Professional Documents
Culture Documents
Patofisiologi CHF
Uploaded by
Hafiz Idul FitranulOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Patofisiologi CHF
Uploaded by
Hafiz Idul FitranulCopyright:
Available Formats
PHARMACOMECHANICAL APPROACH TO CHF TREATMENT
Pathophysiology of Congestive Heart Failure
Gary S. Francis, MD,* W.H. Wilson Tang, MD
*George M. and Linda H. Kaufman Center for Heart Failure, Department of Cardiology, Cleveland Clinic Foundation, Cleveland, OH
Heart failure is a clinical syndrome characterized by impaired structure and/or function of the heart, leading to dyspnea and fatigue at rest or with exertion. The pathophysiology of heart failure is complex, and there is no single lesion. Any form of heart disease can lead to heart failure. Most heart failure can be explained by well-recognized etiologic factors, though ostensibly healthy patients may harbor risk factors for the later development of heart failure. A fundamental response to myocardial injury or altered loading conditions includes remodeling of the heart, so that the size, shape, and function of the affected chamber is grossly distorted. This is accompanied by a constellation of biologic changes, best recognized in advanced cases of heart failure. These multiple alterations may be primary or secondary events but, nonetheless, add importantly to the morbidity and mortality of the patients. More emphasis should be placed on recognition and correction of risk factors related to the development of heart failure. [Rev Cardiovasc Med. 2003;4(suppl 2):S14S20]
2003 MedReviews, LLC
Key words: Heart failure Dilated cardiomyopathy Systolic dysfunction Cardiac myocyte hypertrophy
T
S14 VOL. 4 SUPPL. 2 2003
here are few clinical syndromes that have undergone both the conceptual and epidemiologic transformation that has occurred with heart failure. The entire concept of what the heart failure syndrome is, how it begins, how it naturally unfolds, and how its primary phenotypic expression is manifested has undergone radical change since the 1970s. This has occurred simultaneously with a large increase in incidence, largely attributed to the aging of the Western population. For many years, heart failure was defined as a clinical syndrome in which the amount of blood pumped by the heart was insufficient
REVIEWS IN CARDIOVASCULAR MEDICINE
Pathophysiology of Congestive Heart Failure
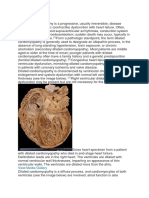
to meet the demands of the various organ systems. Although this concept persists in some circles and is generally correct, we now know that the syndrome of heart failure is far more complex. Heart failure is now more broadly defined as a clinical syndrome characterized by dyspnea and fatigue, at rest or with exertion, due to structural and/or functional abnormalities of the heart. Strictly speaking, there must be a fundamental problem with the heart in the syndrome of heart failure. Like all common clinical syndromes, including anemia and renal failure, heart failure is not a standalone diagnosis. There is always an etiology, though in many cases the precise etiology is not uncovered. For example, it is now clear that as many as 30% of patients with idiopathic dilated cardiomyopathy have genetic bases for their heart failure.1 However, there are multiple genes involved, with a large number of molecular changes that are not apparent under the microscope. Our understanding of the basis of heart failure at the cellular and molecular levels is still evolving. Although it is likely that any form of heart disease can ultimately lead to heart failure, the more common causes in the Western world continue to be coronary artery disease, poorly controlled hypertension, valvular heart disease, primary idiopathic cardiomyopathy, genetic cardiomyopathies, and cardiomyopathies due to lymphocytic inflammatory myocarditis or other infiltrative disorders. Toxic cardiomyopathy due to cocaine, amphetamine, and ephedrine use and various chemotherapies is also recognized as a growing problem.
and classify heart failure. More recently, the syndrome of heart failure has been divided into two broad classes: cases in which systolic function is preserved (so-called diastolic heart failure) and cases in which there is obvious impairment of systolic function with a low ejection fraction and a dilated left ventricle. The definition and natural history of these two broad categories of heart failure have been the subject of numerous reports.25 Diastolic heart failure is largely a product of the aging population, occurring in patients with longstanding hypertension and left ventricular hypertrophy; however, it may also occur in patients with hypertrophic and restrictive cardiomy-
syndrome are well described in a number of recent publications4 and, therefore, will not be addressed.
The Beginning of the Problem: The Index Event
Heart failure syndrome always begins with an index event. The index event may be clinically silent, such as the expression of a genetic mutation, or obvious, such as the catastrophic, sudden loss of a large mass of contractile tissue from acute myocardial infarction. The index event could be the explosive onset of fulminant viral myocarditis, or it may be prolonged and insidious, such as occurs with valvular heart disease. The phenotypic expression of left ventricle dilation and
It is now clear that as many as 30% of patients with idiopathic dilated cardiomyopathy" have genetic bases for their heart failure.
