Professional Documents
Culture Documents
Clinical Evaluation of Aavaraiyathi Churnam in The Management of Type 2 Diabetes Mellitus
Uploaded by
Velpandian PathyOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Clinical Evaluation of Aavaraiyathi Churnam in The Management of Type 2 Diabetes Mellitus
Uploaded by
Velpandian PathyCopyright:
Available Formats
Available online at www.ijpcr.
com International Journal of Pharmaceutical and Clinical Research 2012; 4(4): 37-40 ISSN- 0975 1556 Research Article
Clinical Evaluation of Aavaraiyathi churnam in the Management of Type 2 Diabetes Mellitus
*1Anbu N , 2Velpandian V
1 2
Department of Maruthuvam (Medicine), Government Siddha Medical College, Chennai-106. Department of Gunapadam (Pharmacology), Government Siddha Medical College, Chennai.
ABSTRACT Diabetes mellitus is a heterogeneous disorder of carbohydrate, protein and fat metabolism in which sugars in the body are not oxidized to produce energy due to lack of pancreatic hormone insulin leads to disturbances of the acid base balance. There are two types. Type 1 (IDDM) patients have little or no ability to produce the hormone and are entirely dependent on insulin, whereas Type II (NIDDM) results from inadequate production of insulin which can be controlled by the oral hypoglycemic drugs. Aavaraiyathi churnam is one of the herbal based Siddha anti diabetic formulation for Type II maturity onset diabetes mellitus. So the present research work was carried out to confirm the hypoglycemic effect of Aavaraiyathi churnam clinically on patients with DM (Type II). Open clinical trial was conducted in 50 diabetic patients for the period of six months. Blood glucose level (both fasting and post prandial) were estimated before the enrollment of the study during the trial period and after the completion of the study. At the end of the study period all these parameters were statistically analyzed. The clinical studies revealed that Aavaraiyathi churnam (AC) was well tolerated in high dose and moderate diabetic cases (180 300 mg / dl). The blood sugar level is controlled within 4 16 week, depending upon its initial blood sugar level. No side effects were noted during the study period which confirms the traditional use Aavaraiyathi churnam for the treatment of diabetes mellitus. Key Words: Diabetes mellitus, insulin, Type II NIDDM, Aavaraiyathi churnam, blood glucose level, anti diabetic activity. INTRODUCTION Diabetes is one of the ancient disease. The history of diabetes is stated that in the Ebers papyrus ( 1500 BC )[1] . Polyuria and honey urine was noted as early as 400 BC by the Indian Physician Susrutha. He has described this disease as Madhumeham which means- the honey in the urine [2]. Diabetes mellitus is an absolute or relative deficiency of insulin due to hyperglycemia is characterized by polyuria, polyphagia, and polydipsia. Diabetes mellitus is the commonest endocrine disorder that affects more than 171 million people worldwide [3]. If it is not controlled by medicine it will affect most of the internal organs such as nephropathy, neuropathy, retinopathy etc.[4] Plants are always an excellent source of drugs; in fact many of the currently available drugs were derived either directly or indirectly from them. According to world ethno botanical information reports, almost 800 plants may possess anti-diabetic potential [5]. In the past decade, research has been forced on scientific evaluation of traditional drugs of plant origin and screening of more effective and safe hypoglycemic agents has continued to be an important area of present day research. In developing countries 80 % of population is using traditional medicine in primary medical problems [6]. In the present study, an attempt has been made to investigate the anti-diabetic activity of Aavaraiyathi churnam in NIDDM patients clinically. Aavaraiyathi churnam is a poly herbal formulation, comprising of 5 indigenous medicinal plants namely Cassia auriculata leaves, flower, seed, bark and root bark, Odina wodier bark, Coscinium fenestratum stem, Ficus glomerata tender leaves and Cocculus cordifolius stem. [7] All these plants are well known in the traditional system of medicine from time immortal for their various therapeutic properties especially hypoglycemic activity. [9-13] MATERIALS AND METHODS The Open clinical based trial was conducted at Arignar Anna Government Hospital of Indian Medicine, Chennai from July 2011 to December 2011. The protocol of this clinical study was approved by the Institutional Ethical Committee and conducted according to the guidelines of the Declaration of Helsinki [14]. Total number of 50 cases of either sex and irrespective of socio economic status was selected for this trial from OPD and IPD from the hospital in which 27 males (54%) and 23 females (46%) chosen for the study as shown in Table No.1. All the patients recruited in this study were categorized in different class interval ranging from 35 years of age to 65 years of age as shown in Table No.1. 36 patients were treated as out-patient department and 14 patients who were very sick and were hospitalized in Arignar Anna Hospital, Arumbakkam, Chennai-106. They were all under the medical care on direct supervision of the author. After written informed consent (both English and Tamil) was obtained from the
Author for correspondence: Email: nanbu.sumi@gmail.com
Anbu N, Velpandian V / Clinical Evaluation of...
