Professional Documents
Culture Documents
2012 HCI H2 Chemistry Paper 3 Answers For Other JCs
Uploaded by
Ken JiaOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
2012 HCI H2 Chemistry Paper 3 Answers For Other JCs
Uploaded by
Ken JiaCopyright:
Available Formats
Suggested Answers Page 1 of 9
Hwa Chong Institution 2012 9647 / 03 / JC2 Prelim 2012
Hwa Chong Institution
2012 C2 H2 Chemistry Prelim Paper 3 Answers
1(a)(i)
(ii) P
4
is highly reactive due to strong repulsion between bond pairs of electrons / highly
strained bond angle of 60
o
(iii) P
4
is a simple non-polar covalent molecule and is unable to form favorable interactions
with polar water molecules but able to form favorable interactions with non-polar
carbon disulfide through dispersion forces.
(iv) White phosphorus burns with a bright white flame / light.
(v) P
4
(s) + 3O
2
(g) P
4
O
6
(s) or P
4
(s) + 5O
2
(g) P
4
O
10
(s)
P
4
O
6
(s) + 6H
2
O(g) 4H
3
PO
3
(s) or P
4
O
10
(s) + 6H
2
O(g) 4H
3
PO
4
(s)
(b)(i) - negligible intermolecular forces of attraction or negligible forces of attraction between
the gas particles
- volume of gas particle/molecules is negligible compared to the volume of its container
(ii) Each gaseous P
4
molecule must have dissociated / decomposed / disintegrated into
two gaseous molecules
(c)(i) They consist of metal atoms with few valence electrons which can be readily lost to
form the delocalised sea of electrons to act as mobile charges (resulting in metallic
bonding) & have high electrical conductivity.
(ii) sblock elements can only lose 1-2 es per atom while TM can lose more es from
both 3d and 4s subshells per atom to form metallic bonding due to close proximity of
the 3d and 4s subshells. Thus TMs have stronger metallic bonding and more energy
is needed to melt the metal and thus they have higher mp.
(iii) electronic configurations of Fe: [Ar] 3d
6
4s
2
; of Mn: [Ar] 3d
5
4s
2
or electronic configurations of Fe
2+
: [Ar] 3d
6
; of Mn
2+
: [Ar] 3d
5
Due to inter-electronic repulsion in 3d subshell of Fe
2+
, 3d electrons are higher in
energy, thus the 3
rd
es are easier to be removed
(d)(i)
formed MnO of mol of No
formed KMnO of mol of No
2
4
=
16.0 x 2 54.9
0.174
1.00 x
1000
20.0
x
5
1
+
=
1
2
Let O.N of Mn in A be n+
[R] Mn
n+
+ 2H
2
O + (n 4)e
MnO
2
+ 4H
+
(i)
[O] Mn
n+
+ 4H
2
O MnO
4
+ 8H
+
+ (7 n)e
(ii)
Suggested Answers Page 2 of 9
Hwa Chong Institution 2012 9647 / 03 / JC2 Prelim 2012
From equations (i) & (ii):
2
4
MnO of mol of no
MnO of mol of no
=
n 7
4 n
=
1
2
n = 6
Oxidation number of Mn = +6 A is K
2
MnO
4
(ii) Equation: 3MnO
4
2
+ 4H
+
MnO
2
+ 2MnO
4
+ 2H
2
O
or 3K
2
MnO
4
+ 2H
2
SO
4
MnO
2
+ 2KMnO
4
+ 2K
2
SO
4
+ 2H
2
O
or 3MnO
3
+ H
2
O MnO
2
+ 2MnO
4
+ 2H
+
Disproportionation (MnO
4
2
is simultaneously reduced to MnO
2
and oxidised to MnO
4
)
(iii) Mn is a TM and can form compounds of variable oxidation numbers due to close
proximity of energy levels of 4s and 3d subshells and electrons can be easily lost or
gained.
K and Ca can only form compounds of +1 and +2 respectively as a lot more energy is
required to remove more electrons from an inner quantum (3p) shell, and thus they do
not form compounds with more than 1 oxidation state.