opathy, some of whom may be quite young. Patients with diastolic heart failure tend be older, more often female, have increased left ventricular wall thickness, smaller left ventricular cavities, preserved systolic function by echocardiography, and impaired diastolic function, often with some element of mitral regurgitation. Conversely, patients with systolic heart failure tend to have large, dilated ventricles and markedly impaired systolic function. They frequently manifest mitral regurgitation and tricuspid regurgitation, usually on the basis of dilated ventricles and disruption of the normal papillary muscle architecture (so-called functional regurgitation). The aim of this brief report is to discuss the fundamental pathophysiology of the syndrome of dilated cardiomyopathy with impaired systolic function. The epidemiology and clinical natural history of this impaired systolic function can also be slow to develop, such as a pressureor volume-overloaded state from valvular or coronary heart disease. The clinical picture can also be more rapidly progressive, as sometimes occurs with familial cardiomyopathy.6 By the time patients are seen in the clinic with shortness of breath and fatigue, the late stages of the syndrome are often manifest. Heart failure frequently passes through an asymptomatic, latent phase that is concealed from the patient and the physician.7,8 Unfortunately, because asymptomatic left ventricular dysfunction is often not visible to the practicing physician, these patients do not usually receive treatment. Although it is not certain that the early introduction of drugs such as angiotensin-converting enzyme (ACE) inhibitors and possibly adrenergic receptor blockers slow or reverse the progression of the earli-
Terminology and Semantic Difficulties
There has been a long-standing problem regarding how to define
VOL. 4 SUPPL. 2 2003
REVIEWS IN CARDIOVASCULAR MEDICINE
S15
Pathophysiology of Congestive Heart Failure continued
est form of heart failure, data from the prevention arm of the SOLVD (Studies of Left Ventricular Dysfunction) study suggest that patients with asymptomatic left ventricular dysfunction may benefit from ACE inhibitors.9 Asymptomatic patients with no structural heart disease who are at risk to develop heart failure (stage A) include those with coronary artery disease, diabetes mellitus, hypertension, or a family history of cardiomyopathy, and those who harbor risk factors for the development of coronary artery disease. Early pharmacologic interdiction in such patients may pay broad dividends later, but this remains to be proved.
Myocardial injury
Fall in LV performance ANP BNP
Activation of RAAS, SNS, ET, and others Myocardial toxicity
Peripheral vasoconstriction Hemodynamic alterations Remodeling and progressive worsening of LV function
Morbidity and mortality
Heart failure symptoms
Figure 1. Heart failure: a response to injury. LV, left ventricle; RAAS, renin-angiotensin-aldosterone system; SNS, sympathetic nervous system; ET, endothelin; ANP, atrial natriuretic peptide; BNP, B-type natriuretic peptide.
Response to the Index Event
The response to injury concept suggests that all injured tissue responds in a mechanistically adaptive manner to ensure cellular and, thus, organ survival.10 In the heart, adaptive systemic processes have evolved over hundreds of millions of years and include conservation of effective circulating blood volume and protection of blood pressure and
that some mature cardiac myocytes undergo cell division,11,12 at least more than what was previously considered to be the case.13,14 In addition to myocardial necrosis and subsequent hypertrophy of adjacent viable cells, apoptosis occurs in an unspecified number of cardiac cells,15,16 which likely contributes to cell drop-out and, thus, further impairment of cardiac systolic function.17 The quantitative contribution of apoptosis to impairment of systolic
By the time patients are seen in the clinic with shortness of breath and fatigue, the late stages of the syndrome are often manifest.
flow to vital organs. At the cellular level, the wounded heart is no different than other tissue, in that there is often an initial inflammatory response to the injury followed by a healing phase, which includes the deposition of collagen and, in many cases, hypertrophy of adjacent, viable cardiac myocytes (Figure 1). However, cardiac myocytes, which are highly complex, terminally differentiated cells, do not appear to undergo exuberant cell division in response to injury. Nevertheless, data suggest function and subsequent left ventricular remodeling is still the subject of considerable debate.18,19 The type of myocardial injury response may be highly variable and is dependent on the inciting factors. For example, the myocardial response to chronic, severe mitral regurgitation or chronic aortic regurgitation is quite different from the response to long-term excessive afterload, as might occur in untreated hypertension or severe aortic stenosis. Rather than an acute injury to the heart,
valvular insufficiency imposes chronically perverse loading conditions, in which there are unrelenting, abnormal molecular signals that are sensed by the cardiac myocytes and fibroblasts, leading to myocyte hypertrophy and progressive dilation (remodeling) of the heart with an increase in collagen deposition (Figure 2). In chronically elevated, volume-overloaded states, the cardiac myocytes enlarge mainly by elongation because of an increase of new sarcomeres in series. Chronic volume-overloaded states, which are characteristic of valvular insufficiency and coronary artery disease, typically result in eccentric elongation of cardiac myocytes and obvious chamber enlargement. Changes in the geometry of the left ventricle may distort the relationship of the papillary muscles to the mitral and tricuspid leaflets, leading to additional mitral and tricuspid insufficiency, which only serves to worsen the volume overload. Experimental data suggest that elongation of cardiac myocytes contributes to dilation of the chamber,20 thus providing the structural abnormality that may help form the basis of impaired systolic function. In all likelihood, the increase in
S16
VOL. 4 SUPPL. 2 2003
REVIEWS IN CARDIOVASCULAR MEDICINE
Pathophysiology of Congestive Heart Failure
PRESSURE AND VOLUME OVERLOAD
Activation of angiotensin II synthesis at tissue level
Increases local angiotensin II levels
Cardiac myocyte hypertrophy and growth
Arterial smooth muscle cell hypertrophy
LVH
Remodeling
Figure 2. Response to pressure and volume loading of the left ventricle. LVH, left ventricular hypertrophy. Reproduced from Dahlof B. Effect of angiotensin II blockade on cardiac hypertrophy and remodeling: a review. J Hum Hypertens. 1995;9(suppl 5):S37S44.