Table No.1 showing Age and Sex distribution Category 35 - 43 yrs Type 2 DM Male: 07
44-51 yrs Male: 10
52-59 yrs Male: 06
60 65 yrs Male: 04
Female: 04
Female: 06
Female: 11
Female: 02
Table No.2 showing the signs and symptoms of Type 2 DM patients and showing the clinical improvement at the end of the study Signs and Symptoms 35 -43 44 - 51 52 - 59 years 60 65 Improvement in percentage n = 50 years years years Polyuria 10 15 17 06 96 Polydipsia 09 14 17 06 97 Polyphagia 08 15 16 05 95 General weakness 06 15 15 06 97 Itching 04 06 04 02 94 Loss of weight 02 03 01 01 86 Body pain 01 02 14 05 95 Table No. 3 : Showing the efficacy of Aavaraiyathi churnam on FBGL, PPBGL and HbA1C in treated patients Group (n Fasting glucose (mg/dl) Post Prandial HbA1C (mg %) = 50) (mg/dl) BT AT BT AT BT AT Type 2 152.461.21 96.401.06*** 236.043.09 150.121.34*** 8.560.10 6.160.06 DM Medical College, Chennai. The vouchers of specimen patients before enrollment and proper history samples of the plants were kept in the department for and clinical examination were recorded at future reference. After identification the above plants base line and each follow up. The initial were cleaned well with water and dried in a shadow Fasting Blood Glucose Level (FBGL), Post place. After complete drying, they powdered separately Prandial Blood Glucose Level (PPBGL) and and mixed all together and the trial medicine was HbA1C were estimated at the time of prepared as per the classical text. Good manufacturing enrollment of the study, during the follow up practice was emphasized during the preparation. Then the and at the end of the study. The initial and prepared drug is kept in air tight container which was final readings were compared statistically at used for clinical study. The drug was given to the patients the end of the study and were recorded. at the dose of 3 to 4 gm twice a day with hot water Selection Criteria: Patients selected for this study were depending upon the severity of the disease. The patients based on inclusion criteria and exclusion criteria. were advised to avoid direct sugar, underground root Inclusion criteria for the patients with confirmed vegetables and to take plenty of leafy and fiber diagnosis of type 2 diabetes, patients of either sex vegetables. between the age of 35-65 years, patients with FBGL Laboratory Investigations: Patients under Aavaraiyathi 140 mg/dl , PPBGL 250 mg/dl and HbA1C 7 were churnam trial were subjected to the following laboratory included.[15,16] Patients taking medicines for other health investigations. The initial biochemical analysis of FBGL, conditions, other critical complications like GIT, hepatic, generally done in nearby PPBGL and HbA1C were cardiovascular, renal or endocrine disorder ( other than laboratories and at the hospital of confinement. However, diabetes mellitus ), IDDM, AIDS, STD, HT, pregnancy, to assure comparability of results, blood samples were lactation, smoking, alcohol abuse were excluded from the obtained from each patient prior to, during and after study. Aavaraiyathi churnam administration. About 3 to 5 cc of Formulation of The Trial Drug: The poly herbal blood was obtained from each patient through veneformulation of Aavaraiyathi Churnam described in puncture by using disposable syringes both fasting and Anuboga Vaithiya Navaneetham Part-9. [8] post prandial which was used for the analysis of serum Ingredients of the drug: Cassia auriculata (Aavarai) glucose level. leaves, flower, seed, bark and root bark each 35 gm, Odina wodier (Odhiyam) bark 87.5 gm, Coscinium STATISTICAL ANALYSIS fenestratum (Maramanjal) stem 43.75, Ficus glomerata Statistical analysis was done according to intention-to(Atthi) tender leaves 21.75 gm, Cocculus cordifolius treat principles. Drug concentrations and all derived (Seenthil) stem 183 gm were collected from in and parameters were listed and summarized descriptively. around Chennai. All the plants were identified and authenticated by the botanist, Government Siddha
IJPCR, October-December 2012, Vol 4, Issue 4, 37-40
Page
38
Anbu N, Velpandian V / Clinical Evaluation of...
