Professional Documents
Culture Documents
How Catalytic Converters Reduce Toxins in Car Exhaust
Uploaded by
Neerav Indrajit GadhviOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
How Catalytic Converters Reduce Toxins in Car Exhaust
Uploaded by
Neerav Indrajit GadhviCopyright:
Available Formats
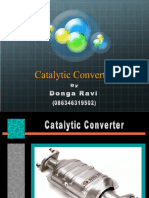
Catalytic Converters
BY: Neerav Gadhvi (09001023) Catalytic converters are devices which convert toxic byproducts of combustion in the exhaust of an internal combustion engine to less toxic substances by way of catalyzed chemical reactions. Exhaust emissions from internal combustion engines contain pollutants mainly, hydrocarbons (CmHn), carbon monoxide (CO), and nitrogen oxides (NO, NO2). By using special catalysts and a suitable air-to-fuel ratio in spark-ignited internal combustion engines, the three classes of pollutants can be removed together in a single catalytic converter. Nowadays, most new cars are equipped with what is called a three-way catalytic converter. A three-way catalytic converter has three simultaneous tasks: 1. Reduction of nitrogen oxides to nitrogen and oxygen: 2NOx xO2 + N2 2. Oxidation of carbon monoxide to carbon dioxide: 2CO + O2 2CO2 3. Oxidation of unburnt hydrocarbons to carbon dioxide & water: CxH2x+2 + [(3x+1)/2]O2 xCO2 + (x+1)H2O There are two main parts to the catalytic converter: Reduction Catalyst The reduction catalyst is the first step that the exhaust goes through in the catalytic converter. The catalysts used in this part are platinum and rhodium to break now NO and NO2 molecules in to N2 and O2 gases which are non-polluting. This process occurs using either of the following two chemical equations: For NO: 2NO N2 + O2 For NO2: 2NO2 N2 + 2O2 Oxidation Catalyst The oxidation catalyst is the second stage of the catalytic converter that oxidizes hydrocarbons and carbon monoxide with palladium and platinum as catalysts in a combustion reaction. The chemical formula for this part is: 2CO + O2 2CO2 Cerium is also used as it promotes oxygen storage to improve oxidation efficiency. Besides NO, CO, & hydrocarbons, diesel engines emit particulate matter & removal of it is a physical separation process. The exhaust gas is forced to flow through a filter material with pores smaller than or similar in size to the particles. The filter must be regenerated by burning the trapped particulate matter with the oxygen present exhaust which requires high temperatures ( 550 C). Since such temperatures are rarely reached in the exhaust of diesel vehicles other methods like catalytically lowering the ignition temperature of the soot are implemented. Also there are systems called continuously regenerating trap (CRT), here naturally occurring NO is oxidized by a strong oxidation catalyst to NO2, which continuously oxidizes the deposited particulate matter. Conclusion A major remaining challenge for the automotive industry is the lowering of fuel consumption and CO2 emissions. This is also a challenge for catalyst development, since the most fuel-efficient engines operate with excess air, & the conventional three-way catalyst does not allow NOx reduction under these conditions. New technologies are being developed that allow fuel-efficient lean operation of the engine and at the same time retain the high emission standards of conventional three-way catalysts.