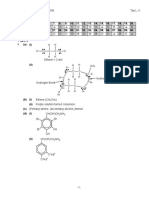
2 (a) (i) Nucleophilic addition
(ii) The negatively charged hydroxide ion is a stronger nucleophile than a
neutral water molecule.
(iii) steric reason (propanone is sterically more hindered as it has two methyl
groups attached to the carbonyl carbon)
electronic reason (propanone has two electron donating methyl groups
lowering the partial positive charge on the carbonyl carbon, making the
carbonyl carbon less susceptible to nucleophilic attack)
(iv)
(b) (i) The protonation of the carbonyl group makes the carbonyl carbon more
electron deficient (greater partial positive charge on carbonyl carbon) and
thus more susceptible for the alcohol to attack, speeding up the rate of
reaction.
(ii) In step IV, there is an elimination of water (dehydration).
With the removal of water, the forward reaction is favoured and thus
drives the equilibrium to the right to produce more acetal.
Suggested Answers Page 3 of 9
Hwa Chong Institution 2012 9647 / 03 / JC2 Prelim 2012
OR
Water can be a competing nucleophile in step II and step V, forming other
side products. Hence, with the removal of water, the formation of acetal
would be favoured over the formation of the diol.
(iii)
[2]
(iv)
1. acetal formation (e.g. ethane-1,2-diol / any alcohol and acid catalyst)
2. reduction of carboxylic acid to alcohol (LiAlH
4
in dry ether)
3. acid hydrolysis of acetal back to ketone (any dilute mineral acids, heat)
(c) (i) Anode: 2Cl
(aq)
Cl
2
(g) + 2e
Cathode: 2H
2
O (l) + 2e
H
2
(g) + 2OH
(aq)
*(ii) O
2
+ 4H
+
+4e
-
2H
2
O +1.23V
Cl
2
+ 2e
2Cl
+1.36V
Since E
(O
2
/H
2
O) is less positive than E
(Cl
2
/Cl
), water is expected to be
discharged.
High concentration of chloride ion in solution causes drop in E
(Cl
2
/Cl
)
to be lower than +1.23V, hence it is preferentially discharged.
(iii) Cold: Cl
2
(g) + 2OH
(aq) Cl
(aq) + ClO
(aq) + H
2
O (l)
Hot: 3Cl
2
(g) + 6OH
(aq) 5Cl
(aq) + ClO
3
(aq) + 3H
2
O (l)
Suggested Answers Page 4 of 9
Hwa Chong Institution 2012 9647 / 03 / JC2 Prelim 2012
3(a)(i)
Exhibits optical isomerism Structure must contain a chiral carbon
Reacts with sodium carbonate to
give CO
2
Structure must contain carboxylic acid functional
group (accept CO
2
H, reject COOH)
Reacts with potassium
manganate to give CO
2
Oxidative cleavage has occurred on a terminal
alkene.
A:
(ii)
B gives a much acidic solution than A
Structure must contain acid chloride
functional group
C is neutral
Alcohol, aldehyde or ketone functional
groups could be present
B and C gives organic ppt with 2,4-DNPH
C gives brick red ppt with Fehlings
solution
Aldehyde functional group present in C
Ketone functional group present in B
B: or
O
Cl
O
Possible Cs (not exhaustive):
(must have aldehyde functional group. Alkene, aldehyde, ketone, ether accepted for rest of
the structure)
Suggested Answers Page 5 of 9
Hwa Chong Institution 2012 9647 / 03 / JC2 Prelim 2012
(iii) B: The acid chloride functional group hydrolyses/dissolves/reacts completely in water
to give HCl(aq) a strong acid/dissociates completely in water. Hence the solution is
highly acidic.
A: The carboxylic acid functional group is only weakly acidic and only partially
dissociates in water, giving a low concentration of H
+
(b) The p orbitals (reject pi-orbitals) of all the carbon atoms/ of every benzene ring in
the structure now overlap and allow the electrons to delocalize over the entire
structure/chain/polymer.
Benzene exists as discrete non polar covalent molecules. They are not charge
carriers and hence do not conduct electricity.
