Professional Documents
Culture Documents
Peds Review: Blood/Neoplastic Disorders: Factor VIII Clotting Prolonged Partial Thromboplastin Time
Uploaded by
Diana HyltonOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Peds Review: Blood/Neoplastic Disorders: Factor VIII Clotting Prolonged Partial Thromboplastin Time
Uploaded by
Diana HyltonCopyright:
Available Formats
Peds Review: Blood/Neoplastic Disorders
Question 1 An 8-week-old breastfed boy is brought to the clinic for his health supervision visit. His mother thinks he may be more pale than her other children, but he has otherwise been healthy. Findings on physical examination and vital signs are normal. He does not appear pale to you. A complete blood count reveals hemoglobin of 9 g/dL (90 g/L) and a mean cell volume of 85 fL. The remainder of the complete blood count is normal. Of the following, the MOST appropriate recommendation for this infant is to a) administer a daily multivitamin and folic acid b) admit the infant for a packed red blood cell transfusion c) measure serum iron and transferrin concentrations
d)
e)
reassure the mother that the anemia will resolve Physiologic anemia of infancy (6 wks 3months) switch feeding to an iron-fortified infant formula
Question 2 A previously healthy 15-year-old girl returns from summer camp in the mountains complaining of dysuria, frequency, and urgency. You diagnose cystitis and prescribe trimethoprim-sulfamethoxazole. Her mother phones 3 days later to report that the girl is very tired and appears pale. You advise her mother to bring her to your office. On examination, she appears pale and your order laboratory tests. The girl's hemoglobin is 8.5 g/dL (85.0 g/L), a decrease from the value of 11.5 g/dL (115.0 g/L) that was measured during her pre-camp physical examination. Her reticulocyte count is 5.0% (0.050), and the red cell indices are normal except for mild microcytosis with a mean corpuscular volume of 76 fL. You review a smear Of the following, the MOST likely cause of this girl's rapid onset of anemia is a) glucose-6-phosphate dehydrogenase deficiency b) hemoglobin SC disease c) hereditary elliptocytosis d) inadequate dietary iron e) pyelonephritis G6PD deficiency is the most common disease-producing enzymopathy in humans. Inherited as an X-linked disorder, primarily as single-base mutations in the G6PD locus at Xq28, G6PD deficiency affects 400 million people worldwide. The highest prevalence rates (with gene frequencies from 5% to 25%) are found in tropical Africa, the Middle East, tropical and subtropical Asia, some areas of the Mediterranean, and Papua New Guinea. The defect occurs in approximately 13% of African American males. Homozygous women are found in populations in which the frequency of G6PD deficiency is high. Heterozygous (carrier) women may develop hemolytic attacks. The gene confers some protection against malaria, which probably accounts for its high gene frequency in modern populations. The G6PD enzyme catalyzes the oxidation of glucose-6-phosphate to 6-phosphogluconate while reducing the oxidized form of nicotinamide adenine dinucleotide phosphate (NADP+) to nicotinamide adenine dinucleotide phosphate (NADPH). NADPH, a required cofactor in many biosynthetic reactions, maintains glutathione in its reduced form. Reduced glutathione is a scavenger for dangerous oxidative metabolites in the cell. Red blood cells depend on G6PD activity as the only protection against oxidative stresses. People deficient in G6PD, therefore, are at risk for hemolysis and its sequelae when exposed to oxidative stress. The degree of hemolysis varies with dose of the inciting agent. The enzymatic defect in Americans of African descent is statistically less severe than that of people of Mediterranean descent. The enzyme also is found in lower quantities in older red blood cells due to senescence of the enzyme. Affected individuals typically do not display symptoms or signs in the absence of oxidative stress. However, those who have very low enzyme concentrations may present with neonatal jaundice and acute hemolytic anemia. Neonatal jaundice usually appears by age 1 to 4 days, at the same time as or slightly earlier than so-called physiologic jaundice; kernicterus is rare. Acute hemolytic anemia results from stress factors such as oxidative drugs or chemicals, infection, or ingestion of fava beans (common to a Mediterranean diet). Gallstones may be a prominent feature in affected individuals. As with hemolysis of different causes, jaundice and splenomegaly may be present during a crisis. Immediately after an episode, younger red blood cells are released that contain a higher concentration of enzyme. Accordingly, testing should be delayed in the event of undiagnosed hemolysis in children for a few weeks after the hemolysis to allow G6PD to decrease to normal concentrations. The clinician should have a high suspicion for G6PD deficiency in immigrants of the ethnic groups noted previously and consider testing potentially affected males who exhibit hemolysis as part of a significant illness (such as diabetic acidosis, hepatitis) or trauma, especially prior to administering