chamber size initially serves to maintain stroke volume despite an obvious reduction in ejection fraction. However, over time, the progressive chamber enlargement is attended by insufficient hypertrophy, leading to increased wall stress and, thus, an obligatory impairment of systolic function.21 Unlike with a chronic volumeoverloaded state, longstanding, severe excessive afterload results in myocyte hypertrophy characterized by an increase in the cross-sectional thickness of the cells. This is often referred to as concentric hypertrophy. Of course, the hypertrophic response of the cardiac myocytes to unusual loading conditions can be a hybrid state between eccentric and concentric hypertrophy. For example, the largest human cardiac myocytes are seen in patients with long-standing, severe aortic regurgitation.22 Myocytes from these patients are much longer and somewhat thicker than normal myocytes. Genetic mutations may lead to abnormal sensing and signaling of loading conditions to the cardiac myocyte cell nucleus.2325 The altered
phenotype may involve microtubules, cytoskeleton, or the interaction of the extracellular matrix and integrins and their transduction of mechanical signals to the cell nucleus. Full expression of the heart failure phenotype in genetically driven dilated cardiomyopathy may require a combination of environmental factors (eg, altered load) and genetically altered structural factors (eg, abnormal cytoskeleton). It is unlikely that genetic cardiomyopathies are due to single mutations. The vari-
heart failure. There is no single molecular lesion of heart failure. Rather, there are many factors that drive the left ventricular remodeling process, which is fundamentally a response to injury and/or abnormal loading conditions and is now considered to be the core lesion of heart failure. The hypothesis that cardiac remodeling is the hallmark of heart failure is supported by the wellknown fact that the only drugs that improve survival in the syndrome also reverse or limit left ventricular remodeling. To date, such drugs include only those that block the renin-angiotensin-aldosterone system (ACE inhibitors, angiotensin-receptor blockers, and aldosterone-receptor blockers) or the sympathetic nervous system ( -adrenergic blockers).
Primary Causes or Secondary Epiphenomena?
There are other important pathophysiologic changes that occur in the heart failure syndrome, but it has been difficult to understand whether these are secondary to the left ventricular remodeling process or primary causes of the heart failure syndrome.26 Such changes include downregulation of the sarcolemma
The hypothesis that cardiac remodeling is the hallmark of heart failure is supported by the well-known fact that the only drugs that improve survival in the syndrome also reverse or limit left ventricular remodeling.
able penetrance may be related to important environmental interaction (ie, altered loading conditions) with dysfunctional proteins. -adrenergic receptor density and, in some cases, uncoupling of the receptoradenyl cyclase system. There is a generalized reversion of the heart to the so-called fetal genetic program. This is associated with observed alterations in -myosin heavy-chain and other contractile proteins; overexpression of counterregulatory hormones, such as B-type
Propagation of the Syndrome
A constellation of many factors converge to provide the abnormalities of heart structure and function that lead to the phenotypic expression of
VOL. 4 SUPPL. 2 2003
REVIEWS IN CARDIOVASCULAR MEDICINE
S17
Pathophysiology of Congestive Heart Failure continued
CNS sympathetic outflow Cardiac sympathetic
activity
Sympathetic activity to kidneys + peripheral vasculature
11receptors receptors
121receptors receptors receptors
Activation of RAAS
Myocardial toxicity Increased arrhythmias
Vasoconstriction Sodium retention
Disease progression
Figure 3. Effects of sympathetic action in heart failure. CNS, central nervous system; RAAS, reninangiotensinaldosterone system. Adapted with permission from Packer M. Beta-adrenergic blockade in chronic heart failure: principles, progress, and practice. Prog Cardiovasc Dis. 1998;41(1 suppl 1):3952.
natriuretic peptide, in the heart; excessive activation of the sympathetic nervous system and reninangiotensin-aldosterone system; reduction of parasympathetic nervous system activity; altered reflex control mechanisms; impaired endothelial function; and alteration of skeletal muscle biochemistry (Figure 3). At the cellular level, there may be changes in cardiac metabolism, including preferential use of glucose over free fatty acids, as a consequence of reversion back to the fetal program. The maintenance of calcium transport across the sarcolemma and the sarcoplasmic reticulum, as well as the subsequent exit of calcium for myocardial contraction, is also grossly altered. This can result in disturbances of electrical synchrony and impaired myocyte contraction. Patchy fibrosis can lead to intraventricular conduction delay, common in 25%30% of patients with heart failure. Such conduction delays are associated with myocardial dyssynchrony, which is believed to cause cellular dysfunction and worsening heart failure.29 Alteration
of calcium transients, coupled with excessive sympathetic activity and diuretic-induced hypokalemia, may also converge to promote cardiac arrhythmias. It is now well recognized that about one third of patients with heart failure die suddenly and unexpectedly, often from lethal arrhythmias. Sudden death from arrhythmias is seemingly more common in less symptomatic New York Heart Association class II patients, often striking down younger
function and clinical outcomes,28 suggesting that desynchronization may be pathophysiologically important in heart failure.29 Another late clinical manifestation of heart failure is impaired reflex control mechanisms. For example, patients with advanced heart failure are unable to mount an appropriate tachycardia in response to exercise, vasodilation, or orthostasis. Parasympathetic nervous system derangement is also abnormal, as demonstrated by impaired heartrate recovery following exercise. Activation of the renin-angiotensinaldosterone system leads to retention of salt and water by the kidney, which can be offset to some extent by release of B-type natriuretic peptide from the heart. As the heart failure syndrome advances, patients become less physically active, and there can be substantial salt and water retention, leading to edema. Many patients eventually die with what is referred to as progressive pump dysfunction, which includes hypotension, low cardiac output, and multiorgan dysfunction. Classification of death is quite problematic in patients with heart failure, with approximately one third dying suddenly, one third dying from progres-
Resynchronization therapy with left-sided pacing can restore cardiac synchrony in some patients and is associated with improved systolic function and clinical outcomes.
