curve is since , when is small. Hence the condition for stable spontaneous magnetization is given by
12
But
, the Curie point. Hence T<
Hence below the Curie point , in the absence of the external field, the domains are spontaneously magnetized to a degree depending upon temperature. Approaching the saturation value as the temperature approaches absolute zero. Above the Curie point , spontaneous magnatisation no longer occurs, the ferromagnetic properties disappear and the substance becomes paramagnetic. Therefore, Curie temperature is transition temperature. Above Curie point, the substance obeys Curie-Weiss law Applications Nuclear Magnetic Resonance A nucleus with a nonzero spin acts like a tiny magnet. When placed in a magnetic field, the magnetic moment vector precesses round the field direction. This is called Larmour precession. This precession is quantized. If this precessing nucleus is placed in the path of beam of electromagnetic radiation there will be coherent interaction between the particle and the radiation provided the frequency of the radiation is equal to that of precession. The phenomenon is thus one of resonance. In the case of nuclei, the phenomenon is called nuclear magnetic resonance (N.M.R) Expression for Resonance Frequency If a nucleus of magnetic moment direction. Then its energy is is placed in a uniform magnetic field in the z-
The magnetic moment
is quantized according to the usual rule
Where is the - factor for the nucleus. Hence the energy levels of the nucleus in the magnetic field are
The energy difference between adjacent energy levels is
The corresponding transition frequency is
13
NMR spectrometer Figure 4 shows a schematic arrangement for observing the nuclear magnetic resonance, and is based on the energy absorption method
Electromagnet Oscilloscope
Rfgenerator
Amplifier mixer and other circuitry
(Static field)
Rf-coil
Figure-5 The specimen S, about 1 c. c. of the material being investigated, is placed between the poles of an electromagnet. This magnet produces the field . An r-f coil surrounding the specimen is carefully positioned to produce a second field perpendicular to . An r-f generator not only serves to drive the coil but also supplies a signal to auxiliary circuitry which measures the r-f power absorbed by the specimen. To trace out the absorption line, an auxiliary low frequency oscillator supplies power to secondary coils wound on the main magnet core. These coils permit the main field. Such a signal is sketched in figure 6 Power absorbed
Figure 6
14
NMR finds a number of practical applications. It can be used for the accurate determination of nuclear moments. In a sensitive form of magnetometer NMR can be used for measuring magnetic fields. In medicine NMR tomography has been developed. In this technique images of tissues are produced by the magnetic resonance. Whole plants, small animals or parts of bigger animals are placed between the pole pieces of suitably designed magnet. Using surface coils of wire radio frequency radiation is directed towards the part under study. Spectra obtained in this way are used for medical diagnosis. This method is supposed to be superior diagnosis by X rays because the potential damage to living tissue by X rays is not present in the case of radio frequency radiation
You might also like