(c)(i) AgCl (s) + e Ag(s) + Cl
(aq)
Na
2
Mn
5
O
10
(s) + 2AgCl (s) 2NaCl(aq) + 5MnO
2
(s) + 2Ag(s)
(ii) no. of moles of Na
+
lost = 5.0 / 23 = 0.2174 mol
no. of moles of electrons used = 0.2174 mol
Q = 0.2174 x 96500 = 20978 C
(iii) Correct electrodes (Na
2
Mn
5
O
10
/MnO
2
(s) and Ag/AgCl (s)) and seawater (accept
0.600 mold dm
3
NaCl) as the electrolyte.
(iv) At the sodium manganese manganate electrode
2Na
+
(aq) + 5MnO
2
(s) + 2e Na
2
Mn
5
O
10
(s)
When the fresh water is replaced with sea water, the [Na
+
] increases, hence by LCP,
the equilibrium is shifted to the right.
Hence the electrode potential becomes more positive.
At the silver/silver chloride electrode
AgCl (s) + e Ag(s) + Cl
(aq)
When the fresh water is replaced with sea water, the [Cl
] increases, hence by LCP,
the equilibrium is shifted to the left.
Hence the electrode potential becomes more negative.
(v) E
cell
at end of charing phase = 0.56 0.34 = +0.22V
E
cell
at start of discharge phase = 0.65 0.29 = +0.36V
Potential gained by system = +0.14V
Suggested Answers Page 6 of 9
Hwa Chong Institution 2012 9647 / 03 / JC2 Prelim 2012
4 (a) (i) Step 1 : Cl + HCH
3
HCl + CH
3
H = 431 + 434 = +3 kJ mol
1
Step 2 : CH
3
+ ClCl CH
3
Cl + Cl
H = 340 + 244 = 96 kJ mol
1
(ii)
(iii
)
The main contributing energetic factors are the decrease in the C-X and H-X
bond strengths, which results in the reaction becoming decreasingly
exothermic / increasingly endothermic, and hence reactivity decreases.
(b
)
(i) (V
n
V
t
) o [C
6
H
5
N
2
Cl] (remaining)
(ii)
horizontal line straight line thr origin curved
(c) (i)
ln
5
3
10 77 . 2
10 14 . 1
x
x
=
R
E
a
|
.
|
\
|
293
1
323
1
Solving, E
a
= 97.5 kJ mol
1
(ii) Since rate has units of mol dm
3
s
1
, and k has the units of s
1
, the order of
reaction must be 1.
Therefore, rate equation is given by : Rate = k [C
6
H
5
N
2
Cl].
(iii
)
Mechanism : S
N
1 or unimolecular nucleophilic substitution
(iv
)
Rate
(V
n
-V
t
)
0
Ist
order
zeroth
order
Rate
(V
n
-V
t
)
E
a
(2)
E
a
(1)
+3
96
Cl + HCH
3
+ Cl
2
HCl + CH
3
+ Cl
2
HCl + CH
3
Cl + Cl
Enthalpy/kJ mol
-1
reaction pathway
Not 0 or
1st order
Rate
(V
n
-V
t
)
0
Suggested Answers Page 7 of 9
Hwa Chong Institution 2012 9647 / 03 / JC2 Prelim 2012
For info : Mechanism consistent with first order kinetics :
Step 1 : (slow)
Step 2 : (fast)
(d
)
(i) The Cu
+
catalyst converts to a Cu
2+
intermediate before reverting back to its
original +1 oxidation state.
(ii)
1. Diazotisation : HNO
2
, HCl, 10
o
C
2. Sandmeyer reaction : CuBr, heat
3. Alkylation : CH
3
Cl, AlCl
3
, heat
4. Oxidation : KMnO
4
, H
2
SO
4
, heat
Alternative scheme :
1. Bromination : Br
2
(l)
2. Diazotisation : HNO
2
, HCl, 10
o
C
3. Sandmeyer reaction : CuCN, heat
4. Hydrolysis : dilute H
2
SO
4
, heat
Suggested Answers Page 8 of 9
Hwa Chong Institution 2012 9647 / 03 / JC2 Prelim 2012
5(a)(i) H
sol
= LE + H
hyd
= (2833) + (1891) + (1004)
= 62 kJ mol
1
(ii) No. of moles of MgSO
4
= 2/ 120.4 = 1.661 x 10
2
mol
Heat released by solution of MgSO
4
= 62 x 1.661 x 10
2
= 1029.9 J
Heat gained by water = 15(4.18)(T)
15(4.18)(T) = 1029.0
T = 16.4 K
Final temp = 25 + 16.4 = 41.4
o
C
(b)(i) A: BaCO
3
and B: MgCO
3
MCO
3
(s) MgO (s) + CO
2
(g)
Mg
2+
has greater charge density, hence polarizing the anion to a greater extent. This
causes magnesium carbonate to be less stable to heat.