You might also like
- The Diesel Catalytic ConverterDocument4 pagesThe Diesel Catalytic ConvertersayeemNo ratings yet
- Unit II - Engine Emission Control and 3 Way Catalytic ConverterDocument28 pagesUnit II - Engine Emission Control and 3 Way Catalytic ConverterdrkbalaNo ratings yet
- Reduce Emissions with Catalytic ConvertersDocument8 pagesReduce Emissions with Catalytic ConvertersEugeneNo ratings yet
- Novel Catalytic Technologies For Car Emission ReductionDocument52 pagesNovel Catalytic Technologies For Car Emission ReductionNguyenHuanNo ratings yet
- Diesel Engines: Vehicle Emission RegulationsDocument11 pagesDiesel Engines: Vehicle Emission Regulationssheetal62No ratings yet
- CatalysatorDocument8 pagesCatalysatorslowspeedysNo ratings yet
- Post CombustionDocument15 pagesPost CombustionThe UltimateNo ratings yet
- Diesel Catalytic Converters As Emission Control DevicesDocument19 pagesDiesel Catalytic Converters As Emission Control Deviceskk ppNo ratings yet
- Lecture - 6 Exhaust Gas RecirculationDocument47 pagesLecture - 6 Exhaust Gas RecirculationUmar ChNo ratings yet
- ICE - Bharat Stage Vs Euro Emission NormsDocument27 pagesICE - Bharat Stage Vs Euro Emission Normsmaa1333No ratings yet
- Formation of CO and NOx in IC EnginesDocument44 pagesFormation of CO and NOx in IC EnginesGautam GunjanNo ratings yet
- Technical Seminar on Catalytic ConvertersDocument21 pagesTechnical Seminar on Catalytic ConvertersDiksha MishraNo ratings yet
- Pollutants Produced by A Car Engine: GasolineDocument16 pagesPollutants Produced by A Car Engine: GasolineHarpreet RandhawaNo ratings yet
- Seminar ReportDocument17 pagesSeminar Reportapi-3706848100% (1)
- Catalytic ConverterDocument18 pagesCatalytic Converterblinkingsun30No ratings yet
- How catalytic converters reduce vehicle emissionsDocument2 pagesHow catalytic converters reduce vehicle emissionsRosetaCNo ratings yet
- Chemistry Sulphur and Catalyst (AutoRecovered)Document3 pagesChemistry Sulphur and Catalyst (AutoRecovered)Nelsen GabrielNo ratings yet
- EGR-Systems For Diesel EnginesDocument57 pagesEGR-Systems For Diesel EnginesRanil Fernando100% (1)
- Everything You Need to Know About Catalytic ConvertersDocument16 pagesEverything You Need to Know About Catalytic ConvertersRahul PrasadNo ratings yet
- Catalytic ConverterDocument9 pagesCatalytic ConverterVishnuNo ratings yet
- Catalytic ConverterDocument14 pagesCatalytic ConverterRavi DongaNo ratings yet
- ENV315 - Online Air Pollution Control Lecture 11Document23 pagesENV315 - Online Air Pollution Control Lecture 11Samim JubayerNo ratings yet
- Emission Control: Exhaust Emissions Are Produced by Cars, Buses, andDocument10 pagesEmission Control: Exhaust Emissions Are Produced by Cars, Buses, andsonabrar10100% (1)
- Iss PresentatCATALYTIC CONVERTERSionDocument30 pagesIss PresentatCATALYTIC CONVERTERSionamulya_sainiNo ratings yet
- Catalytic Converter Based On Non Noble MaterialDocument4 pagesCatalytic Converter Based On Non Noble Materialpamela garciaNo ratings yet
- Vehicle emission control techniquesDocument6 pagesVehicle emission control techniquesmsaqibraza93No ratings yet
- Emissions LectureDocument27 pagesEmissions LectureNandepu Sravan KumarNo ratings yet
- International Journal of Engineering and Science Invention (IJESI)Document10 pagesInternational Journal of Engineering and Science Invention (IJESI)inventionjournalsNo ratings yet