medications that may precipitate hemolysis. Precipitants include antibacterials (especially sulfonamides), antimalarials (chloroquine, primaquine), and other medications (aspirin, vitamin K analogs). Chemicals that may induce hemolysis include naphthalene, which is found in mothballs. Question 3 A newborn male experiences prolonged oozing following circumcision. Hematologic evaluation reveals that he has less than 1% of factor VIII clotting activity and a prolonged partial thromboplastin time, consistent with severe hemophilia A. His family history is negative for any individuals affected by clotting disorders. Of the following, the MOST accurate statement for counseling this child's parents is that a) another family member likely is affected, but the condition is so mild that the person has not been diagnosed b) in families such as this, 50% of affected boys have a spontaneous gene mutation c) molecular genetic testing can detect mutations in 50% of affected individuals d) severe hemophilia is the rarest of all types e) there is an 80% chance that the mother is a hemophilia carrier One third to one half of all males who have hemophilia A have no family history of the disorder, as described for the infant in the vignette. In these cases, it is important to recognize that multiple genetic scenarios can result in such an outcome. In about 20% of the cases involving no previous family history for hemophilia, the mother is not a carrier, in which case her son has a de novo disease-causing mutation. In some of these situations, the son is a somatic mosaic for the mutation, ie, the mutation is not present in all of the cells of his body. In 80% of such cases, the mother is a carrier, and she might have a de novo gene mutation. This mutation could have occurred in the egg or sperm cell from which she was
conceived (in which case it is present in all of her cells). Alternatively, it may be due to a somatic mutation that occurred in early embryogenesis or to germline mosaicism, in which case it is present in only some of her germ cells. The mother could have inherited the disease-causing mutation from her mother or unaffected father (if he is a somatic or germline mosaic). Finally, the mother could have inherited the mutation from a previous generation, which may have been passed on only through daughters, for example, so that no one was affected with hemophilia A. Cases in which the mutation for hemophilia A is present in a mosaic state are unusual, and the gene mutation runs true in families. Therefore, every boy in a family who has the same mutation will have the same severity of disease. Severe hemophilia (<1% normal factor VIII activity) is the most common of all types of hemophilia A. Molecular genetic testing can detect mutations in 98% of those who have severe disease. Question 4 When a 14-year-old girl had frequent complaints of shoulder pain made worse by pitching softball a few months ago, you diagnosed overuse injury. Nonsteroidal antiinflammatory drugs and rest have provided some relief. She presents today with complaints of recurrent upper arm pain that is unrelated to exercise and sometimes awakens her from sleep. Physical examination reveals a slightly larger circumference of the left proximal humerus compared with the right. There is minimal tenderness on palpation over the area, although the girl reports a constant ache. She has full range of motion of the arm at the shoulder and elbow. You obtain a shoulder radiograph Of the following, the MOST likely diagnosis is a) acromioclavicular separation b) acute osteomyelitis c) chronic osteomyelitis d) osteosarcoma e) supracondylar fracture of the humerus The differential diagnosis of bone pain and swelling includes stress fracture, hematoma, bone cysts, and other bony tumors such as Ewing sarcoma. Initial evaluation of an adolescent or older child presenting with bone pain and swelling, especially with a palpable mass, should include: Plain radiographs (two views) of the suspected lesions, although no single feature on radiographs is diagnostic. Osteosarcomatous lesions can be purely osteolytic (about 30% of patients), purely osteoblastic (about 45% of patients), or a mixture of both. Elevation of the periosteum may appear as the characteristic Codman triangle. Extension of tumor through the periosteum may result in a socalled "sunburst appearance" (about 60% of patients). Both magnetic resonance imaging of the primary lesion and computed tomography scan of the chest are necessary to confirm the diagnosis and for staging purposes. Such scans frequently are performed at the tertiary center using their protocols. Question 5 You are treating a 2-year-old girl who has suspected meningococcal bacteremia and meningitis. Over the past 2 hours, she has required multiple fluid boluses and inotropic support to help maintain her blood pressure. She has been intubated due to respiratory failure. Her temperature is 96F (35.6C), and she is covered in a petechial and purpuric rash (Item Q44A). Her most recent laboratory results reveal a white blood cell count of 1.2x103/mcL (1.2x109/L) with 80% lymphocytes, 10% neutrophils, and 10% band forms and a platelet count of 32x103/mcL (32x109/L). Of the following, the MOST important additional laboratory test is a) erythrocyte sedimentation rate b) measurement of creatine kinase c) measurement of fibrinogen d) measurement of lactic acid e) review of peripheral blood smear