BaO (s) + H
2
O (l) Ba(OH)
2
(aq)
(c)(i) Ba
2+
is precipitated first
When BaCO
3
precipitates; [CO
3
2-
]
min
= 5.1 10
8
mol dm
3
(ii) As Mg(OH)
2
starts to precipitate, [CO
3
2-
] = 3.5 10
7
mol dm
3
[Ba
2+
] remaining in solution =
7
10 x 3.5
x10 5.1
9
= 0.0146 mol dm
3
(iii)
% of Ba
2+
remained in solution = 100 x
0.10
0.0145
= 14.6 % >> 1%
separation is not effective
(d)(i) hydrogen bond: between CH
2
OH, CO
2
H of lysozyme and CH
2
OH, CO
2
H of the
polysaccharide
or ionic interaction: between (CH
2
)
4
NH
3
+
of lysozyme and CO
2
of the
polysaccharide
(ii) Correct drawing of a tripeptide (correct peptide bond)
Correct choice of amino acids (with at least one of the amino acids being able to
form hydrogen bond or ionic interactions
(iii) water / H
2
O
(e)(i) 1. Acid group will react with ammonia hence excess ammonia is needed. OR
2. To lower the chances of the amine product undergoing multiple substitutions
(ii) HO
2
CCH(CH
3
)NHCH(CH
3
)CO
2
H
(f)(i) HO
2
CCH
2
CHO
(ii) Elimintation
Suggested Answers Page 9 of 9
Hwa Chong Institution 2012 9647 / 03 / JC2 Prelim 2012
(iii) Dilute acid, heat
You might also like
- Ri h2 Chem p2 AnswersDocument7 pagesRi h2 Chem p2 Answersdragon slayerNo ratings yet
- Topic 9.4 2009 Transition Elements Prelim SolnDocument17 pagesTopic 9.4 2009 Transition Elements Prelim SolndeadbeanNo ratings yet
- 2010 A Level H2 P3 Suggested AnswersDocument10 pages2010 A Level H2 P3 Suggested AnswersMichelle LimNo ratings yet
- 2011 H2 Chemistry Paper 3 Suggested SolutionsDocument7 pages2011 H2 Chemistry Paper 3 Suggested SolutionsLee Jun Hui0% (1)
- Form Four (4) Chemistry 233/1 Marking SchemeDocument3 pagesForm Four (4) Chemistry 233/1 Marking SchemeTwinomujuniNo ratings yet
- VJC 2007Document14 pagesVJC 2007sswee_1No ratings yet
- P2 Answer SchemeDocument10 pagesP2 Answer Schemesherry_christyNo ratings yet
- SMK Dato Jaafar, JohorDocument8 pagesSMK Dato Jaafar, JohorJun Hao ChongNo ratings yet
- Chem PP1, PP2 & PP3 MSDocument19 pagesChem PP1, PP2 & PP3 MSNgechiiNo ratings yet
- Chemistry (Theory) : General InstructionsDocument8 pagesChemistry (Theory) : General InstructionsDeepali SinghNo ratings yet
- RJC 2011 Chem Prelim Paper3ANSDocument12 pagesRJC 2011 Chem Prelim Paper3ANSJean HomeNo ratings yet
- Perfect Score Chemistry SBP 2012 - ANSWERDocument61 pagesPerfect Score Chemistry SBP 2012 - ANSWERAhmad RawiNo ratings yet
- MARKING SCHEME (2021-22) Term - Ii Chemistry Theory (043) Set ADocument5 pagesMARKING SCHEME (2021-22) Term - Ii Chemistry Theory (043) Set ASyed ShammazNo ratings yet
- IJC H2 Paper 1 and 2 Answers (For Sharing)Document9 pagesIJC H2 Paper 1 and 2 Answers (For Sharing)Sharon HowNo ratings yet
- Previous Year Chemistry Question Paper For CBSE Class 12 - 2014Document11 pagesPrevious Year Chemistry Question Paper For CBSE Class 12 - 2014GouravNo ratings yet
- Chemistry Common Answer KeyDocument10 pagesChemistry Common Answer KeyiskypiskybruhNo ratings yet
- CAPE Chemistry U2 P2 2004 2018 Solutions PDFDocument108 pagesCAPE Chemistry U2 P2 2004 2018 Solutions PDFvalrie bryan100% (4)