patients in the earlier stages of heart failure. Medical therapy to prevent serious arrhythmias has been lacking, but implantable cardiac defibrillators can prolong life in certain subsets of patients.27 Resynchronization therapy with left-sided pacing can restore cardiac synchrony in some patients and is associated with improved systolic sive pump failure, and one third dying in unclassified manners, usually from arrhythmias occurring in the setting of terminal hemodynamic decompensation. The epidemiologic factors that drive the heart failure syndrome are well understood and include coronary artery disease, hypertension, valvular heart disease, and infiltra-
S18
VOL. 4 SUPPL. 2 2003
REVIEWS IN CARDIOVASCULAR MEDICINE
Pathophysiology of Congestive Heart Failure
tive heart disease. However, many patients with dilated cardiomyopathy demonstrate no inciting factor. It remains unclear why the genetic expression of dilated cardiomyopathy occurs when it does in any given patient; it may occur early in life, during midlife, or even late in life. Expression of polymorphism and mutations can be strongly influenced by environmental factors. This may explain why two patients with similar-sized myocardial infarction may develop dissimilar degrees of heart failure.
strategy would likely be far more successful and economical than the current process of waiting for symptomatic heart failure to occur and then prescribing multiple drugs and devices. Currently, physicians are merely delaying the progression of heart failure for a few months without being given a chance to actually prevent its root causes. Clearly, a strategy of aggressive, front-loaded management of risk factors is needed for the prevention of heart failure.
Summary
Heart failure is a clinical syndrome characterized by impaired heart structure and/or function, leading to dyspnea and fatigue at rest or with exertion. Its pathophysiology is complex, and there is no single lesion. Any form of heart disease can lead to heart failure. Most heart failure can be explained by wellrecognized etiologic factors, though ostensibly healthy patients may harbor risk factors for the later development of heart failure. Early recognition and correction of these
The Prevention of Heart Failure
We now know that certain risk factors put patients at risk for the development of heart failure. Such factors include hypercholesterolemia, coronary artery disease, poorly controlled hypertension, left ventricular hypertrophy, and diabetes mellitus. Although unproven, it is likely that aggressive, early management of these risk factors may delay the onset of heart failure or, in some cases, prevent heart failure. Such a
risk factors would likely provide for a more robust favorable outcome. Many cases previously considered to be idiopathic dilated cardiomyopathy are now recognized to be due to a variety of genetic mutations, many of which likely require some interaction of environmental factors to express the full phenotype. A fundamental response to myocardial injury or altered loading conditions includes remodeling of the heart, so that the size, shape, and function of the affected chamber is grossly distorted. This is accompanied by a vast constellation of biologic changes, best recognized in advanced cases of heart failure. These multiple alterations may be primary or secondary events but, nonetheless, add substantially to the morbidity and mortality of patients. Unfortunately, most of our current therapies are provided to the patient toward the end of the natural history of the syndrome, rather than at the beginning. Ultimately, more emphasis should be placed on recognition and correction of risk factors related to
Main Points
Heart failure is broadly defined as a clinical syndrome characterized by dyspnea and fatigue, at rest or with exertion, due to structural and/or functional abnormalities of the heart. The more common causes of heart failure in the Western world are coronary artery disease, poorly controlled hypertension, valvular heart disease, primary idiopathic cardiomyopathy, genetic cardiomyopathies, and cardiomyopathies due to lymphocytic inflammatory myocarditis or other infiltrative disorders. Patients with systolic heart failure tend to have large, dilated ventricles and markedly impaired systolic function. They frequently manifest mitral regurgitation and tricuspid regurgitation, usually on the basis of dilated ventricles and disruption of the normal papillary muscle architecture. There is no one index event in heart failure: index events can be clinically silent (eg, expression of a genetic mutation) or obvious (acute myocardial infarction), explosive (fulminant viral myocarditis) or prolonged and insidious (valvular heart disease). Heart failure often passes through an asymptomatic, latent phase that is frequently concealed from the patient and the physician. As heart failure syndrome advances, patients become less physically active, and there can be substantial salt and water retention, leading to edema. Many patients eventually die from progressive pump dysfunction, which includes hypotension, low cardiac output, and multiorgan dysfunction. Aggressive, early management of the risk factors of heart failure (hypercholesterolemia, coronary artery disease, poorly controlled hypertension, left ventricular hypertrophy, and diabetes mellitus) may result in the delay of onset of heart failure or, in some cases, the prevention of heart failure.
VOL. 4 SUPPL. 2 2003
REVIEWS IN CARDIOVASCULAR MEDICINE
S19
Pathophysiology of Congestive Heart Failure continued
the development of heart failure. This will likely prove to have the most profound public health effect.
References
1. Baig MK, Goldman JH, Caforio AL, et al. Familial dilated cardiomyopathy: cardiac abnormalities are common in asymptomatic relatives and may represent early disease. J Am Coll Cardiol. 1998;31:195201. Chen HH, Lainchbury JG, Senni M, et al. Diastolic heart failure in the community: clinical profile, natural history, therapy, and impact of proposed diagnostic criteria. J Card Fail. 2002;8:279287. Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:21442150. Tang WHW, Francis GS. Natural history of heart failure. In: Kukin ML, Fuster V, eds. Oxidative Stress and Cardiac Failure. Armonk, NY: Futura Publishing, 2003:147. Vasan RS, Benjamin EJ, Levy D. Prevalence, clinical features and prognosis of diastolic heart failure: an epidemiologic perspective. J Am Coll Cardiol. 1995;26:15651574. Valantine HA, Hunt SA, Fowler MB, et al. Frequency of familial nature of dilated cardiomyopathy and usefulness of cardiac transplantation in this subset. Am J Cardiol. 1989;63:959963. Vasan RS, Larson MG, Benjamin EJ, et al. Left ventricular dilatation and the risk of congestive heart failure in people without myocardial infarction. N Engl J Med. 1997;336:13501355. Thomas KG, Redfield MM. Asymptomatic left ventricular dysfunction. Heart Fail Rev. 1997;2:1122.
