Professional Documents
Culture Documents
States of Matter Assignment
Uploaded by
gsr54Original Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
States of Matter Assignment
Uploaded by
gsr54Copyright:
Available Formats
STATES OF MATTER (ASSIGNMENT)
1. A gas is collected in theflask shown here.
Whatis the pressure exertedby the gas if the
atmospheric pressure is735 mmHg?
A) 42 mmHg B) 693 mmHg
C) 735 mmHg D) 777 mmHg
2. A commercial gas cylinder contain 100 litres of helium at a pressure of 11 atm.Howmany
2.5 litres balloones at 1 atm may be filled by the gas in the cylinder
A) 150 B)450 C)500 D)400
3. The density of SO2 at STP is 2.86 kg/m3.then its density at 819
0C
and 2 atm pressure is
A) 0.715 B) 1.43 C) 2.86 D) 4.26kg/m3
4. An open vessel at
0
27 c heated until 2/5 of the air in it has been expelled. Assuming that
the volume of the vessel remains constant. Find the temperature to which the vessel has
been heated?
A)
0
227 C B)
0
477 C C) 750K D)
0
500 C
5. 0.2g of gas X occupies 320 ml but under the same conditions 0.1 g of oxygen occupies
290ml .Molar mass of the gas
A) 64 B)29 C)58 D)45
6. The number of molecules present in one lit flask having nitrogen gas at 27
0
C and
5`
7.6 10 mm
pressure.
1)
15
2.4 10 2)
16
2.4 10
3)
16
4.2 10 4)
16
4.2 10
7. When 4g of agas X is introduced into an evacuated flask at 30
0
Cthe pressure is found to be
1.6 atm.When 12 g another gas is added to the same flask the total pressure is
2.8atm.Molecular weight ratio of the gases
1) 1:4 2) 2:3 3) 1:2 4) 4:1
8. The density of air is 0.0013 g/mL. The vapor density of air will be
(1) 14.23 (2) 14.56 (3) 14.48 (4) 14.65
9. How much should the pressure be increased in order to decrease the volume of a gas by
5% at constant temperature?
(1) 25% (2) 10% (3) 4.26% (4) 5.26%
10. A small bubble rises from the bottom of a lake, where the temperature and pressure are
0
8 C and 6.0atm, to the waters surface, where the temperature is
0
25 C and pressure is
1.0atm. Calculate the final volume of the bubble if its initial volume was 2mL.
A) 14mL B) 12.72mL C) 11.21mL D) 15mL
11. A air column of length 10cm is trapped by a column of Hg 4cm long in a capillary tube
of uniform bore when tube is held horizontally in a room at 1 atm . Length of air column
when the tube is held vertically with open end up
A) 9.5 B)10.52
C) 3.53 D) 4.6
12. A gaseous mixture contains 1 g of H
2
, 4 g of He, 7 g of N
2
, and 10 g O
2
. The gases
having highest and lowest partial pressures respectively are
(1) He, N
2
(2) H
2
, O
2
(3) O
2
, N
2
(4) He, H
2
13. Two flasks X and Y of volumes 250ml and 300ml respectively at the same temperature
are connected by a stop cock of negligible volume. The flask X contains nitrogen gas at a
pressure of 660 torr and the flask Y contains neon gas at a pressure of 825torr. If the stop
cock is opened to allow the two gases to mix, the partial pressure of neon gas and total
pressure of the system will be
(A) 300 torr,700torr (B) 400 torr,700torr (C) 450 torr,750torr (D) 300 torr,750torr
14. A container contains certain gas of mass m at high pressure. Some of the gas has been
allowed to escape from the container and after some time the pressure of gas becomes half
and its absolute temperature 2/3 rd. The amount of gas escaped is
(1) m
3
2
(2) m
2
1
(3) m
4
1
(4) m
6
1
15. Consider three gases at same temperature and pressure having the molar masses M
1
, M
2
and
M
3
; rates of diffusion r
1
, r
2
, r
3
; times of diffusion t
1
, t
2
, t
3
; volumes diffused V
1
, V
2
, V
3
;
masses diffused m
1
, m
2
, m
3
.
LIST I LIST II
A. t
1
: t
2
: t
3
(equal volumes) 1.
3
M :
2
M :
1
M
B. V
1
: V
2
: V
3
(same times) 2.
1
M :
2
M :
3
M
C. r
1
: r
2
: r
3
3.