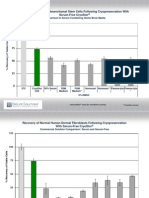
Fig No.1. Serum glucose level (fasting)
Fig No.2. Serum glucose level (PP)
Fig No.3. Glycated haemoglobin level in mg percentage The changes in various parameters in the post-treatment values were carried out by Pairedt test. P values 0.05 were considered statistically significant which is shown in Table No. 3. RESULTS AND DISCUSSION Diabetic care needs patience, compassion optimism and from the physician. Patient must be actively involved in his own management and should develop confidence to bring about adjustment in day to day life management. The poly herbal formulation Aavaraiyathi churnam used in this study was selected on the basis of their traditional use in Siddha system of medicine. All the herbs used in this formulation have independent hypoglycemic activity which was explored scientifically by various research works. For example Cassia auriculata leaves, flower, seed, bark and root bark, Odina wodier bark, Coscinium fenestratum stem, Ficus glomerata tender leaves, Cocculus cordifolius stem were used to treat diabetes mellitus. The primary criteria are evaluating the efficacy of the drug. Total number of 50 Type 2 diabetes mellitus patients were treated with Aavaraiyathi churnam 3-4 gm
IJPCR, October-December 2012, Vol 4, Issue 4, 37-40
Page
two times a day with hot water for one month duration. The improvement in clinical features and bio chemical analysis of this open clinical study was summarized in the Table No. 2 and Table No.3 respectively. Based on the results it was observed that there was an extremely significant reduction in fasting blood glucose level, post prandial blood glucose level and HbA1C level in patients who were completed the clinical trial successfully. All the patients were found symptomatic improvement by clinically. Patients reported improvement was observed in frequency of urine were reduced. Nocturia, unquenched thirst was subsided and the general feeling of well being was reported by all the patients, polyphagia and weakness were subsided. At the time of admission, the initial mean FBGL was 152.461.21 and PPBGL was 236.043.09. After completion of the trial the mean FBGL and PPBGL were 96.401.06*** and 150.121.34*** respectively. The declined trend was observed at a constant level. In addition, there was significant reduction of glycated hemoglobin (HbA1C) was observed which is summarized in Table No.3. Based on this, the initial level of glycated hemoglobin was 8.560.10 %, and after treatment the level was 6.16 0.06 % which confirmed the
39
Anbu N, Velpandian V / Clinical Evaluation of...