9.
10.
11.
2.
12.
3.
13.
14.
4.
15.
5.
16.
6.
17.
18.
7.
8.
19.
The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327:685691. Francis GS, McDonald KM, Cohn JN. Neurohumoral activation in preclinical heart failure. Remodeling and the potential for intervention. Circulation. 1993;87:IV90IV96. Kajstura J, Leri A, Finato N, et al. Myocyte proliferation in end-stage cardiac failure in humans. Proc Natl Acad Sci U S A. 1998;95:88018805. Quaini F, Cigola E, Lagrasta C, et al. End-stage cardiac failure in humans is coupled with the induction of proliferating cell nuclear antigen and nuclear mitotic division in ventricular myocytes. Circ Res. 1994;75:10501063. Anversa P, Kajstura J. Ventricular myocytes are not terminally differentiated in the adult mammalian heart. Circ Res. 1998;83:114. Pfeffer MA, Pfeffer JM. Adult myocyte hyperplasia: divided they fail? J Am Coll Cardiol. 1994;24:150151. Kang PM, Izumo S. Apoptosis and heart failure: a critical review of the literature. Circ Res. 2000;86:11071113. Olivetti G, Abbi R, Quaini F, et al. Apoptosis in the failing human heart. N Engl J Med. 1997;336:11311141. Beltrami CA, Finato N, Rocco M, et al. Structural basis of end-stage failure in ischemic cardiomyopathy in humans. Circulation. 1994;89:151163. Francis GS, Anwar F, Bank AJ, et al. Apoptosis, Bcl-2, and proliferating cell nuclear antigen in the failing human heart: observations made after implantation of left ventricular assist device. J Card Fail. 1999;5:308315. Tamura T, Said S, Lu W, et al. Is apoptosis present in progression to chronic hypertensive heart failure? J Card Fail. 2000;6:3742.
20.
21.
22.
23.
24.
25.
26.
27.
28.
29.
Tamura T, Onodera T, Said S, Gerdes AM. Correlation of myocyte lengthening to chamber dilation in the spontaneously hypertensive heart failure (SHHF) rat. J Mol Cell Cardiol. 1998;30:21752181. Grossman W. Cardiac hypertrophy: useful adaptation or pathologic process? Am J Med. 1980;69:576584. Linzbach AJ. Heart failure from the point of view of quantitative anatomy. Am J Cardiol. 1960;5:370382. Shaw T, Elliott P, McKenna WJ. Dilated cardiomyopathy: a genetically heterogeneous disease. Lancet. 2002;360:654655. Vatta M, Stetson SJ, Perez-Verdia A, et al. Molecular remodeling of dystrophin in patients with end-stage cardiomyopathies and reversal in patients on assistance-device therapy. Lancet. 2002;359:936941. Kamisago M, Sharma SD, DePalma SR, et al. Mutations in sarcomere protein genes as a cause of dilated cardiomyopathy. N Engl J Med. 2000;343:16881696. Francis GS, Gassler JP, Sonnenblick EH. Pathophysiology and diagnosis of heart failure. In: Fuster V, ed. Hursts The Heart. 10th ed. New York: McGraw-Hill, 2001:665685. Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877883. Abraham WT, Fisher WG, Smith AL, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:18451853. Wilkoff BL, Cook JR, Epstein AE, et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: The Dual Chamber and VVI Implantable Defibrillator (DAVID) trial. JAMA. 2002;288:31153123.
S20
VOL. 4 SUPPL. 2 2003
REVIEWS IN CARDIOVASCULAR MEDICINE
You might also like
- CHF PathDocument7 pagesCHF PathGeraldine Gallaron - CasipongNo ratings yet
- Medicine in Brief: Name the Disease in Haiku, Tanka and ArtFrom EverandMedicine in Brief: Name the Disease in Haiku, Tanka and ArtRating: 5 out of 5 stars5/5 (1)
- Congestive Heart FailureDocument16 pagesCongestive Heart FailureRahmat Burhanudin RamdaniNo ratings yet
- Cardiorenal Syndrome in Heart FailureFrom EverandCardiorenal Syndrome in Heart FailureW. H. Wilson TangNo ratings yet
- Congestive Heart Failure: Diagnosis, Pathophysiology, Therapy, and Implications For Respiratory CareDocument10 pagesCongestive Heart Failure: Diagnosis, Pathophysiology, Therapy, and Implications For Respiratory CareKevinDilianSugandaNo ratings yet
- Causes of Heart FailureDocument4 pagesCauses of Heart Failurejana7-7No ratings yet
- CHFDocument10 pagesCHFPowpOw SangalangNo ratings yet
- Chronic Heart Failure: Overview#a0101Document2 pagesChronic Heart Failure: Overview#a0101Erickson OcialNo ratings yet
- Chronic Heart Failure-Part IDocument18 pagesChronic Heart Failure-Part IdrtpkNo ratings yet
- Jurnal NCBI Gagal JantungDocument16 pagesJurnal NCBI Gagal JantungMAS MANTRINo ratings yet
- 403 Full PDFDocument10 pages403 Full PDFKuroto YoshikiNo ratings yet
- Hypertrophic CardiomyopathyDocument10 pagesHypertrophic CardiomyopathySam SonNo ratings yet
- View Media GalleryDocument9 pagesView Media Galleryjenrimz33No ratings yet
- Congestive Heart Failure: Diagnosis, Pathophysiology, Therapy, and Implications For Respiratory CareDocument10 pagesCongestive Heart Failure: Diagnosis, Pathophysiology, Therapy, and Implications For Respiratory CareIntan PurnamasariNo ratings yet