1
M
1
:
2
M
1
:
3
M
1
D. m
1
: m
2
: m
3
(same times) 4.
3
M
1
:
2
M
1
:
1
M
1
The correct match is
A)A-2,B-3,C-3,D-2 B) A-4,B-3C-1,D-4 C)A-2 ,B-2, C-4,D-2 D) A-3,B-2,C-4,D-1
10cm 4cm
air
16. 4gm of sulphur dioxide gas diffuses from a container in 8min. Mass of helium gas
diffusing from the same container over the same time interval is
A) 0.5gm B) 1gm C) 2gm D) 4gm
17. The ratio of mean speeds of a gas at temperatures 173 C, 127 C and 627 C is
(A) 6:3:2 (B) 1:2:3 (C) 3:2:1 (D) 1:4:9
18. Two glass bulbs A and B at same temperature are connected by a very small tube having
a stop-cock. Bulb A has a volume of
3
100cm and contained the gas while bulb B was
empty. On opening the stop-cock, the pressure fell down to 20%. The volume of the bulb
B is
A)
3
100cm B)
3
200cm C)
3
250cm D)
3
400cm
19.
A mixture of hydrogen and oxygen at one bar pressure contain 20% by weight of hydrogen .
Calculate the partial pressure of hydrogen
a) 1.0 b) 0.8 c) 1.2 d) 1.8 bar
20.. Which of the following expressions between the vander waals constant b and the radius
r of spherical molecules is true?
A)
3
4
3
A
b r N t
| |
=
|
\ .
B)
3
4
3
b r t
| |
=
|
\ .
C)
3
4
2
3
A
b r N t
| |
=
|
\ .
D)
3
4
4
3
A
b r N t
| |
=
|
\ .
21. Which of the following expressions of compression factor of a real gas is applicable at
high pressure?
A)
1 /
m
Z a V RT =
B)
1 /
m
Z a V RT = +
C)
1 / Z pb RT = +
D)
1 / Z pb RT =
22. The ratio of Boyle's temperature (Tb) and critical temperature (T
C
)for a gas is
A) 8/27 B) 27/8 C) 1/2 D) 2/1
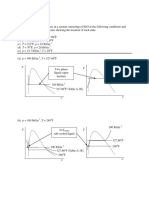
23. The boiling points of three liquids A,B ,C are 350K , 490 K and 630K respectively.The
correct diagram of graphs representing their vapour pressures against different
temperatures
A)
temperature
p
r
e
s
s
u
r
e
B
A
C
B)
temperature
p
r
e
s
s
u
r
e
A
B
C
C)
temperature
p
r
e
s
s
u
r
e
C
B
A
D)
temperature
p
r
e
s
s
u
r
e
A C
B
24. A gas obeys the equation of state P(V-b)=RT(The parameter b is a constant). The slop for an
isochore will be:
(A) Negative (B) Zero (C) R/(V-b) (D) R/P
25. From the following values of the critical constants. Find out b of the van der Waals
equation for C Tc atm P H
c
= = 7 . 33 ., 4 . 12 :
2
.
(A) 0253 . 0 (B) 0212 . 0 (C) 0174 . 0 (D) 0192 . 0
Explanatory Key
7. Molar ratio= Partial pressure
4 12 1.6 4
:
1.2 3 x y
= =
4 4 4
12 3 3 3
y y
or
x x
= = or
3 3
4
x
y
= OR : 1: 4 x y =
OR
1 1 2 2 1 2
1` 1 2` 2 1` 2`
PV PV P P
or
n T n T n n
= = or
1 1 2 2
1 2
PM P M
W W
= or
1 2
1.6 1.2
4 12
M M
= and
1
2
1: 4
M
M
=
10. The volume of the gas in the bubble remains constant, so that
1 2
n n = . To calculate the
final volume,
2
V
1 2
2 1
2 1
6.0 298
2.0 12.72
1.0 281
P T
V V mL
P T
= = =
11. AWhen open end up the wt of Hg represents an increase in the P of gas of 4cm Hg .
Final pressure =760+40=800mm
P1V1=P2V2 or P1A1l1=P2A2l2 Since area of cross section is constant
P1l1=P2l2 or 760x10= 800xl or l= 9.5 cm
16.
Explanation:
1 2 2 1 2 2
2 1 1 1 2 1
.