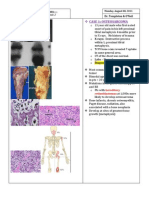
Question 6 A male infant is born without a right thumb. Laboratory testing reveals a platelet count of 60 x 103/mcL (60 x 109/L). There is no evidence of bleeding. Of the following, the BEST management of this patient is a) bone marrow biopsy b) computed tomography of the head c) genetics consultation d) intravenous corticosteroid therapy e) intravenous immunoglobulin G The newborn who has thrombocytopenia may be at risk for spontaneous hemorrhage, especially if platelet counts decrease to less than 10 to 15 x 103/mcL (10 to 15 x 109/L). Thrombocytopenia may be associated with numerous chromosomal and genetic syndromes, and one of the most readily discernible is the thrombocytopenia-absent radius (TAR) syndrome (Item C196A). Affected patients have bilateral absence of the radius, abnormal carpal bones and phalanges, clubbed hands, or phocomelia. Thumbs typically are present. Additionally, the thrombocytopenia in infants who have TAR becomes apparent in the first week after birth with spontaneous hemorrhage (90% of patients are symptomatic by 4 months of age). Intracranial hemorrhage may be present with seizure, lethargy, poor feeding, apnea, or hemodynamic collapse. The symptomatic patient should have platelet counts evaluated and imaging of the central nervous system (eg, computed tomography). Petechiae are common, and any procedure that could be associated with bleeding (eg, circumcision, phlebotomy) should be avoided. The thrombocytopenia is associated with a hyporegenerative marrow that is unresponsive to corticosteroids or intravenous immunoglobulin G. Bone marrow aspiration is both risky and unnecessary. Thrombopoietin levels are normal to elevated. Management of infants who have TAR syndrome includes avoidance of trauma and elective surgeries and maintaining a platelet count greater than 15 x 103/mcL (15 x 109/L) using platelet transfusions. The thrombocytopenia typically is self-limited, with normal platelet counts being established by age 2 years. The limb anomalies require physical therapy, occupational therapy, pediatric orthopedic and physical medicine consultation, and rehabilitation over a number of years. Genetics consultation is required because some cases of TAR syndrome are autosomal recessive, and recurrence risks should be addressed. Question 7 An 18-month-old infant presents with a skin eruption that consists of discrete red, orange, and yellow-brown papules (Item Q2A). On physical examination, you note scaling dermatitis of the scalp, postauricular, perineal, and axillary areas. A skin biopsy reveals CD1a-positive monocyte macrophages that contain Birbeck granules. Of the following, the MOST likely diagnosis for this infant is
a) b) c) d) e)
acute monocytic leukemia familial erythrophagocytic lymphohistiocytosis infection-associated hemophagocytic syndrome Langerhans cell histiocytosis malignant histiocytosis
The infant described in the vignette exhibits physical findings and skin biopsy characteristics consistent with Langerhans cell histiocytosis (LCH). The name of LCH has been applied to the class I histiocytoses (Item C2A), previously known as histiocytosis X, and includes the clinical entities of eosinophilic granuloma, Hand-SchllerChristian disease, and Letterer-Siwe disease. The normal Langerhans cell is an antigen-presenting cell of the skin. The hallmark of LCH is the Birbeck granule (Item C2B), a tennis racket-shaped bilamellar granule, which when seen in the cytoplasm of lesional cells in LCH, is diagnostic of the disease. Alternatively, LCH can be diagnosed definitively by demonstrating CD1a (surface markers on T cells) positivity of lesional cells. Class II histiocytoses are characterized by accumulation of antigen-processing cells (ie, macrophages). These phagocytic cells lack the two class I markers (Birbeck granules and CD1a positivity) characteristic of LCH cells. The two major diseases of class II histiocytoses are familial erythrophagocytic lymphohistiocytosis and infection-associated hemophagocytic syndrome. In both diseases, disseminated lesions involve many organ systems. The lesions are characterized by infiltration of the involved organ with activated phagocytic macrophages and lymphocytes. Class III histiocytoses are characterized by neoplastic proliferation of cells that have characteristics of monocytes/macrophages or their precursors. Acute monocytic leukemia and malignant histiocytosis are included in this category. In both conditions, Birbeck granules are absent, and cells are CD1a negative. Question 8 An 18-month-old girl comes to your clinic with her mother, who reports that the girl has seemed pale over the last week. The mother explains that there has been no fever, change in appetite, or weight loss. The girl has been healthy, but she always has been a picky eater. On physical examination, you find an alert girl