- Congestive Heart Failure: Diagnosis, Pathophysiology, Therapy, and Implications For Respiratory CareDocument10 pagesCongestive Heart Failure: Diagnosis, Pathophysiology, Therapy, and Implications For Respiratory CareMirachel AugustNo ratings yet
- Etiology: Table 279-1Document31 pagesEtiology: Table 279-1CatharinaNoviaNo ratings yet
- Chronic Heart FailureDocument11 pagesChronic Heart FailurelaydyNo ratings yet
- The Pace at Which The Base of Knowledge Has ExpandedDocument4 pagesThe Pace at Which The Base of Knowledge Has ExpandedRita ErmuracheNo ratings yet
- Pediatric Dilated CardiomyopathyDocument10 pagesPediatric Dilated CardiomyopathyRiduan Adoro Lumban GaolNo ratings yet
- Case Study-Congestive Heart FailureDocument71 pagesCase Study-Congestive Heart FailureKentTangcalagan92% (13)
- High output heart failure: a reviewDocument7 pagesHigh output heart failure: a reviewChanvira Aria CandrayanaNo ratings yet
- CV in ElderlyDocument6 pagesCV in ElderlySeli MandianganNo ratings yet
- Congestive Heart Failure in The ElderlyDocument13 pagesCongestive Heart Failure in The ElderlyKezia MarsilinaNo ratings yet
- Hypertensive Vascular DiseaseDocument5 pagesHypertensive Vascular DiseaseGenoMacaraanNo ratings yet
- ICC Cronica Fisiopatologia 2014Document11 pagesICC Cronica Fisiopatologia 2014Karen Grissell Serrano RamosNo ratings yet
- Heart FailureDocument28 pagesHeart FailureNabeel ShahzadNo ratings yet
- Heart DiseasesDocument73 pagesHeart DiseasesturbomurboNo ratings yet
- Valvular Heart Disease: Zorana Mrsic,, Scott P. Hopkins,, Jared L. Antevil,, Philip S. MullenixDocument14 pagesValvular Heart Disease: Zorana Mrsic,, Scott P. Hopkins,, Jared L. Antevil,, Philip S. Mullenixdr_antonio81No ratings yet
- Seminar: John J V Mcmurray, Marc A PfefferDocument13 pagesSeminar: John J V Mcmurray, Marc A Pfefferjvasco_santos8178No ratings yet
- CHFDocument61 pagesCHFAngeline Lareza-Reyna VillasorNo ratings yet
- Lemone 6e Chapter 31Document47 pagesLemone 6e Chapter 31Husein Fatih ArafatNo ratings yet
- Diagnosing Syncope in PetsDocument12 pagesDiagnosing Syncope in PetsSuha AbdullahNo ratings yet
- WJC 12 7Document20 pagesWJC 12 7leidyalexserraoNo ratings yet
- Pharmacokinetics and PharmacodynamicsDocument166 pagesPharmacokinetics and PharmacodynamicsEvgeny BadmaevNo ratings yet
- Heart Failure Treatment in The Intensive Care Unit in ChildrenDocument28 pagesHeart Failure Treatment in The Intensive Care Unit in ChildrenNatalia Alejandra Cardozo GomezNo ratings yet
- Jurnal Isu Keperwatan Tentang Jantung: Oleh: Retno NadyaDocument11 pagesJurnal Isu Keperwatan Tentang Jantung: Oleh: Retno NadyazizijasinNo ratings yet
- Congestive Heart Failure FinalxxxxDocument12 pagesCongestive Heart Failure FinalxxxxDoneva Lyn MedinaNo ratings yet
- Falla Cardiaca ReviewDocument9 pagesFalla Cardiaca ReviewYasmin CarhuamacaNo ratings yet
- Enfermedad CV en El BoxeoDocument12 pagesEnfermedad CV en El BoxeoOmar Fernando Hijar FraustoNo ratings yet
- SINCOPE Journal of Internal MedicineDocument9 pagesSINCOPE Journal of Internal MedicineIlse MarquezNo ratings yet
- Heart Failure Epidemiology Pathophysiology and Dia PDFDocument37 pagesHeart Failure Epidemiology Pathophysiology and Dia PDFLolitaNo ratings yet
- Failure in Infants and Children: HeartDocument9 pagesFailure in Infants and Children: HeartAzizatul AuliaNo ratings yet
- Approach To Cardiac PatientDocument6 pagesApproach To Cardiac PatientDemuel Dee L. BertoNo ratings yet
- Case 12 Stroke Rev'd ShorterDocument3 pagesCase 12 Stroke Rev'd Shorterng100% (1)
- Congenital Heart Disease (2011)Document17 pagesCongenital Heart Disease (2011)drheay100% (1)
- Tasks:: For StudentDocument3 pagesTasks:: For StudentlalitrajindoliaNo ratings yet
- Constrictive Pericarditis: There Are Some Disease As A DifferentialDocument3 pagesConstrictive Pericarditis: There Are Some Disease As A DifferentialPradnya ParamitaNo ratings yet
- Diagnostico HF 2004Document8 pagesDiagnostico HF 2004Carlos Alan Lopez100% (1)
- Heart Failure & Cor PulmunaleDocument12 pagesHeart Failure & Cor PulmunaleShrestha AnjivNo ratings yet
- Seminar ON: Cardiomyopat HYDocument43 pagesSeminar ON: Cardiomyopat HYmerin sunilNo ratings yet
- 1.3 Heart FailureDocument30 pages1.3 Heart Failuresn_b5No ratings yet
- Dilated Cardiomyopathy and Hypertrophic CardiomyopathyDocument23 pagesDilated Cardiomyopathy and Hypertrophic CardiomyopathygibreilNo ratings yet
- Congestive Heart FailureDocument25 pagesCongestive Heart FailureAristya Rahadiyan BudiNo ratings yet
- Myocardial Infarction: BackgroundDocument9 pagesMyocardial Infarction: BackgroundNor Faizah Ahmad SadriNo ratings yet
- Guidelines: Management of Stable Angina PectorisDocument20 pagesGuidelines: Management of Stable Angina PectorisyudaNo ratings yet