;
.
n t M w M M
n t M M w M
= =
1 1 1
2 2
4
;
4 64
w M w
w M
= =
1
1 w gm =
19. Let hydrogen = 20g and oxygen = 80g Number of moles = 20/2+80/32 =10+2.5 = 12.5
PP of hydrogen = 10/12.5x1=100/125 = 0.8 bar
23) Explanation: p(v-b)=RT
RT R
P T
V b V b
| |
= =
|
\ .
Slope =
R
V b
25. We have
0253 . 0
8 4 . 12
0821 . 0 ) 7 . 33 273 (
8
=
+
=
=
C
C
P
R T
b
You might also like
- Gas Well LoadingDocument9 pagesGas Well LoadingalyshahNo ratings yet
- Chang's Test Bank (Chapters 5, 7, 8, 9)Document27 pagesChang's Test Bank (Chapters 5, 7, 8, 9)asfaNo ratings yet
- NSS Chemistry Part 9 Rate of ReactionsDocument26 pagesNSS Chemistry Part 9 Rate of ReactionsFelix YueNo ratings yet
- Malcolm P. Kennett - Essential Statistical Physics 2020Document263 pagesMalcolm P. Kennett - Essential Statistical Physics 2020seneca76No ratings yet
- Matter in Our Surroundings - Shobhit NirwanDocument17 pagesMatter in Our Surroundings - Shobhit NirwanDibya Biswal94% (65)
- States of MatterDocument21 pagesStates of MatterRegina Mae Narciso NazarenoNo ratings yet
- 02 Kinetic Theory of Gases Practice Problem1Document12 pages02 Kinetic Theory of Gases Practice Problem1Ashok PradhanNo ratings yet
- Calculation of Air-Fuel RatioDocument16 pagesCalculation of Air-Fuel RatiorajeshNo ratings yet
- Chemistry-Gas Laws Multiple ChoiceDocument5 pagesChemistry-Gas Laws Multiple ChoiceGeorge Isaac McQuilesNo ratings yet
- Guidance On Eng. MTD Separator DSGNDocument55 pagesGuidance On Eng. MTD Separator DSGNLeonard AlvinNo ratings yet
- Exercise GasesDocument4 pagesExercise GasesAri AdiantariNo ratings yet
- Gases & The Kinetic-Molecular TheoryDocument20 pagesGases & The Kinetic-Molecular TheoryAshley Marie ChildersNo ratings yet
- PDM-P-CS-002 - HP Prod SeparatorDocument5 pagesPDM-P-CS-002 - HP Prod Separatorfirman adiyantoNo ratings yet
- Gaseous StateDocument39 pagesGaseous Statesourabhmaths100% (1)
- CH 5 Practice ProblemsDocument16 pagesCH 5 Practice Problemsjaskaran singhNo ratings yet
- Gaseous StateDocument8 pagesGaseous StateGadde Gopala KrishnaNo ratings yet
- Supplementary Ans Gaseous StateDocument5 pagesSupplementary Ans Gaseous Statekik leeNo ratings yet
- 7.12-States of Matter-2 IITDocument5 pages7.12-States of Matter-2 IITNikhilesh PrabhakarNo ratings yet
- 03 - Ans To Gaseous State Supplemtary QN - 2012Document4 pages03 - Ans To Gaseous State Supplemtary QN - 2012caspersoongNo ratings yet
- Test Bank Chapter 5Document7 pagesTest Bank Chapter 5Ahmed ZakiNo ratings yet
- 2 Part Gas Law Practice!!Document28 pages2 Part Gas Law Practice!!ahix123No ratings yet
- 1617 Level L Chemistry Revision Sheet T1 Wk11Document8 pages1617 Level L Chemistry Revision Sheet T1 Wk11Hisham El KanayatiNo ratings yet
- Test Bank Chapter 5Document8 pagesTest Bank Chapter 5teafNo ratings yet
- Practice Questions For Ch. 5: Name: - Class: - Date: - Id: ADocument23 pagesPractice Questions For Ch. 5: Name: - Class: - Date: - Id: APrem MehrotraNo ratings yet
- Unit 11 Test Review KeyDocument5 pagesUnit 11 Test Review KeyRyanGargantillaNo ratings yet
- Chemistry Form 6 Sem 1 04Document64 pagesChemistry Form 6 Sem 1 04Ng Swee Loong Steven100% (6)
- Chapter 5Document27 pagesChapter 5sidra89No ratings yet
- Chapter 5 GasesDocument37 pagesChapter 5 GasesNeally WeallyNo ratings yet