- A Sad Story of Artificial Animal in Natural Environment ..: IC Engine EmissionsDocument33 pagesA Sad Story of Artificial Animal in Natural Environment ..: IC Engine EmissionsdhavalNo ratings yet
- CDGI’s CHAMELI DEVI SCHOOL OF ENGINEERING, INDORE Department of Mechanical Engineering Session: 2015-16 Subject Code:ME-607 SEMINAR ON CATALYTIC CONVERTERDocument20 pagesCDGI’s CHAMELI DEVI SCHOOL OF ENGINEERING, INDORE Department of Mechanical Engineering Session: 2015-16 Subject Code:ME-607 SEMINAR ON CATALYTIC CONVERTERSanskarrRathiiNo ratings yet
- Engine Emissions and Combustion StrategiesDocument32 pagesEngine Emissions and Combustion StrategiesVaratharaju NamakkalNo ratings yet
- Me 303 CH12Document47 pagesMe 303 CH12Osman KutluNo ratings yet
- Chemical Lopping CombustionDocument19 pagesChemical Lopping CombustionAstha YadavNo ratings yet
- "Effect of Exhaust Gas Recirculation (Egr) On Nox Emission From C.I. Engine" - A Review StudyDocument5 pages"Effect of Exhaust Gas Recirculation (Egr) On Nox Emission From C.I. Engine" - A Review StudyShreyash BalpandeNo ratings yet
- Case Study 1Document7 pagesCase Study 1Muhammad AtifNo ratings yet
- Diesel Exhaust Gas AftertreatmentDocument16 pagesDiesel Exhaust Gas AftertreatmentlukhmanNo ratings yet
- Catalytic ConverterDocument19 pagesCatalytic ConverterParth Batra50% (2)
- Catalytic Converter - Wikipedia, The Free EncyclopediaDocument11 pagesCatalytic Converter - Wikipedia, The Free EncyclopediaazamrashdiNo ratings yet
- "Effect of Exhaust Gas Recirculation (Egr) On Nox Emission From C.I. Engine" - A Review StudyDocument5 pages"Effect of Exhaust Gas Recirculation (Egr) On Nox Emission From C.I. Engine" - A Review Studyslv_prasaadNo ratings yet
- 9 - Emissions and ControlsDocument32 pages9 - Emissions and ControlsAshik HasanNo ratings yet
- Fuel AnalysisDocument34 pagesFuel AnalysisYedla Santosh kumar100% (2)
- Everything You Need to Know About Catalytic ConvertersDocument25 pagesEverything You Need to Know About Catalytic ConvertersCharlie Tej0% (1)
- Catalytic ConverterDocument17 pagesCatalytic ConverterRavi Donga93% (14)
- Emission Standard DetailsDocument25 pagesEmission Standard DetailsbalamuraliNo ratings yet
- Catalytic ConverterDocument11 pagesCatalytic ConverterAJ MukunNo ratings yet
- Presentation topic:HC, CO and Nox Emission: Instructor:Engr - Altaf Subject:IC - Engine Mechanical 7thDocument11 pagesPresentation topic:HC, CO and Nox Emission: Instructor:Engr - Altaf Subject:IC - Engine Mechanical 7thharoon ashrafNo ratings yet
- Structured Catalysts For Soot Combustion For Diesel Engines: E.D. Banús, M.A. Ulla, E.E. Miró and V.G. MiltDocument26 pagesStructured Catalysts For Soot Combustion For Diesel Engines: E.D. Banús, M.A. Ulla, E.E. Miró and V.G. MiltYuva RajNo ratings yet
- Catalytic ConverterDocument35 pagesCatalytic ConverterVikas SalveNo ratings yet
- Recent Development in Marine EnginesDocument81 pagesRecent Development in Marine EnginesShashidhar ChandraiahNo ratings yet
- Coal and Coal ChemicalsDocument54 pagesCoal and Coal ChemicalsVishal DhapaNo ratings yet
- On IC EnginesDocument29 pagesOn IC EnginesBabu JonnalagaddaNo ratings yet
- FUEL TECHNOLOGY: PETROLEUM PROCESSING AND FRACTIONSDocument22 pagesFUEL TECHNOLOGY: PETROLEUM PROCESSING AND FRACTIONSsubham kunduNo ratings yet