- Advanced Functional Materials: A Perspective from Theory and ExperimentFrom EverandAdvanced Functional Materials: A Perspective from Theory and ExperimentNo ratings yet
- Magnetism Notes CompleteDocument11 pagesMagnetism Notes CompleteSathya Sai Kumar Yeluri100% (1)
- Langevin of DiamagnetismDocument6 pagesLangevin of Diamagnetismlakshmi2811No ratings yet
- Molecular Magnetism ExplainedDocument13 pagesMolecular Magnetism ExplainedAkongseh NgwanaNo ratings yet
- Ferrofluid CharactersDocument10 pagesFerrofluid CharactersPondok Huda100% (1)
- ParamagnetismDocument29 pagesParamagnetismKousik DubeyNo ratings yet
- FerrimagnetismDocument6 pagesFerrimagnetismca_luisgNo ratings yet
- Magnetochemistry: Synthesis and Determination of The Magnetic Moment of Some Iron and Nickel ComplexesDocument9 pagesMagnetochemistry: Synthesis and Determination of The Magnetic Moment of Some Iron and Nickel ComplexeskawtherahmedNo ratings yet
- Module2 Lesson2 ElectromagDocument5 pagesModule2 Lesson2 ElectromagJerald AlvaradoNo ratings yet
- Lab Manual 2 (Magnetism Phase Transition)Document14 pagesLab Manual 2 (Magnetism Phase Transition)Furqan Ali CheemaNo ratings yet
- VocabDocument17 pagesVocabEJHNo ratings yet
- Magnetic and Optical Prop. of MaterialsDocument75 pagesMagnetic and Optical Prop. of MaterialsNiña Marie RoblesNo ratings yet
- Magnetic Particle CharacterizationDocument30 pagesMagnetic Particle Characterizationtesfahun mollaNo ratings yet
- Magnetic Materials Explained: Diamagnetic, Paramagnetic and FerromagneticDocument4 pagesMagnetic Materials Explained: Diamagnetic, Paramagnetic and FerromagneticpannNo ratings yet
- Magnetism Is A Property of Materials That Respond at An Atomic or Subatomic Level To An Applied Magnetic FieldDocument13 pagesMagnetism Is A Property of Materials That Respond at An Atomic or Subatomic Level To An Applied Magnetic Fieldprasanth20101987No ratings yet
- Magnetic Properties of MaterialsDocument31 pagesMagnetic Properties of MaterialssoumyajyotideyNo ratings yet
- Diamagnetism: Submitted By: Group 1Document25 pagesDiamagnetism: Submitted By: Group 1Rida FatimaNo ratings yet
- The Ising Model: Indrek MandreDocument20 pagesThe Ising Model: Indrek MandremebadboyNo ratings yet
- Para MagnetismDocument7 pagesPara MagnetismNima ChenNo ratings yet
- Magnetism and MatterDocument29 pagesMagnetism and MatterRitesh AgarwalNo ratings yet
- Magnet Presentation - EMT PhysicsDocument24 pagesMagnet Presentation - EMT PhysicsletsjoyNo ratings yet
- Chapter 5 - Magnetism and Matter - WatermarkDocument23 pagesChapter 5 - Magnetism and Matter - WatermarkKABIR KALWANINo ratings yet
- Magnetism and Matter Short NotesDocument4 pagesMagnetism and Matter Short NotesgokulravirgNo ratings yet
- Magnetochemistry Principles for Transition Metal ComplexesDocument7 pagesMagnetochemistry Principles for Transition Metal ComplexesYousuf Raza100% (1)
- Permanent MagnetDocument16 pagesPermanent MagnetDamiano ZitoNo ratings yet
- Introduction to Magnetochemistry: An Overview of Magnetic Properties and States of MatterDocument8 pagesIntroduction to Magnetochemistry: An Overview of Magnetic Properties and States of MatterMuhammad ZubairNo ratings yet
- Introduction to Magnetochemistry in Transition Metal ComplexesDocument4 pagesIntroduction to Magnetochemistry in Transition Metal Complexespeoples1231697No ratings yet
- 1 Assignment: Name: Muhammad RizwanDocument14 pages1 Assignment: Name: Muhammad RizwanMuhammad RizwanNo ratings yet