who has marked conjunctival pallor. Her weight is at the 24th percentile, and her heart rate is 160 beats/min. There is no hepatosplenomegaly. You note a c-cm bruise on her left buttock and several smaller bruises on her upper arms and abdomen. Findings on the remainder of her examination are within normal limits. Of the following, the MOST likely diagnosis is a) acute lymphoblastic leukemia b) child abuse c) immune thrombocytopenic purpura d) iron-deficiency anemia e) transient erythroblastopenia of childhood The clinical features of pallor and bruising in the child described in the vignette suggest anemia and thrombocytopenia. In such a case, when two cell lines appear to be affected, an impairment of normal bone marrow function (ie, erythropoiesis and thrombopoiesis) is likely. Viral infections are a common cause of bone marrow suppression in children. IN addition, any disease that affects the bone marrow can cause marrow failure, including aplastic anemia, myelofibrosis, and leukemias and other malignancies, such as neuroblastoma. Therefore, of the conditions listed, the most likely diagnosis in this child is acute lymphoblastic leukemia. Children who have acute lymphoblastic leukemia typically present with symptoms suggestive of bone marrow failure, with the involvement of more than one cell line, as well as bone and joint pain lymphadenopathy, and organomegaly. Occasionally, affected children present with an abnormality of only one cell line, such as neutropenia or thrombocytopenia. Lymphoblasts may be seen on peripheral smear, but the absence of these cells does not rule out the diagnosis. A bone marrow examination and biopsy are necessary to make the diagnosis. Child abuse should be suspected in any child who presents with bruising in atypical areas of the body, such as the trunk, face, or buttocks. However, marked pallor would not be seen unless massive bleeding from occult trauma was present. Immune thrombocytopenic purpura is a common cause of bruising in young children. Hemoglobin levels typically are normal in this disease; mild anemia due to bleeding may be present, but it is not severe enough to cause significant pallor. Iron-deficiency anemia and transient erythroblastopenia of childhood are diseases that affect only the erythroid cell line, so bruising is not present. Question 9 A 15-month-old infant has been breastfed since birth. He eats finger foods (eg, peas, carrots) and occasionally some cereal. His mother adheres to a vegan diet and plans the same for her child. A complete blood count documents anemia. Of the following, the MOST likely cause of this infants anemia is a deficiency of a) folic acid b) niacin c) riboflavin d) thiamine
e)
vitamin B12
The infant described in the vignette has been exclusively breastfed by a strict vegan mother and is at risk for anemia due to vitamin B12 deficiency. The appearance of hypersegmented neutrophils in the peripheral blood precedes the development of classic megaloblastic (macrocytic) anemia. The infants deficiency may be caused by reduced placental transfer of vitamin B12 in utero and lower vitamin content in the mothers milk. In addition, the infant is being offered other foods that are consistent with a vegan diet and contain little vitamin B12. Deficiency of vitamin B12, including dietary deficiency, is unusual in infants and children in the United States. Other causes of deficiency are pernicious anemia; impaired gastric and small bowel absorption, including resections; and rare inherited metabolic disorders such as methylmalonic aciduria and intrinsic factor deficiency. Vitamin B12 deficiency is diagnosed by recognition of risk factors, documentation of low serum B12 concentrations in the infant, and a subsequent response to treatment. Demonstration of low serum vitamin B12 values in the mother supports the diagnosis. Folic acid deficiency is an unlikely cause of the anemia for the infant described in the vignette. Folate is present in many foods consumed by vegans, and human milk provides adequate folate for the breastfed infant. Folate deficiency rarely is present in newborns because the fetus extracts adequate folate from the mother, even in the presence of maternal deficiency. Defects of folate metabolism are rare, although infants exclusively fed goat milk are at risk for folate deficiency and possible megaloblastic anemia. A diagnosis of folate deficiency should be confirmed, including the exclusion of vitamin B12 deficiency, before initiating folate replacement therapy. Treatment with therapeutic doses of folate may delay the diagnosis of vitamin B12 deficiency and increase the risk of neurologic complications. Erythrocyte folic acid concentration is a better measure of folate sufficiency than serum folate concentrations. Deficiencies of thiamine (vitamin B1), riboflavin (vitamin B2), and niacin (vitamin B3) are rare and not associated with anemia. Thiamine deficiency is associated with beriberi (cardiomyopathy, peripheral neuropathy, and encephalopathy). Riboflavin deficiency is associated with cheilosis and glossitis. Pellagra (dementia, diarrhea, and dermatitis) is associated with niacin deficiency.