- Marking Scheme: ChemistryDocument11 pagesMarking Scheme: ChemistryVinay TyagiNo ratings yet
- Chemistry Class 12th CBSE Sample PaperDocument9 pagesChemistry Class 12th CBSE Sample PaperSiddhi GoplanNo ratings yet
- 2012 A Level H2 Chem P3 Worked Solutions - 2014Document25 pages2012 A Level H2 Chem P3 Worked Solutions - 2014Jerald Lim Yi Heng25% (4)
- ChemistryDocument4 pagesChemistryRaghav KaranNo ratings yet
- Nov 2008Document13 pagesNov 2008dharshanaabNo ratings yet
- Tenkasi District Schools .Qu - KeyDocument16 pagesTenkasi District Schools .Qu - Keydevilssworld143No ratings yet
- HKALE Chemistry 2001 Marking SchemeDocument7 pagesHKALE Chemistry 2001 Marking SchemeHon KwanNo ratings yet
- NEET 2015 Question Paper With Answers (Code A) PDF DownloadDocument56 pagesNEET 2015 Question Paper With Answers (Code A) PDF Downloadharsharma5636No ratings yet
- Chem Unit 5 Transition Metals AnswersDocument13 pagesChem Unit 5 Transition Metals Answersareyouthere9250% (2)
- Answers To Examination Style QuestionsDocument5 pagesAnswers To Examination Style QuestionsClayanne KnottNo ratings yet
- Actual Repeat Paper 2013Document10 pagesActual Repeat Paper 2013Jasmeet Kaur SandhuNo ratings yet
- Chemistry Perfect Score 2011 Module AnswerDocument43 pagesChemistry Perfect Score 2011 Module Answersarahrozaimi100% (1)
- Asm1 Chemistry 253147Document6 pagesAsm1 Chemistry 253147deek_jNo ratings yet
- Chemistry-TermII-Set2 21649Document4 pagesChemistry-TermII-Set2 21649Mridula MishraNo ratings yet
- Model Paper 6 SchemeDocument11 pagesModel Paper 6 SchemeKalyan ReddyNo ratings yet
- Lesson 8 - Electrolysis Part 3Document16 pagesLesson 8 - Electrolysis Part 3Dishna KarunasekaraNo ratings yet
- Class - Xii Chemistry Sample Paper - 3 Time: Three Hours Max. Marks: 70 General InstructionsDocument17 pagesClass - Xii Chemistry Sample Paper - 3 Time: Three Hours Max. Marks: 70 General Instructionssoumya mazumdarNo ratings yet
- Electrochemistry Mittal Sir: Worksheet-I Objective QuestionsDocument3 pagesElectrochemistry Mittal Sir: Worksheet-I Objective QuestionstarunNo ratings yet
- 2014 12 Lyp Chemistry 04 Outside Delhi Sol 9neDocument8 pages2014 12 Lyp Chemistry 04 Outside Delhi Sol 9neDivyansh WaghmareNo ratings yet
- BT1 Inorganic Chem Solutions 2012Document40 pagesBT1 Inorganic Chem Solutions 2012Shrabonti MohammedNo ratings yet
- Kendriya Vidyalaya Sangathan, Kolkata Region 2 Pre Board Examination - 2014-15Document5 pagesKendriya Vidyalaya Sangathan, Kolkata Region 2 Pre Board Examination - 2014-15NareshNo ratings yet
- The Transition Elements: Practice ExamplesDocument15 pagesThe Transition Elements: Practice Exampleskennethleo69No ratings yet
- Crclho Clho: Material Downloaded From and Portal For Cbse Notes, Test Papers, Sample Papers, Tips and TricksDocument12 pagesCrclho Clho: Material Downloaded From and Portal For Cbse Notes, Test Papers, Sample Papers, Tips and TricksChandan PatraNo ratings yet