hypoglycemic effect of the poly herbal formulation of Aavaraiyathi churnam regardless of age and sex. Untoward effects: There was no side effects were noticed during the study period. CONCLUSION Aavaraiyathi churnam is more effective in the treatment of type 2 diabetes mellitus as determined by extreme statistically significant p-value < 0.0001. The patients were very clear improvement of clinically and biochemical investigations. The test drug did not display or show any untoward manifestations associated with the use of this medication. The mechanism of the hypoglycemic activity of poly herbal formulation Aavaraiyathi churnam may be due to enhance the peripheral utilization of glucose, normalize the hepatic glycolysis and gluconeogenesis. So this study suggests that the Aavaraiyathi churnam had very good hypoglycemic effects proved by clinical improvement and bio-chemical analysis. ACKNOWLEDGEMENT The authors are thankful to the Principal, Government Siddha Medical College, Chennai 106 and the Head of the department of Medicine, Government Siddha Medical College, Chennai for their constant help and support. REFERENCES 1. Kathryn M Kiag, Greg Rubin. British Journal of Nursing, Vol.12, issue 18.1091-1095 2. Charaka Samhita Vol II. Chikitsasthana. P.V. Sharma. Chaukhamba Orientalia, Varanasi (India), 1983; 118. 3. American Diabetes Association. Diagnosis and Classification of diabetes mellitus. Diabetic Care .2008: 31:S55-60. 4. Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes. Estimates for the year 2000 and projections for 2030. Diabetes Care 2004; 27: 1047-1053. 5. Edwards CRW, Boucheir IAD, Haslett C, Chilvers ER. Davidsons Principles and Practice of Medicine. British Library Cataloguing in Publication Data Seventeenth edition 1995; 724-774.
6.
7.
8.
9.
10.
11.
12.
13.
14.
15. 16.
Alarcon-Aguilara, F.J., R.Roman-Ramas,S.Peroz Gutierrez, A.Aguilator-Contreras, C.C.Conteras Weber and J.L.Flores Saenz,1998. Study of antihyperglycemic effect of Plants used as anti-diabetics. J.E.Ethnopharmacol, 61:101-110. Grover J.K and SP Yadav. Pharmacological actions and potential uses of Momordica charantior. A review J. Ethnopharmacol, 2004: 93:123-132 Hakkim Abdulla Sahibu. Anuboga Vaidhya Navaneetham, Part 9, Thamarai publications, September 1995: 90. Pari L, Latha M. Effect of Cassia auriculata flowers on Blood Sugar Levels, Serum and Tissue Lipids in Streptozotocin Diabetic Rats, Vol 43(12), Singapore Med J 2002: 617-621. Subramanian S, Rajeswari S, Sriram Basanth G. Antidiabetic, Antilipidemic and Antioxident Nature of Tridax procumbens studied in Alloxon- induced Experimental Diabetes in Rats, a Biochemical Approach. Asian Journal of Research in Chemistry, Volume 04, issue 11; 1732-1738. Sirinton Yibchok-anun, Wanlaya Jittaprasatsin, Darorong Somtir, Wijit Bunlunara., Sirichai Adisakwattana. Insulin secreting and glucoside inhibitory activity of Coscinium fenestratum and postprandial hyperglycemia in normal and diabetic rats. Journal of Medicinal Plants Research; Vol 3(9), 2009: 646-651. Monique SJ Simmonds, Melanie-Jayne R.Howes. 2 plants used in the Traditional of Diabetes. Traditional medicines for Modern Times Anti diabetic; 2005: 1969. Padma M Paarakh. Ficus racemosa Linn, an overview Natural Product Radiance; Vol 8(1), 2009: 84-90. World Medical Association. Declaration at Helsinki. Recommendations guiding physicians in biochemical research involving human subjects. Cardiovasc.Res.1997; 35: 2-3 WHO, 1992. Diabetes Mellitus, report of study group, Geneva WHO technical report series: 727. Nobert W Tielt. 1976, Fundamentals of clinical chemistry, Philadelphia: W.B.Saunders company 2nd ed.