Question 10 You are asked to see a 7-year-boy in whom medulloblastoma (primitive neuroectodermal tumor) was diagnosed at age 3 years. Treatment at that time consisted of chemotherapy and craniospinal irradiation. During the past year, he grew 2 cm, although he is eating normally, and his weight is appropriate for height. Despite spinal irradiation, the upper-to-lower segment ratio is normal for his age. Of the following, the MOST likely diagnosis is a) acquired growth hormone deficiency b) chemotherapy-induced renal failure c) Cushing syndrome d) irradiation-induced epiphyseal fusion e) tumor recurrence Question 11 You are seeing a 30-year-old multigravid woman for prenatal counseling. She has had immune thrombocytopenic purpura for the past 5 years, and her spleen was removed 2 years ago. She asks you about the effects that her disease might have on her unborn child. Of the following, you are MOST likely to tell her that a) if her newborn has thrombocytopenia, he or she will be treated with intravenous immunoglobulin b) maternal platelet counts predict fetal risks of intracranial hemorrhage c) maternal platelet transfusion during pregnancy will minimize the risk for neonatal thrombocytopenia d) operative delivery of the newborn will reduce the risk of intracranial hemorrhage e) the newborn will require a platelet transfusion soon after birth Question 12 A 6-year-old child presents for a health supervision visit. On physical examination, his weight is 18 kg and height is 102 cm (<3rd percentile), his pulse rate is 90 beats/min, respiratory rate is 18 breaths/min, and blood pressure is 134/88 mm Hg. Of note, he has pale conjunctivae and mild edema. Among the results of laboratory evaluation are: Hemoglobin, 7.5 g/dL (75 g/L)
Serum creatinine, 12.1 mg/dL (1,070 mcmol/L) The stool is negative for occult blood. Of the following, the MOST likely explanation for this patient's anemia is a) chronic gastrointestinal blood loss b) erythropoietin deficiency c) folic acid deficiency d) hemolysis e) iron deficiency Question 13 A 6-month-old girl, who was born in Nigeria, presents for an urgent visit as soon as the family arrives in the United States because of fever and irritability. Physical examination reveals a fussy infant who has anorexia, a temperature of 100F (37.8C), and swelling of all of the fingers of the right hand (Item Q228). The remainder of the examination findings are negative.
White blood cell count, 6x103/mcL (6x109/L) Platelet count, 275x103/mcL (275x109/L) Mean cell volume, 82 fL Reticulocyte count, 0.4% (0.004) Blood urea nitrogen, 94 mg/dL (33.6 mmol/L)
Of the following, the MOST likely cause of this pattern of swelling in this child is a) cellulitis b) juvenile idiopathic arthritis c) malaria d) sickle cell disease e) trauma
You might also like
- MRCPCH Guide EndoDocument7 pagesMRCPCH Guide EndoRajiv KabadNo ratings yet
- Prep For USMLEDocument96 pagesPrep For USMLEdrhassam90No ratings yet
- Neonatal PresenatestionDocument32 pagesNeonatal PresenatestionMerwan KemalNo ratings yet
- Azazim 2012 PDFDocument27 pagesAzazim 2012 PDFShowmik PaulNo ratings yet
- Hematology McqsDocument63 pagesHematology McqsGalaleldin AliNo ratings yet
- 1 Answer by DR - Hayder Al-Nashmei 1-: Kallmann's SyndromeDocument3 pages1 Answer by DR - Hayder Al-Nashmei 1-: Kallmann's SyndromeAladdin Ali Abu DanielNo ratings yet
- SickleDocument6 pagesSickleNalini T GanesanNo ratings yet
- Adolescent Gynecologic Care QUESTIONS 1, 2, 3Document7 pagesAdolescent Gynecologic Care QUESTIONS 1, 2, 3patelkn_2005No ratings yet
- Antiphospholipid Antibody Syndrome and PregnancyDocument17 pagesAntiphospholipid Antibody Syndrome and Pregnancybidan22No ratings yet
- File 195 - PAX6 Aug 2019Document8 pagesFile 195 - PAX6 Aug 2019asmaarabee50No ratings yet
- Genes and Human Disease: Monogenic DiseasesDocument8 pagesGenes and Human Disease: Monogenic DiseasesE.R.ONo ratings yet