- Air and Water ChemistryDocument24 pagesAir and Water ChemistryShaman Samuel GodfreyNo ratings yet
- Steam Reforming Converts Hydrocarbons to Syngas for Hydrogen ProductionDocument4 pagesSteam Reforming Converts Hydrocarbons to Syngas for Hydrogen Productiontriatmi lusitaNo ratings yet
- What Is Emission Control System?Document14 pagesWhat Is Emission Control System?nahomNo ratings yet
- Reduction of No Emission in Biodiesel Engines by Exhaust Gas Aftertreatment MethodDocument12 pagesReduction of No Emission in Biodiesel Engines by Exhaust Gas Aftertreatment MethodVijay Kumar DanapalNo ratings yet
- Energies: Dimethyl Carbonate As A Promising Oxygenated Fuel For Combustion: A ReviewDocument20 pagesEnergies: Dimethyl Carbonate As A Promising Oxygenated Fuel For Combustion: A ReviewMátyás DalnokiNo ratings yet
- Carbon Capture Technologies for Gas-Turbine-Based Power PlantsFrom EverandCarbon Capture Technologies for Gas-Turbine-Based Power PlantsNo ratings yet
- Clean Ironmaking and Steelmaking Processes: Efficient Technologies for Greenhouse Emissions AbatementFrom EverandClean Ironmaking and Steelmaking Processes: Efficient Technologies for Greenhouse Emissions AbatementNo ratings yet
- Lab Experiment RubricDocument3 pagesLab Experiment RubricNeerav Indrajit Gadhvi100% (1)
- Exam Rubrics Final PDFDocument1 pageExam Rubrics Final PDFNeerav Indrajit GadhviNo ratings yet
- CBSE Class X (Science) Page 1 30Document30 pagesCBSE Class X (Science) Page 1 30Nitesh Bhardwaj0% (1)
- Exam Rubrics Final PDFDocument1 pageExam Rubrics Final PDFNeerav Indrajit GadhviNo ratings yet
- General Industry Safety Manual Final3Document142 pagesGeneral Industry Safety Manual Final3danni1No ratings yet
- Schedule For Analyst Inst MeetDocument20 pagesSchedule For Analyst Inst MeetNeerav Indrajit GadhviNo ratings yet
- ch16 PDFDocument5 pagesch16 PDFNeerav Indrajit GadhviNo ratings yet
- ch16 PDFDocument5 pagesch16 PDFNeerav Indrajit GadhviNo ratings yet
- Chapter 3Document35 pagesChapter 3navneetNo ratings yet
- CH 2Document5 pagesCH 2Lawrence LeeNo ratings yet
- Janmangal Stotra Large FontsDocument5 pagesJanmangal Stotra Large FontsNeerav Indrajit GadhviNo ratings yet
- 3350503 (2)Document2 pages3350503 (2)Neerav Indrajit GadhviNo ratings yet
- Micro-Crossflow Membrane Filtration SystemsDocument2 pagesMicro-Crossflow Membrane Filtration SystemsNeerav Indrajit GadhviNo ratings yet
- Manufacturing & Effluent Treatment ProcessesDocument28 pagesManufacturing & Effluent Treatment ProcessesNeerav Indrajit GadhviNo ratings yet
- Manufacturing & Effluent Treatment ProcessesDocument28 pagesManufacturing & Effluent Treatment ProcessesNeerav Indrajit GadhviNo ratings yet
- 17-34-ET-V1-S1 Module Theory of Penicillin FermentationDocument8 pages17-34-ET-V1-S1 Module Theory of Penicillin FermentationNeerav Indrajit GadhviNo ratings yet
- List of Booker Prize Winners - by Neerav GadhviDocument45 pagesList of Booker Prize Winners - by Neerav GadhviNeerav Indrajit GadhviNo ratings yet
- Micro-Crossflow Membrane Filtration SystemsDocument2 pagesMicro-Crossflow Membrane Filtration SystemsNeerav Indrajit GadhviNo ratings yet
- How To Wear A TieDocument1 pageHow To Wear A TieNeerav Indrajit GadhviNo ratings yet
- Advt 08 2015Document7 pagesAdvt 08 2015Neerav Indrajit GadhviNo ratings yet
- 16 FL - vinegarFermentationUnitsDocument2 pages16 FL - vinegarFermentationUnitsNeerav Indrajit GadhviNo ratings yet