- CBSE 12 Chemistry Solution Term2Document5 pagesCBSE 12 Chemistry Solution Term2R roseNo ratings yet
- CHEM5 June 2013 Unofficial MSDocument5 pagesCHEM5 June 2013 Unofficial MSMNo ratings yet
- Answer Scheme Term 2 TrialDocument3 pagesAnswer Scheme Term 2 TrialTing TCNo ratings yet
- TruechemDocument10 pagesTruechemaNo ratings yet
- ChemDocument10 pagesChemAnshika singh sisodiyaNo ratings yet
- Xi-Chem With Solution +1Document21 pagesXi-Chem With Solution +1Níkhíl Bansal100% (1)
- Que Bank 12 ChemDocument8 pagesQue Bank 12 Chemtechblogger098No ratings yet
- Suggested Answer For STPM 2013 Paper 2 (U)Document4 pagesSuggested Answer For STPM 2013 Paper 2 (U)Jin Yee Tan100% (2)
- 2009 RI Prelims Chem H2 P2 AnsDocument7 pages2009 RI Prelims Chem H2 P2 AnsJasonNo ratings yet
- Raffles Junior College JC2 H2 Chemistry 2007/8 Suggested Answers To Nov 2007 Chemistry 9746 Paper 1Document16 pagesRaffles Junior College JC2 H2 Chemistry 2007/8 Suggested Answers To Nov 2007 Chemistry 9746 Paper 1Ah XiuNo ratings yet
- Hsslive Xi Chem Pyq Ans 2. EletrochemistryDocument12 pagesHsslive Xi Chem Pyq Ans 2. EletrochemistryPritika RajendranNo ratings yet
- Ahs Preliminary Examination 2008 2Document5 pagesAhs Preliminary Examination 2008 2QM007No ratings yet
- Assignment On Co-Ordination CompoundsDocument2 pagesAssignment On Co-Ordination CompoundsMayank MundadaNo ratings yet
- XII CHEM RT - 9 Answer KeyDocument7 pagesXII CHEM RT - 9 Answer KeyEVAN GERSHONNo ratings yet
- Tranisition Elements-02 - Solved ProblemsDocument9 pagesTranisition Elements-02 - Solved ProblemsRaju SinghNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Graphene Oxide: Fundamentals and ApplicationsFrom EverandGraphene Oxide: Fundamentals and ApplicationsAyrat M. DimievNo ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersFrom EverandPractice Makes Perfect in Chemistry: Oxidation-Reduction with AnswersNo ratings yet
- Novel Carbon Materials and Composites: Synthesis, Properties and ApplicationsFrom EverandNovel Carbon Materials and Composites: Synthesis, Properties and ApplicationsXin JiangNo ratings yet
- ACJC JC2 H2 Maths 2012 Year End Exam Question Paper 1Document5 pagesACJC JC2 H2 Maths 2012 Year End Exam Question Paper 1Ken JiaNo ratings yet
- Gce A Level 2011 h2 Maths 9740 Paper 1 SolutionsDocument17 pagesGce A Level 2011 h2 Maths 9740 Paper 1 SolutionsKen JiaNo ratings yet
- Many Developed Counties Are Paying Increasing Attention To The Needs of The Disadvantaged. How Far Is This True in SingaporeDocument4 pagesMany Developed Counties Are Paying Increasing Attention To The Needs of The Disadvantaged. How Far Is This True in SingaporeKen JiaNo ratings yet
- 2012 Intro Lecture (H1 Geo)Document20 pages2012 Intro Lecture (H1 Geo)Ken JiaNo ratings yet
- Analytical Report - Soil: Physical Properties - GranulometryDocument2 pagesAnalytical Report - Soil: Physical Properties - GranulometryGabriela Salinas ChávezNo ratings yet
- DitiocarbamatosDocument15 pagesDitiocarbamatosLaura GuarguatiNo ratings yet