- Genes and Human Disease: Monogenic DiseasesDocument8 pagesGenes and Human Disease: Monogenic DiseasesE.R.ONo ratings yet
- Genetic Disease in HumansDocument5 pagesGenetic Disease in Humanslastjoe71No ratings yet
- Pediatric Endocrinology Review Mcqs - Part 2: April 2020Document39 pagesPediatric Endocrinology Review Mcqs - Part 2: April 2020Bhagwani LohanaNo ratings yet
- Antiphospholipid SyndromeDocument8 pagesAntiphospholipid SyndromeVijeyachandhar DorairajNo ratings yet
- 21Document28 pages21Mateen ShukriNo ratings yet
- Diagnosis and Management of Autoimmune Hepatitis: A Clinical GuideFrom EverandDiagnosis and Management of Autoimmune Hepatitis: A Clinical GuideMark W. RussoNo ratings yet
- Pediatric Endocrinology Review MCQS: Part - 6Document29 pagesPediatric Endocrinology Review MCQS: Part - 6wanjala francis xavierNo ratings yet
- MCC QE1 Questions Answers 4Document8 pagesMCC QE1 Questions Answers 4BasirQidwai100% (3)
- Antiphospholipid Antibody Syndrome and PregnancyDocument7 pagesAntiphospholipid Antibody Syndrome and PregnancystepepsNo ratings yet
- Case+Study AGN MINEDocument66 pagesCase+Study AGN MINEJm BernardoNo ratings yet
- Thesis Statement On Sickle Cell AnemiaDocument7 pagesThesis Statement On Sickle Cell Anemiacoawokugg100% (1)
- Pre EclampsiaDocument12 pagesPre EclampsiaJohn Mark PocsidioNo ratings yet
- Favism Mti ResearchDocument3 pagesFavism Mti Researchlobna adelNo ratings yet
- Nej MoDocument9 pagesNej MoNadia Mrz Mattew MuseNo ratings yet
- Manuscript 2Document5 pagesManuscript 2maryNo ratings yet
- Question: 1 of 10 / Overall Score: 60%: True / FalseDocument11 pagesQuestion: 1 of 10 / Overall Score: 60%: True / FalseGalaleldin AliNo ratings yet
- SMFM Sickle Cell Disease in PregnancyDocument24 pagesSMFM Sickle Cell Disease in PregnancyAngela FernandezNo ratings yet
- Prolactinoma MEdscapeDocument12 pagesProlactinoma MEdscapeHenryNo ratings yet
- Antiphospholipidsyndrome 141019122948 Conversion Gate01Document50 pagesAntiphospholipidsyndrome 141019122948 Conversion Gate01asri khazaliNo ratings yet
- Endocrine DisordersDocument51 pagesEndocrine DisordersavisenicNo ratings yet
- Hipofisis 3Document23 pagesHipofisis 3RafaelPetitNo ratings yet
- JaundiceDocument20 pagesJaundicelourdesfercab_at_msnNo ratings yet
- Nephrology Best RDocument6 pagesNephrology Best Rfrabzi100% (1)
- Reading Test 2 Part A: TIME: 15 MinutesDocument22 pagesReading Test 2 Part A: TIME: 15 Minutesçiğdem ünsalNo ratings yet
- A Model for Gene Therapy: Gene Replacement in the Treatment of Sickle Cell Anemia and ThalassemiaFrom EverandA Model for Gene Therapy: Gene Replacement in the Treatment of Sickle Cell Anemia and ThalassemiaNo ratings yet
- Tall StatureDocument24 pagesTall StaturedianmutiaNo ratings yet
- Precocious Puberty With Primary Hypothyroidism Due To Autoimmune ThyroiditisDocument3 pagesPrecocious Puberty With Primary Hypothyroidism Due To Autoimmune ThyroiditisShohel RanaNo ratings yet
- Wikipedia: 3.1.-Una Patologia On Està Implicada. Modificació Estructural I Funcional (100 Paraules) (Jose)Document4 pagesWikipedia: 3.1.-Una Patologia On Està Implicada. Modificació Estructural I Funcional (100 Paraules) (Jose)jcasanovas1995No ratings yet
- Aplastic Anemia, 2008Document10 pagesAplastic Anemia, 2008j.doe.hex_87No ratings yet
- CF and SidsDocument7 pagesCF and SidsEira LopezNo ratings yet
- Sharief F 2011Document14 pagesSharief F 2011wiwiNo ratings yet
- Background: Special DietsDocument4 pagesBackground: Special DietsGusti YoandaNo ratings yet
- Recurrent Non-Immune Fetal Hydrops: A Case ReportDocument3 pagesRecurrent Non-Immune Fetal Hydrops: A Case ReportHervi LaksariNo ratings yet
- 2006 FRACP Written Examination Paediatrics & ChildDocument59 pages2006 FRACP Written Examination Paediatrics & ChildMedicEdNo ratings yet