- Magnetic susceptibility measurement and classification of materialsDocument8 pagesMagnetic susceptibility measurement and classification of materialsthisispasswordNo ratings yet
- Magnetism III: Magnetic Ordering: Physics 355Document27 pagesMagnetism III: Magnetic Ordering: Physics 355Engr Arfan Ali DhamrahoNo ratings yet
- Lab Report OnDocument10 pagesLab Report Onbhaski88No ratings yet
- Types of MagnetismDocument5 pagesTypes of MagnetismNithish KumarNo ratings yet
- Magnetism NotesDocument5 pagesMagnetism Noteskalalund2562006No ratings yet
- Fundamenals of MagnetismDocument13 pagesFundamenals of MagnetismBewnet GetachewNo ratings yet
- DiamagnetismDocument6 pagesDiamagnetismMis ter StudenttNo ratings yet
- Unit-4 - Magnetic Properties - notes@VNR VJIET PDFDocument29 pagesUnit-4 - Magnetic Properties - notes@VNR VJIET PDFVamshiNo ratings yet
- Electricity & MagnetismDocument26 pagesElectricity & MagnetismM HASIN ISHMAM JEETNo ratings yet
- 3-1 Classification of Magnetic MaterialsDocument4 pages3-1 Classification of Magnetic MaterialsSattwik MannaNo ratings yet
- Material ScienceDocument20 pagesMaterial ScienceVarsha PraburamNo ratings yet
- Class NotesDocument18 pagesClass NotesRajaul Morshad SaikotNo ratings yet
- Classes of Magnetic MaterialsDocument3 pagesClasses of Magnetic MaterialsVĩnh PhạmNo ratings yet
- Magnetic Materials - 1Document72 pagesMagnetic Materials - 1Amit JangirNo ratings yet
- Magnetic Properties of Matter ExplainedDocument6 pagesMagnetic Properties of Matter ExplainedAtiqur Rahman SakibNo ratings yet
- Magnetic PropertiesDocument29 pagesMagnetic PropertieshrsreenathNo ratings yet
- FerromagnetismDocument48 pagesFerromagnetismkawtherahmedNo ratings yet
- MagnetismDocument221 pagesMagnetismBharat Ramkumar63% (8)
- Magnetic Properties of MatterDocument8 pagesMagnetic Properties of MatterRobin SinghNo ratings yet
- Magnet 2Document12 pagesMagnet 2peoples1231697No ratings yet
- Material (Magnetic Material) 211269143036Document11 pagesMaterial (Magnetic Material) 211269143036Sani perryNo ratings yet
- 604b0e32cae10d00af2efddb - ## - Magnetism & Matter Lect 03 NotesDocument70 pages604b0e32cae10d00af2efddb - ## - Magnetism & Matter Lect 03 NotesMishthi SinghNo ratings yet
- Dielectrics and Magnetic Materials GuideDocument128 pagesDielectrics and Magnetic Materials GuideSankalp NistalaNo ratings yet
- Clasification of Magnetic MaterialsDocument16 pagesClasification of Magnetic MaterialsdirtycutfreakNo ratings yet
- MC-511, Part 3: Nuclear Magnetic Resonance (NMR) Lecture NotesDocument57 pagesMC-511, Part 3: Nuclear Magnetic Resonance (NMR) Lecture NotesMaheshNo ratings yet
- Introduction To MagnetochemistryDocument46 pagesIntroduction To Magnetochemistrypathisharma100% (1)
- Superparamagnetism - WikipediaDocument31 pagesSuperparamagnetism - Wikipediatufanbanerjee000No ratings yet
- Chapter - 6 Engineering MaterialsDocument5 pagesChapter - 6 Engineering MaterialsSanjiv BadheNo ratings yet
- ES2: Electron orbitals and magnetic propertiesDocument34 pagesES2: Electron orbitals and magnetic propertiesTam Jun HuiNo ratings yet
- Physics Project Netural Point of A MagneDocument26 pagesPhysics Project Netural Point of A MagneOshnic100% (1)
- Notes On Magnetic MaterialDocument12 pagesNotes On Magnetic MaterialGauri Deshmukh KaranjgaokarNo ratings yet