- Microsoft Word - 4-State of Matter - Gaseous StateDocument5 pagesMicrosoft Word - 4-State of Matter - Gaseous StateSatya KamNo ratings yet
- General Chemistry Principles and Modern Applications Petrucci 10th Edition Test BankDocument25 pagesGeneral Chemistry Principles and Modern Applications Petrucci 10th Edition Test Bankronaldgraytajnmisrxw100% (32)
- Phy 1321 Assignment SolutionsDocument2 pagesPhy 1321 Assignment SolutionsNupur VijNo ratings yet
- Chapter Practice QuestionsDocument12 pagesChapter Practice QuestionsNoranisza MahmudNo ratings yet
- YogDocument24 pagesYogYogesh khandelwal0% (1)
- Ideal Gas Equation and Related Gas LawsDocument6 pagesIdeal Gas Equation and Related Gas LawsNshjdibNo ratings yet
- Assignment Gaseous State JH Sir-2621Document38 pagesAssignment Gaseous State JH Sir-2621Noob Iplay100% (1)
- Ebook Chemistry The Central Science 11Th Edition Brown Test Bank Full Chapter PDFDocument67 pagesEbook Chemistry The Central Science 11Th Edition Brown Test Bank Full Chapter PDFformatbalanoidyxl100% (12)
- Chem 1A Chapter5 Exercises PDFDocument5 pagesChem 1A Chapter5 Exercises PDFJoela Faith Ming GongNo ratings yet
- Ideal Gas Law Extra Practice CHALLENGE QUESTIONSDocument7 pagesIdeal Gas Law Extra Practice CHALLENGE QUESTIONSTanisha DamleNo ratings yet
- Gaseous State PDFDocument4 pagesGaseous State PDFramanji1021No ratings yet
- Behavior of Pure Substances: Than One Phase, But Each Phase Must Have The Same Chemical CompositionDocument18 pagesBehavior of Pure Substances: Than One Phase, But Each Phase Must Have The Same Chemical CompositionDharmesh PatelNo ratings yet
- 1694771313-FlattenedDocument14 pages1694771313-Flattenedaarushigusain25No ratings yet
- Sheet-1-Gaseous StateDocument3 pagesSheet-1-Gaseous StateHarshit SinghNo ratings yet
- Chemistry Gaseous StateDocument6 pagesChemistry Gaseous Stateraghavendra jNo ratings yet
- 2012 Gaseous State Tutorial TeacherDocument10 pages2012 Gaseous State Tutorial Teacherjzhong_7No ratings yet
- Physics ALMCDocument185 pagesPhysics ALMClavina rachelNo ratings yet
- Gas Laws AssignmentDocument5 pagesGas Laws AssignmentShweta SharmaNo ratings yet
- Cpp-Gaseous State - RGVDocument2 pagesCpp-Gaseous State - RGVGauri KabraNo ratings yet
- B18pa1 NHN 08 PDFDocument4 pagesB18pa1 NHN 08 PDFMohamed AbdullaNo ratings yet
- Eu3c6 by Adel KhamisDocument31 pagesEu3c6 by Adel KhamisAdel KhamisNo ratings yet
- 3rd Quarter Review Honors ChemDocument27 pages3rd Quarter Review Honors Chemjkomtil7No ratings yet
- Phy Chem Topics Set 2Document8 pagesPhy Chem Topics Set 2Anonymous RbmGbYvNo ratings yet
- Chapter 5 - Ideal GasesDocument71 pagesChapter 5 - Ideal GasesRabbitNo ratings yet
- IIT Jee Mayank Test-2Document5 pagesIIT Jee Mayank Test-2kamalkantmbbsNo ratings yet
- 11.Fisika1-Kalor KinetikDocument47 pages11.Fisika1-Kalor KinetikWahyu Septriandi SaktiNo ratings yet
- Taller de GasesDocument20 pagesTaller de GasesAle Cruz DNo ratings yet
- Jitsin P1 Trial 2015Document21 pagesJitsin P1 Trial 2015Lam WEn SiangNo ratings yet
- The Celsius, Fahrenheit, and Kelvin Temperature Scales: Problems of Chapter 1Document8 pagesThe Celsius, Fahrenheit, and Kelvin Temperature Scales: Problems of Chapter 1Nguyễn Quốc Khánh100% (1)
- 4.1 GasesDocument23 pages4.1 GasesVasanth Kumar BatumalaiNo ratings yet