- Ischemic Heart Disease Literature ReviewDocument4 pagesIschemic Heart Disease Literature Reviewafdtvovhb100% (1)
- Heart FailureDocument13 pagesHeart Failuremildred alidon100% (2)
- Heart Failure DDx and WorkupDocument3 pagesHeart Failure DDx and WorkupAizel ManiagoNo ratings yet
- Justin A. Zivin: Cerebrovascular Disease 2Document21 pagesJustin A. Zivin: Cerebrovascular Disease 2Henry SsentongoNo ratings yet
- Management of NutritionDocument1 pageManagement of NutritionHafiz Idul FitranulNo ratings yet
- ModuleDocument15 pagesModuleQariahMaulidiahAminNo ratings yet
- Explanation of The Students' Module: Growth-Development Disturbance Medical Faculty Hasanuddin University MakassarDocument10 pagesExplanation of The Students' Module: Growth-Development Disturbance Medical Faculty Hasanuddin University MakassarHafiz Idul FitranulNo ratings yet
- 17.hematological Disorders in Geriatric PatientsDocument18 pages17.hematological Disorders in Geriatric PatientsHafiz Idul FitranulNo ratings yet
- P2 EnglishDocument9 pagesP2 EnglishHafiz Idul FitranulNo ratings yet
- Anatomy of The Head: Nervous System (PNS) - The Former Consists of The Brain and Spinal Cord, While The LatterDocument6 pagesAnatomy of The Head: Nervous System (PNS) - The Former Consists of The Brain and Spinal Cord, While The LatterHafiz Idul FitranulNo ratings yet
- Riatric CardioDocument23 pagesRiatric CardioHafiz Idul FitranulNo ratings yet
- Muhammad Ilyas Pulmonology Division on Community Acquired Pneumonia (CAPDocument22 pagesMuhammad Ilyas Pulmonology Division on Community Acquired Pneumonia (CAPHafiz Idul FitranulNo ratings yet
- Anatomy of The Head: Nervous System (PNS) - The Former Consists of The Brain and Spinal Cord, While The LatterDocument6 pagesAnatomy of The Head: Nervous System (PNS) - The Former Consists of The Brain and Spinal Cord, While The LatterHafiz Idul FitranulNo ratings yet
- NIOSH Investigation - CR 337 Fire LODDDocument32 pagesNIOSH Investigation - CR 337 Fire LODDRamblingChiefNo ratings yet
- Acute Liver Injury and FailureDocument14 pagesAcute Liver Injury and FailureWeslei ChaconNo ratings yet
- How Can We Use Neurotransmitters in Emotion and Reward System To Study DepressionDocument22 pagesHow Can We Use Neurotransmitters in Emotion and Reward System To Study DepressionGlobal Research and Development ServicesNo ratings yet
- Come Thirsty Chapter1Document4 pagesCome Thirsty Chapter1Natalia Lupasco100% (1)
- The Integumentary SystemDocument11 pagesThe Integumentary SystemHamdy Pagilit DimaporoNo ratings yet
- Center For Prehospital Care: Benjamin E. EsparzaDocument10 pagesCenter For Prehospital Care: Benjamin E. EsparzasenjahipgabiNo ratings yet
- B CellDocument10 pagesB CellSonia Elizabeth SimonNo ratings yet
- Chlorhexidine - An Antiseptic in PeriodonticsDocument4 pagesChlorhexidine - An Antiseptic in PeriodonticsInternational Organization of Scientific Research (IOSR)No ratings yet
- Branches of ZoologyDocument3 pagesBranches of ZoologyVivek Morya100% (1)
- NCP of CavDocument3 pagesNCP of CavHenry Roque TagalagNo ratings yet
- Mobilization NotesDocument239 pagesMobilization NotesSuganya Balachandran100% (6)
- SP FinalDocument18 pagesSP Finalapi-344954734No ratings yet
- Template POMRDocument8 pagesTemplate POMRPPDS IPD ULMNo ratings yet
- Posture and AlignmentDocument1 pagePosture and AlignmentNell KeeneNo ratings yet
- T2DMDocument24 pagesT2DMXyra BadangayonNo ratings yet
- SGD Physiology Endocrine and MetabolismDocument7 pagesSGD Physiology Endocrine and MetabolismTinesh RajahNo ratings yet
- Modul Strategik Bab 1 Ting 5Document6 pagesModul Strategik Bab 1 Ting 5SK Pos TenauNo ratings yet
- Fuel Metabolism in StarvationDocument27 pagesFuel Metabolism in Starvationilluminel100% (2)
- Comprehensive Review of Temporal Lobe EpilepsyDocument36 pagesComprehensive Review of Temporal Lobe Epilepsyyohanes0gadiNo ratings yet
- Drug StudyDocument11 pagesDrug StudyKimberly Ann MendozaNo ratings yet
- CSIR UGC JRF Subjetive Model Test PaperDocument17 pagesCSIR UGC JRF Subjetive Model Test PaperManu Mallahalli ShanthappaNo ratings yet
- Biology #KautilyaDocument79 pagesBiology #KautilyapankajNo ratings yet
- H2S Training Slides ENGLISHDocument46 pagesH2S Training Slides ENGLISHf.B100% (1)
- Pregnancy - Anatomy and PhysiologyDocument5 pagesPregnancy - Anatomy and PhysiologyNiSCHENo ratings yet
- Abdominal SurgeryDocument166 pagesAbdominal SurgeryIndera VyasNo ratings yet
- Alternative Modes of Mechanical Ventilation - A Review of The HospitalistDocument14 pagesAlternative Modes of Mechanical Ventilation - A Review of The HospitalistMichael LevitNo ratings yet
- GIT Physio D&R AgamDocument67 pagesGIT Physio D&R Agamvisweswar030406No ratings yet