- PEBjournalDocument8 pagesPEBjournalLisa Linggi'AlloNo ratings yet
- Glucos-6 Phosphate: Regional Government of Kurdistan University of Salahaddin Department of BiologyDocument13 pagesGlucos-6 Phosphate: Regional Government of Kurdistan University of Salahaddin Department of BiologyMohammed MohammedNo ratings yet
- MRCPass Notes For MRCP 1 - HEMATOLOGYDocument9 pagesMRCPass Notes For MRCP 1 - HEMATOLOGYsabdali100% (1)
- Hiperparatiroidismo PrimarioDocument10 pagesHiperparatiroidismo Primariopruebaprueba321765No ratings yet
- Jaundice: Nccu Clinical Guidelines S: 10Document3 pagesJaundice: Nccu Clinical Guidelines S: 10Gomathi Sankara Narayanan VenkataramanNo ratings yet
- Case Study PaediatricsDocument8 pagesCase Study PaediatricsDaudNo ratings yet
- WHO - Thalassaemia and Other HaemoglobinopathiesDocument8 pagesWHO - Thalassaemia and Other HaemoglobinopathiesZahra MaulidaNo ratings yet
- Adrenal MedullaDocument32 pagesAdrenal MedullaAmanda MartinezNo ratings yet
- Maple Syrup Urine DiseaseDocument14 pagesMaple Syrup Urine DiseasefantasticoolNo ratings yet
- Reading Test - 19 Part - A: TIME: 15 MinutesDocument17 pagesReading Test - 19 Part - A: TIME: 15 MinutesKelvin KanengoniNo ratings yet
- Hematologic Disorders PowerpointDocument51 pagesHematologic Disorders PowerpointjoycechicagoNo ratings yet
- Endocrine MCQDocument51 pagesEndocrine MCQMayyada ShihadaNo ratings yet
- Sickle-Cell Anaemia EssayDocument4 pagesSickle-Cell Anaemia Essayapi-299807117No ratings yet
- Metabolism Q and ADocument86 pagesMetabolism Q and AFlowerNo ratings yet
- Complementary and Alternative Medical Lab Testing Part 9: GynecologyFrom EverandComplementary and Alternative Medical Lab Testing Part 9: GynecologyNo ratings yet
- 1.05.12 Depression in Elderly OutlineDocument8 pages1.05.12 Depression in Elderly OutlineDiana HyltonNo ratings yet
- Fetal Alcohol Syndrome: Clinical Diagnosis Smooth Philtrum: Thin Vermillion Small Palpebral FissuresDocument5 pagesFetal Alcohol Syndrome: Clinical Diagnosis Smooth Philtrum: Thin Vermillion Small Palpebral FissuresDiana HyltonNo ratings yet
- Benjamin Moore Pain TDocument7 pagesBenjamin Moore Pain TDiana HyltonNo ratings yet
- Op Thom OlogyDocument2 pagesOp Thom OlogyDiana HyltonNo ratings yet
- 12.14.11 Geriatric Immunization & ScreeningDocument6 pages12.14.11 Geriatric Immunization & ScreeningDiana HyltonNo ratings yet
- IC Midterm Exam ADocument18 pagesIC Midterm Exam ADiana HyltonNo ratings yet
- Biostatis Tics (Recovered)Document3 pagesBiostatis Tics (Recovered)Diana Hylton100% (1)
- Bone & Joint Path IIDocument4 pagesBone & Joint Path IIDiana HyltonNo ratings yet
- My BiostatsDocument11 pagesMy BiostatsDiana HyltonNo ratings yet
- Hy Kaplan PulmDocument9 pagesHy Kaplan PulmDiana HyltonNo ratings yet
- Cerebrovascular DiseaseDocument6 pagesCerebrovascular DiseaseDiana HyltonNo ratings yet
- Kapla N OncologyDocument4 pagesKapla N OncologyDiana HyltonNo ratings yet
- Cerebrovascular DiseaseDocument6 pagesCerebrovascular DiseaseDiana HyltonNo ratings yet
- Arterial Blood Gas Cases: Case 1Document4 pagesArterial Blood Gas Cases: Case 1Diana HyltonNo ratings yet
- 3.26.12 Treatment of GI DisordersDocument6 pages3.26.12 Treatment of GI DisordersDiana Hylton100% (4)
- AFM 2013 JoyinPracticeDocument7 pagesAFM 2013 JoyinPracticeDiana HyltonNo ratings yet
- Arterial Blood Gas Cases: Case 1Document4 pagesArterial Blood Gas Cases: Case 1Diana HyltonNo ratings yet
- My Renal Rounds NotesDocument4 pagesMy Renal Rounds NotesDiana HyltonNo ratings yet
- ShockDocument1 pageShockDiana HyltonNo ratings yet
- Abg CaseAnswersDocument7 pagesAbg CaseAnswersDiana HyltonNo ratings yet
- Check Patient Name-Correct Patient? 2. What Is The Rate? 3. Is This Sinus Rhythm? A. If Not, What Is It? 4. 5. 6. A. B. C. D. 7. 8. 9. 10Document1 pageCheck Patient Name-Correct Patient? 2. What Is The Rate? 3. Is This Sinus Rhythm? A. If Not, What Is It? 4. 5. 6. A. B. C. D. 7. 8. 9. 10Diana HyltonNo ratings yet