- Answer Key HMWK - 1 CHPT 9 - 10Document11 pagesAnswer Key HMWK - 1 CHPT 9 - 10jts399No ratings yet
- A Unique Product For Optimal Friction and Anticorrosion PerformanceDocument2 pagesA Unique Product For Optimal Friction and Anticorrosion PerformanceKaran ChadhaNo ratings yet
- Chemistry: Classification of MatterDocument29 pagesChemistry: Classification of MatterRamzen Raphael DomingoNo ratings yet
- Periodic TableDocument1 pagePeriodic TableAisyah AlkatiriNo ratings yet
- Neccesity of Auxiliaries in DyeingDocument3 pagesNeccesity of Auxiliaries in DyeingMohammed Atiqul Hoque ChowdhuryNo ratings yet
- En-006-Compatibilita OssigenoDocument24 pagesEn-006-Compatibilita OssigenoMauro Costa100% (1)
- H2S - Is.11255.4.2006Document11 pagesH2S - Is.11255.4.2006khyatithackerNo ratings yet
- Rationalise List Welding ConsumablesDocument19 pagesRationalise List Welding ConsumablesMayank SadaniNo ratings yet
- Chapter 6 - Chang Test BankDocument22 pagesChapter 6 - Chang Test BankDariusz MilewskiNo ratings yet
- Titrimetric Methods of AnalysesDocument10 pagesTitrimetric Methods of AnalysesJason BakerNo ratings yet
- Small Scale Gold Refining by Zinc PrecipitationDocument4 pagesSmall Scale Gold Refining by Zinc Precipitationpakde jongkoNo ratings yet
- Answers Equilibrium Solutions and KSPDocument10 pagesAnswers Equilibrium Solutions and KSPrajNo ratings yet
- D AND F BLOCK ELEMENT NotesDocument5 pagesD AND F BLOCK ELEMENT NotesM AroNo ratings yet
- IIT gUWAHATI NOTESDocument25 pagesIIT gUWAHATI NOTESआदेश मीणाNo ratings yet
- Assertion Reasoning Questions All Chapters - AnswersDocument10 pagesAssertion Reasoning Questions All Chapters - AnswersSheeba Sathyan Mohan70% (10)
- SF 0654Document4 pagesSF 0654QA LAB ISMNo ratings yet
- Reactivity SeriesDocument5 pagesReactivity SeriesTAKUNDA MARIMENo ratings yet
- Test Procedure Potassium Ion ConcentrationDocument1 pageTest Procedure Potassium Ion ConcentrationjalalNo ratings yet
- Chemistry Higher Level Paper 2: Instructions To CandidatesDocument24 pagesChemistry Higher Level Paper 2: Instructions To CandidatesJuan Camilo VargasNo ratings yet
- CH 8. P-Block (Chem - 2)Document77 pagesCH 8. P-Block (Chem - 2)Pradeep KumarNo ratings yet
- Slides Laser Beam WeldingDocument14 pagesSlides Laser Beam WeldingManuel Sebastian Navarro MosqueraNo ratings yet
- S Block ChemhaackDocument13 pagesS Block ChemhaackDrushya SalunkeNo ratings yet
- Formation of Heavier Elements in The Evolution of The UniverseDocument14 pagesFormation of Heavier Elements in The Evolution of The UniverseSimon Joseph ManceraNo ratings yet
- Types of Reversible CellsDocument16 pagesTypes of Reversible CellsKaran RavalNo ratings yet
- Astm D 5091 - 95 (2014)Document2 pagesAstm D 5091 - 95 (2014)Sur Vani100% (1)
- LFP Study PDFDocument31 pagesLFP Study PDFAltinNo ratings yet
- Covalent Bonding - Multiple Choice QPDocument9 pagesCovalent Bonding - Multiple Choice QPmourees karanNo ratings yet
- History of Periodic TableeDocument12 pagesHistory of Periodic TableeMuh HanifNo ratings yet