IJPCR, October-December 2012, Vol 4, Issue 4, 37-40
Page
40
You might also like
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- NIMH CatalogDocument76 pagesNIMH CatalogJFNo ratings yet
- Module 2 USP Impurities - 0501Document74 pagesModule 2 USP Impurities - 0501sreekanthsharmaNo ratings yet
- Obat GlaukomaDocument8 pagesObat GlaukomaMuhammad Farrash HadyanNo ratings yet
- CRO ListDocument18 pagesCRO ListVidhyaRIyengarNo ratings yet
- Sodium+Bicarbonate Neo v2 0 PDFDocument3 pagesSodium+Bicarbonate Neo v2 0 PDFApres SyahwaNo ratings yet
- How To Critically Appraise An Article PDFDocument14 pagesHow To Critically Appraise An Article PDFPaul Joseph100% (1)
- Intramuscular Injection GuidelinesDocument2 pagesIntramuscular Injection GuidelinesPauline MartinezNo ratings yet
- Clinical Research OrganizationDocument2 pagesClinical Research OrganizationnehacodeslabNo ratings yet
- CryoStor Biopreservation EfficacyDocument9 pagesCryoStor Biopreservation EfficacyAna AsmaraNo ratings yet
- Alpha-Calcidol Vs CalcitriolDocument8 pagesAlpha-Calcidol Vs Calcitriolamin138irNo ratings yet
- Upadacitinib AR SELECT NEXT TrialDocument10 pagesUpadacitinib AR SELECT NEXT TrialMr. LNo ratings yet
- Constraint Induced Manual TherapyDocument165 pagesConstraint Induced Manual TherapyPrabhu Chinna Gounder100% (1)
- PrefixDocument6 pagesPrefixbingkydoodle1012No ratings yet
- Interventions of ImpetigoDocument97 pagesInterventions of ImpetigoAfiani JannahNo ratings yet
- Parte 1Document3 pagesParte 1Jaciel PereiraNo ratings yet
- Logemann and Vitalstim Therapy PDFDocument2 pagesLogemann and Vitalstim Therapy PDFjoygarridoNo ratings yet
- Jacobs 2007Document9 pagesJacobs 2007aiakobyNo ratings yet
- KER48201059583796394Document3 pagesKER48201059583796394fahad fahadNo ratings yet
- Amgen Inc. v. F. Hoffmann-LaRoche LTD Et Al - Document No. 292Document4 pagesAmgen Inc. v. F. Hoffmann-LaRoche LTD Et Al - Document No. 292Justia.comNo ratings yet
- Perbedaan NNT Pada Trial Effectiveness Dan NNT Trial Adverse EffectDocument8 pagesPerbedaan NNT Pada Trial Effectiveness Dan NNT Trial Adverse EffectUlquiorra SchifferNo ratings yet
- About A Metered-Dose Inhaler (MDI)Document6 pagesAbout A Metered-Dose Inhaler (MDI)Sasgia ArumNo ratings yet
- SCLC LancetDocument15 pagesSCLC LancetLeeTomNo ratings yet
- Directly Observed Therapy For Treating Tuberculosis (Review)Document34 pagesDirectly Observed Therapy For Treating Tuberculosis (Review)Karol M.No ratings yet
- EU Physiotherapy Guideline For Parkinson's DiseaseDocument29 pagesEU Physiotherapy Guideline For Parkinson's DiseaseAulia Zerlinda SyihabNo ratings yet
- Brochure 3rd World Congress On Pharmacology and Clinical TrialsDocument9 pagesBrochure 3rd World Congress On Pharmacology and Clinical TrialsbapaknyanadlirNo ratings yet
- CramsDocument14 pagesCramsJasveen SawhneyNo ratings yet
- Chapter 01Document38 pagesChapter 01Jason WalshNo ratings yet
- Dialog About Hospital PersonDocument7 pagesDialog About Hospital PersonLulu Afifah OctaviaNo ratings yet
- Kuwat Suk A 2016Document5 pagesKuwat Suk A 2016DenySidiqMulyonoChtNo ratings yet
- Cover Letter Template NIH 1Document3 pagesCover Letter Template NIH 1James RodrickNo ratings yet