- GI Dysmotility IIIDocument11 pagesGI Dysmotility IIIDiana HyltonNo ratings yet
- EKG Primer2woEKGsDocument26 pagesEKG Primer2woEKGsDiana HyltonNo ratings yet
- GI PretestDocument1 pageGI PretestDiana HyltonNo ratings yet
- Usmle Step1 FilesDocument9 pagesUsmle Step1 FilesDiana HyltonNo ratings yet
- Viral Midterm ReviewDocument7 pagesViral Midterm ReviewDiana HyltonNo ratings yet
- PolymyositisDocument1 pagePolymyositisDiana HyltonNo ratings yet
- ShockDocument1 pageShockDiana HyltonNo ratings yet
- Congenital Heart DiseaseDocument2 pagesCongenital Heart DiseaseDiana HyltonNo ratings yet
- Guidance Document - Nutritional Care & Support For TB Patients in India PDFDocument107 pagesGuidance Document - Nutritional Care & Support For TB Patients in India PDFekalubisNo ratings yet
- Home Environmental AssessmentDocument2 pagesHome Environmental AssessmentSelvi Puspa SariNo ratings yet
- Down SyndromeDocument20 pagesDown SyndromeJessa DiñoNo ratings yet
- Gastric Varices Management 2020Document14 pagesGastric Varices Management 2020Oscar ArdilaNo ratings yet
- MSPE Example File 2018Document5 pagesMSPE Example File 2018ingridNo ratings yet
- Aarc 2002Document10 pagesAarc 2002Yolanda ShintaNo ratings yet
- Wu2016 Article ClinicalComparisonBetweenTwoHyDocument7 pagesWu2016 Article ClinicalComparisonBetweenTwoHyRhelvis1No ratings yet
- VaccinationDocument6 pagesVaccinationapi-19916399No ratings yet
- Concept of Rasa (Taste) Order in Usage As Medicine and FoodDocument4 pagesConcept of Rasa (Taste) Order in Usage As Medicine and FoodShubham MakwanaNo ratings yet
- Matary Surgical RadiologyDocument304 pagesMatary Surgical RadiologyRaouf Ra'fat Soliman80% (10)
- Medical TechnologistDocument2 pagesMedical Technologistapi-307429612No ratings yet
- Nutrition Assessment Lab 2Document10 pagesNutrition Assessment Lab 2Lee Yann LynnNo ratings yet
- OctreotideDocument12 pagesOctreotiderpadmakrishnanNo ratings yet
- Project CharterDocument9 pagesProject CharterTatianaObregon100% (1)
- Links To Clinical Info About The VaccineDocument9 pagesLinks To Clinical Info About The VaccinekarunaNo ratings yet
- Tom Rogers: A Life Worth LivingDocument4 pagesTom Rogers: A Life Worth LivingWilliam MacfadyenNo ratings yet
- PneumoniaDocument27 pagesPneumoniamameekasim75No ratings yet
- Stress Affects Us AllDocument4 pagesStress Affects Us AllOmayma ChaoukiNo ratings yet
- Annotated BibliographyDocument10 pagesAnnotated Bibliographyapi-338437548100% (1)
- Atonia UteriDocument22 pagesAtonia UteriDarra KurniasariNo ratings yet
- Biology Project Class 12Document16 pagesBiology Project Class 12Anshika Singh100% (1)
- Persuasive LetterDocument2 pagesPersuasive Letterapi-341527188No ratings yet
- Tickborne Diseases of The United States: A Reference Manual For Healthcare ProvidersDocument52 pagesTickborne Diseases of The United States: A Reference Manual For Healthcare ProvidersJorge Navarrete LozanoNo ratings yet
- Assessment Diagnosis Planning Intervention Rationale EvaluationDocument1 pageAssessment Diagnosis Planning Intervention Rationale EvaluationPamela Angelica Obaniana FisicoNo ratings yet
- Patient CareDocument24 pagesPatient CareMuhammad ShahZad100% (1)
- Updated Ico Residency CurriculumDocument219 pagesUpdated Ico Residency CurriculumEdoga Chima EmmanuelNo ratings yet
- Chn-Jessie DaclisDocument3 pagesChn-Jessie DaclisJasmine JarapNo ratings yet
- Er DR Ward ScriptDocument3 pagesEr DR Ward ScriptChristine Mae MacatangayNo ratings yet
- Medicinal PlantsDocument214 pagesMedicinal PlantsArnold Jr Bautista100% (1)
- 4.1 Prevail - Tan - Hi-Tech vs. Hi-Touch Understanding The Patient's Expectations in The Real WorldDocument38 pages4.1 Prevail - Tan - Hi-Tech vs. Hi-Touch Understanding The Patient's Expectations in The Real WorldMark Anthony EllanaNo ratings yet