- Diagnostic TestDocument1 pageDiagnostic TestAyesh Nayana GunawardanaNo ratings yet
- Inter Stellar MediumDocument18 pagesInter Stellar MediumAyesh Nayana GunawardanaNo ratings yet
- My QFT NoteDocument16 pagesMy QFT NoteAyesh Nayana GunawardanaNo ratings yet
- Advance level physics conceptsDocument32 pagesAdvance level physics conceptsAyesh Nayana GunawardanaNo ratings yet
- Problem 9:: Solutions For Class #3 From Yosumism WebsiteDocument9 pagesProblem 9:: Solutions For Class #3 From Yosumism WebsiteHiya MukherjeeNo ratings yet
- Runge Kutta - MatlabDocument6 pagesRunge Kutta - MatlabAyesh Nayana GunawardanaNo ratings yet
- Matlab Code For Heat EquationDocument2 pagesMatlab Code For Heat EquationAyesh Nayana GunawardanaNo ratings yet
- Water Quality ManagementDocument77 pagesWater Quality ManagementAyesh Nayana GunawardanaNo ratings yet
- Vector and Matrix NormDocument17 pagesVector and Matrix NormpaivensolidsnakeNo ratings yet
- Speak Test BookDocument128 pagesSpeak Test BookAyesh Nayana GunawardanaNo ratings yet
- Group TheoryDocument48 pagesGroup TheoryAyesh Nayana GunawardanaNo ratings yet
- Magnetic Properties of MaterialsDocument15 pagesMagnetic Properties of MaterialsAyesh Nayana GunawardanaNo ratings yet
- Heat Temp and 0th Law of ThermodynamicsDocument3 pagesHeat Temp and 0th Law of ThermodynamicsAyesh Nayana GunawardanaNo ratings yet
- Taylor and Laurent series convergenceDocument84 pagesTaylor and Laurent series convergenceAparna ViswanathNo ratings yet
- Mous Song ThesisDocument55 pagesMous Song ThesisAyesh Nayana GunawardanaNo ratings yet
- A Model For Coronal Phase Hydrogen Plasma of Interstellar Medium Part 1Document18 pagesA Model For Coronal Phase Hydrogen Plasma of Interstellar Medium Part 1Ayesh Nayana GunawardanaNo ratings yet
- Result Obtained From Dirac Delta FunctionDocument17 pagesResult Obtained From Dirac Delta FunctionAyesh Nayana GunawardanaNo ratings yet
- Fluid DynamicsDocument6 pagesFluid DynamicsAyesh Nayana GunawardanaNo ratings yet
- Role of DSS in RefineryDocument24 pagesRole of DSS in Refineryramadoss_alwar7307100% (1)
- Review of Essential Oils from Annonaceae PlantsDocument13 pagesReview of Essential Oils from Annonaceae PlantsroxanaNo ratings yet
- As 2278.1-2008 Aerosol Containers Metal Aerosol Dispensers of Capacity 50 ML To 1000 ML InclusiveDocument7 pagesAs 2278.1-2008 Aerosol Containers Metal Aerosol Dispensers of Capacity 50 ML To 1000 ML InclusiveSAI Global - APAC50% (2)
- DESN 10041 - Exam-2020-MainDocument4 pagesDESN 10041 - Exam-2020-MainZikani NyirendaNo ratings yet
- Computational Models For Drug Design and DelivDocument235 pagesComputational Models For Drug Design and DelivIbrahim Al SharabiNo ratings yet
- დ მიქელაძის-ბიოქიმიაDocument201 pagesდ მიქელაძის-ბიოქიმიაJuli JulianaNo ratings yet
- Solvent Extraction Principles and Practice 2nd Ed - Jan Rydberg Et Al. (Marcel Dekker, 2004)Document724 pagesSolvent Extraction Principles and Practice 2nd Ed - Jan Rydberg Et Al. (Marcel Dekker, 2004)Milena Katarina Stojiljkovic100% (6)
- Aldehyde, Ketones and Carboxylic AcidDocument18 pagesAldehyde, Ketones and Carboxylic AcidPRADEEP CNo ratings yet
- Is 2986Document9 pagesIs 2986sreenathaNo ratings yet
- 12 Biology Notes Ch06 Molecular Basis of InheritanceDocument14 pages12 Biology Notes Ch06 Molecular Basis of Inheritancehimanshu kumarNo ratings yet