- Prokaryotes and Eukaryotes: Strategies and Successes: Michael CarlileDocument3 pagesProkaryotes and Eukaryotes: Strategies and Successes: Michael CarlileNurul ShazwaniNo ratings yet
- CONSUMER BEHAVIOUR UpdatedDocument66 pagesCONSUMER BEHAVIOUR UpdatedUmer AzizNo ratings yet
- Altered Body TemperatureDocument27 pagesAltered Body TemperatureAnusikta PandaNo ratings yet
- LIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionFrom EverandLIT: Life Ignition Tools: Use Nature's Playbook to Energize Your Brain, Spark Ideas, and Ignite ActionRating: 4 out of 5 stars4/5 (402)
- Why We Die: The New Science of Aging and the Quest for ImmortalityFrom EverandWhy We Die: The New Science of Aging and the Quest for ImmortalityRating: 3.5 out of 5 stars3.5/5 (2)
- Outlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisFrom EverandOutlive: The Science and Art of Longevity by Peter Attia: Key Takeaways, Summary & AnalysisRating: 4 out of 5 stars4/5 (1)
- Summary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedFrom EverandSummary: The Psychology of Money: Timeless Lessons on Wealth, Greed, and Happiness by Morgan Housel: Key Takeaways, Summary & Analysis IncludedRating: 5 out of 5 stars5/5 (78)
- The Age of Magical Overthinking: Notes on Modern IrrationalityFrom EverandThe Age of Magical Overthinking: Notes on Modern IrrationalityRating: 4 out of 5 stars4/5 (13)
- Think This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeFrom EverandThink This, Not That: 12 Mindshifts to Breakthrough Limiting Beliefs and Become Who You Were Born to BeNo ratings yet
- The Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsFrom EverandThe Ritual Effect: From Habit to Ritual, Harness the Surprising Power of Everyday ActionsRating: 3.5 out of 5 stars3.5/5 (3)
- Techniques Exercises And Tricks For Memory ImprovementFrom EverandTechniques Exercises And Tricks For Memory ImprovementRating: 4.5 out of 5 stars4.5/5 (40)
- Raising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsFrom EverandRaising Mentally Strong Kids: How to Combine the Power of Neuroscience with Love and Logic to Grow Confident, Kind, Responsible, and Resilient Children and Young AdultsRating: 5 out of 5 stars5/5 (1)
- Raising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsFrom EverandRaising Good Humans: A Mindful Guide to Breaking the Cycle of Reactive Parenting and Raising Kind, Confident KidsRating: 4.5 out of 5 stars4.5/5 (169)
- The Obesity Code: Unlocking the Secrets of Weight LossFrom EverandThe Obesity Code: Unlocking the Secrets of Weight LossRating: 5 out of 5 stars5/5 (4)
- The Ultimate Guide To Memory Improvement TechniquesFrom EverandThe Ultimate Guide To Memory Improvement TechniquesRating: 5 out of 5 stars5/5 (34)
- Dark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.From EverandDark Psychology & Manipulation: Discover How To Analyze People and Master Human Behaviour Using Emotional Influence Techniques, Body Language Secrets, Covert NLP, Speed Reading, and Hypnosis.Rating: 4.5 out of 5 stars4.5/5 (110)
- Roxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingFrom EverandRoxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingRating: 5 out of 5 stars5/5 (4)
- Summary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisFrom EverandSummary: Outlive: The Science and Art of Longevity by Peter Attia MD, With Bill Gifford: Key Takeaways, Summary & AnalysisRating: 4.5 out of 5 stars4.5/5 (41)
- By the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsFrom EverandBy the Time You Read This: The Space between Cheslie's Smile and Mental Illness—Her Story in Her Own WordsNo ratings yet
- The Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsFrom EverandThe Garden Within: Where the War with Your Emotions Ends and Your Most Powerful Life BeginsNo ratings yet
- Secure Love: Create a Relationship That Lasts a LifetimeFrom EverandSecure Love: Create a Relationship That Lasts a LifetimeRating: 5 out of 5 stars5/5 (17)
- Roxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingFrom EverandRoxane Gay & Everand Originals: My Year of Psychedelics: Lessons on Better LivingRating: 3.5 out of 5 stars3.5/5 (33)
- The Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaFrom EverandThe Body Keeps the Score by Bessel Van der Kolk, M.D. - Book Summary: Brain, Mind, and Body in the Healing of TraumaRating: 4.5 out of 5 stars4.5/5 (266)
- The Happiness Trap: How to Stop Struggling and Start LivingFrom EverandThe Happiness Trap: How to Stop Struggling and Start LivingRating: 4 out of 5 stars4/5 (1)
- Cult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryFrom EverandCult, A Love Story: Ten Years Inside a Canadian Cult and the Subsequent Long Road of RecoveryRating: 4 out of 5 stars4/5 (44)