- CHE 160 Semester Review Zumdahl CH 5-7Document20 pagesCHE 160 Semester Review Zumdahl CH 5-7Kinal PatelNo ratings yet
- Chapter 10 Sept13Document57 pagesChapter 10 Sept13chandro57No ratings yet
- Chem 181 Chemistry of GasesDocument15 pagesChem 181 Chemistry of GasesJoey PooleNo ratings yet
- NCERT Solutions For Class 11 Chemistry Chapter 5Document18 pagesNCERT Solutions For Class 11 Chemistry Chapter 5Abhishek VermaNo ratings yet
- Exercises: Sections 10.3, 10.4: The Gas Laws The Ideal-Gas EquationDocument6 pagesExercises: Sections 10.3, 10.4: The Gas Laws The Ideal-Gas EquationPcd MickeyNo ratings yet
- ChemTeam - Assorted Gas Law Problems 26-50Document13 pagesChemTeam - Assorted Gas Law Problems 26-50Koh Jiun AnNo ratings yet
- A Modern Course in Statistical PhysicsFrom EverandA Modern Course in Statistical PhysicsRating: 3.5 out of 5 stars3.5/5 (2)
- Generation of Steam at Constant PressureDocument23 pagesGeneration of Steam at Constant PressureASHISH PATILNo ratings yet
- Acentric Factor: Values of Some Common GasesDocument3 pagesAcentric Factor: Values of Some Common GasesYousef SailiniNo ratings yet
- ME 231 Montazami Whharris 9-10-18 SOLUTIONDocument4 pagesME 231 Montazami Whharris 9-10-18 SOLUTIONEduardo Perez UriegasNo ratings yet
- E3. Friction Losses in Pipes and FittingsDocument15 pagesE3. Friction Losses in Pipes and FittingsMuzammil IqbalNo ratings yet
- Activity Problem Set G4Document5 pagesActivity Problem Set G4Cloister CapananNo ratings yet
- 06 - Thermodynamic - Cycles - (Frigo) 1 PDFDocument44 pages06 - Thermodynamic - Cycles - (Frigo) 1 PDFAntonio Di FioreNo ratings yet
- PWOG HZL 6033 PR CAL 001 - New Dust Collector Design and DatasheetDocument3 pagesPWOG HZL 6033 PR CAL 001 - New Dust Collector Design and DatasheetpavanNo ratings yet
- EC1 Problemario de Equilibrio de Fases y Calculos FlashDocument2 pagesEC1 Problemario de Equilibrio de Fases y Calculos FlashRosario Zambrano CoronaNo ratings yet
- Lecture 3a PDFDocument42 pagesLecture 3a PDFUSMAN SARWARNo ratings yet
- P Tran Solution 1Document37 pagesP Tran Solution 1MiguelaTayNo ratings yet
- Properties of SteamDocument7 pagesProperties of SteamRavichandran GNo ratings yet
- Density Height ExercisesDocument4 pagesDensity Height Exercisessol santanaNo ratings yet
- Ideal Gas LawDocument5 pagesIdeal Gas LawChristian Alic KelleyNo ratings yet
- CPD Group 16Document9 pagesCPD Group 16iffatNo ratings yet
- Calculo de Dimension Cajon OverDocument7 pagesCalculo de Dimension Cajon OverJonathan Romero AlfaroNo ratings yet
- Flash Distillation: All Rights Reserved. Armando B. Corripio, PHD, Pe. 2013Document29 pagesFlash Distillation: All Rights Reserved. Armando B. Corripio, PHD, Pe. 2013beshoy naseefNo ratings yet
- First Write UpDocument2 pagesFirst Write UpMadhukar ScribdNo ratings yet
- FM Lab ReportDocument5 pagesFM Lab ReportDinesh Bala KrishnanNo ratings yet
- Raghav Jha Critical XY ModelDocument14 pagesRaghav Jha Critical XY Modelanurag sahayNo ratings yet
- THE IDEAL GAS (Topic6)Document17 pagesTHE IDEAL GAS (Topic6)ch0k3 iiiNo ratings yet
- Diagram PsychrometricDocument4 pagesDiagram PsychrometricJanry EfriyantoNo ratings yet
- Chapter 2 PensyarahDocument75 pagesChapter 2 PensyarahAdi BaddNo ratings yet
- Isentropic ProblemsDocument5 pagesIsentropic ProblemsjecuadranteNo ratings yet