- Synthesis and Discriminative Stimulus Properties of N(6)-Alkyl Norlysergic Acid DerivativesDocument4 pagesSynthesis and Discriminative Stimulus Properties of N(6)-Alkyl Norlysergic Acid Derivativesmik100% (1)
- Wilson Tool Coating Solutions - SMDocument2 pagesWilson Tool Coating Solutions - SMSM TECH SRLNo ratings yet
- Johnson Industrial Screens PDFDocument20 pagesJohnson Industrial Screens PDFjaime palenzuela rodriguezNo ratings yet
- Chapter-1 Classification of MaterialsDocument46 pagesChapter-1 Classification of MaterialstrfuawlachewNo ratings yet
- Syntheses of Soap and DetergentDocument4 pagesSyntheses of Soap and DetergentChin Castro Zabat100% (2)
- Chemical Reactions and Energy ChangesDocument6 pagesChemical Reactions and Energy ChangesMiku HatsuneNo ratings yet
- Advance Engine Timing with STCDocument28 pagesAdvance Engine Timing with STCLuisPupiales100% (1)
- Hydrogen BondsDocument2 pagesHydrogen BondsJohnNo ratings yet
- ZeTo RulesDocument30 pagesZeTo RulesRamli Disa100% (5)
- Toxic Substances and Disease Registry MRLs for March 2016Document16 pagesToxic Substances and Disease Registry MRLs for March 2016KodeChandrshaekharNo ratings yet
- ASPHALT METHOD STATEMENTDocument7 pagesASPHALT METHOD STATEMENTBasem Donia100% (1)
- FuelsDocument22 pagesFuelsADITYA GAURNo ratings yet
- Induction Sealing - Wikipedia, The Free EncyclopediaDocument6 pagesInduction Sealing - Wikipedia, The Free EncyclopediaSohail ShaikhNo ratings yet
- Conocophillips Indonesia - Ramba: Job Description: Area/Unit: Tag No/Equipment Description: ReferencesDocument3 pagesConocophillips Indonesia - Ramba: Job Description: Area/Unit: Tag No/Equipment Description: Referencesaneshse100% (9)
- ASTM D3013 Epoxy Molding CompoundsDocument3 pagesASTM D3013 Epoxy Molding CompoundsNelson RomeroNo ratings yet
- Flexible Packaging Solutions For Liquids: Packaging and Specialty PlasticsDocument12 pagesFlexible Packaging Solutions For Liquids: Packaging and Specialty PlasticsSupermobile 786No ratings yet
- Chemistry SBA7 ReportDocument6 pagesChemistry SBA7 ReportSam ChanNo ratings yet
- Determination of Moisture ContentDocument21 pagesDetermination of Moisture ContentasadaltafgillNo ratings yet
- ASTM G5-94 Standard Practice PDFDocument12 pagesASTM G5-94 Standard Practice PDFMarcela BaronaNo ratings yet
- Adam Lechter: Resident Course in Confectionery TechnologyDocument73 pagesAdam Lechter: Resident Course in Confectionery TechnologyJulio KinenNo ratings yet
- Quantum Physics: What Everyone Needs to KnowFrom EverandQuantum Physics: What Everyone Needs to KnowRating: 4.5 out of 5 stars4.5/5 (48)
- Quantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessFrom EverandQuantum Spirituality: Science, Gnostic Mysticism, and Connecting with Source ConsciousnessRating: 4 out of 5 stars4/5 (6)
- Quantum Physics for Beginners Who Flunked Math And Science: Quantum Mechanics And Physics Made Easy Guide In Plain Simple EnglishFrom EverandQuantum Physics for Beginners Who Flunked Math And Science: Quantum Mechanics And Physics Made Easy Guide In Plain Simple EnglishRating: 4.5 out of 5 stars4.5/5 (18)
- Too Big for a Single Mind: How the Greatest Generation of Physicists Uncovered the Quantum WorldFrom EverandToo Big for a Single Mind: How the Greatest Generation of Physicists Uncovered the Quantum WorldRating: 4.5 out of 5 stars4.5/5 (8)
- Summary and Interpretation of Reality TransurfingFrom EverandSummary and Interpretation of Reality TransurfingRating: 5 out of 5 stars5/5 (5)
- The Tao of Physics: An Exploration of the Parallels between Modern Physics and Eastern MysticismFrom EverandThe Tao of Physics: An Exploration of the Parallels between Modern Physics and Eastern MysticismRating: 4 out of 5 stars4/5 (500)
- Midnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterFrom EverandMidnight in Chernobyl: The Story of the World's Greatest Nuclear DisasterRating: 4.5 out of 5 stars4.5/5 (409)
- A Brief History of Time: From the Big Bang to Black HolesFrom EverandA Brief History of Time: From the Big Bang to Black HolesRating: 4 out of 5 stars4/5 (2193)
- The Physics of God: How the Deepest Theories of Science Explain Religion and How the Deepest Truths of Religion Explain ScienceFrom EverandThe Physics of God: How the Deepest Theories of Science Explain Religion and How the Deepest Truths of Religion Explain ScienceRating: 4.5 out of 5 stars4.5/5 (23)
- Packing for Mars: The Curious Science of Life in the VoidFrom EverandPacking for Mars: The Curious Science of Life in the VoidRating: 4 out of 5 stars4/5 (1395)
- The End of Everything: (Astrophysically Speaking)From EverandThe End of Everything: (Astrophysically Speaking)Rating: 4.5 out of 5 stars4.5/5 (155)
- The Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifeFrom EverandThe Magick of Physics: Uncovering the Fantastical Phenomena in Everyday LifeNo ratings yet
- Lost in Math: How Beauty Leads Physics AstrayFrom EverandLost in Math: How Beauty Leads Physics AstrayRating: 4.5 out of 5 stars4.5/5 (125)
- Bedeviled: A Shadow History of Demons in ScienceFrom EverandBedeviled: A Shadow History of Demons in ScienceRating: 5 out of 5 stars5/5 (5)
- The Holographic Universe: The Revolutionary Theory of RealityFrom EverandThe Holographic Universe: The Revolutionary Theory of RealityRating: 4.5 out of 5 stars4.5/5 (75)
- Strange Angel: The Otherworldly Life of Rocket Scientist John Whiteside ParsonsFrom EverandStrange Angel: The Otherworldly Life of Rocket Scientist John Whiteside ParsonsRating: 4 out of 5 stars4/5 (94)
- The Beginning of Infinity: Explanations That Transform the WorldFrom EverandThe Beginning of Infinity: Explanations That Transform the WorldRating: 5 out of 5 stars5/5 (60)
- Starry Messenger: Cosmic Perspectives on CivilizationFrom EverandStarry Messenger: Cosmic Perspectives on CivilizationRating: 4.5 out of 5 stars4.5/5 (158)
- What is Life?: With Mind and Matter and Autobiographical SketchesFrom EverandWhat is Life?: With Mind and Matter and Autobiographical SketchesRating: 4 out of 5 stars4/5 (139)
- The Sounds of Life: How Digital Technology Is Bringing Us Closer to the Worlds of Animals and PlantsFrom EverandThe Sounds of Life: How Digital Technology Is Bringing Us Closer to the Worlds of Animals and PlantsRating: 5 out of 5 stars5/5 (5)
- Quantum Physics for Beginners: Simple Illustrated Guide to Discover with Practical Explanations the Paradoxes of the Life and Universe Reconsidering RealityFrom EverandQuantum Physics for Beginners: Simple Illustrated Guide to Discover with Practical Explanations the Paradoxes of the Life and Universe Reconsidering RealityRating: 2 out of 5 stars2/5 (1)
- Black Holes: The Key to Understanding the UniverseFrom EverandBlack Holes: The Key to Understanding the UniverseRating: 4.5 out of 5 stars4.5/5 (13)