- Advt. For GETs Through GATE-2013Document5 pagesAdvt. For GETs Through GATE-2013rathour2No ratings yet
- List of Booker Prize Winners - by Neerav GadhviDocument45 pagesList of Booker Prize Winners - by Neerav GadhviNeerav Indrajit GadhviNo ratings yet
- LARR Bill 2015 PDFDocument9 pagesLARR Bill 2015 PDFNeerav Indrajit GadhviNo ratings yet
- ThoughtsDocument3 pagesThoughtsNeerav Indrajit GadhviNo ratings yet
- IIT Gandhinagar Timetable Second Semester 2012Document17 pagesIIT Gandhinagar Timetable Second Semester 2012Neerav Indrajit GadhviNo ratings yet
- RTIDocument1 pageRTINeerav Indrajit GadhviNo ratings yet
- List of Good BooksDocument15 pagesList of Good BooksNeerav Indrajit Gadhvi100% (1)
- Comprehensive Guide to Topics Covered on Science Direct DatabaseDocument8 pagesComprehensive Guide to Topics Covered on Science Direct DatabaseNeerav Indrajit GadhviNo ratings yet
- HW2 SolutionsDocument8 pagesHW2 SolutionsPamela HulseNo ratings yet
- Heliarc 250 Ac/Dc Power Sources For 230/460/575 VOLT SERVICEDocument4 pagesHeliarc 250 Ac/Dc Power Sources For 230/460/575 VOLT SERVICEDeepan MNo ratings yet
- Report On Sae SupraDocument21 pagesReport On Sae SupraSanchit Agarwal0% (1)
- 08 DRG - Protection SLD 220kV SarigamDocument1 page08 DRG - Protection SLD 220kV SarigamAmarjit Kulkarni100% (1)
- PCB1043 Tutorial 2 ENERGY TRANSFER BY HEAT, WORK & MASSDocument4 pagesPCB1043 Tutorial 2 ENERGY TRANSFER BY HEAT, WORK & MASSAlex CoolNo ratings yet
- ThePowerOfPositiveYou 7StepPlan PDFDocument11 pagesThePowerOfPositiveYou 7StepPlan PDFaaa bbb cccNo ratings yet
- SD 0100CT1502 Sec-06Document18 pagesSD 0100CT1502 Sec-06royvindasNo ratings yet
- Knief Chapter 3 Nuke EngineeringDocument40 pagesKnief Chapter 3 Nuke EngineeringJohn W HollandNo ratings yet
- DCE Biodiesel Information BrochureDocument10 pagesDCE Biodiesel Information BrochureIshwar ChandraNo ratings yet
- Lưu ý: - Thí sinh làm bài trên tờ giấy thi, ghi theo đúng thứ tự câu từ 1 đến 32Document4 pagesLưu ý: - Thí sinh làm bài trên tờ giấy thi, ghi theo đúng thứ tự câu từ 1 đến 32nang chieu Lung linh muon hoa vangNo ratings yet
- DPS2400B-48 Fact Sheet PDFDocument2 pagesDPS2400B-48 Fact Sheet PDFmazenkhodr1983No ratings yet
- Final Copy of Thesis - RTDocument86 pagesFinal Copy of Thesis - RTMuhammad Sohail AbidNo ratings yet
- Environment Iced II Fact Sheet EngDocument2 pagesEnvironment Iced II Fact Sheet EngDeni IstiawanNo ratings yet
- What is transformer rating based on temperature and insulation limitsDocument2 pagesWhat is transformer rating based on temperature and insulation limitsSugeng SumarnoNo ratings yet
- PSP, Modern Technologies and Large Scale PDFDocument11 pagesPSP, Modern Technologies and Large Scale PDFDeepak GehlotNo ratings yet
- Eaton 12v 540w Battery Brochure PA162003ENDocument2 pagesEaton 12v 540w Battery Brochure PA162003ENRafael AguileraNo ratings yet
- Chapter 1. Review of Fundamentals: Essential Graduate Physics CM: Classical MechanicsDocument14 pagesChapter 1. Review of Fundamentals: Essential Graduate Physics CM: Classical MechanicsplfratarNo ratings yet
- Earth and Life Science Diagnostic ExamDocument3 pagesEarth and Life Science Diagnostic ExamJohn Mark JarencioNo ratings yet
- CV SukiDocument3 pagesCV SukiGusti Ryandi AriefNo ratings yet
- Enrique Alvarez Vita Ensayo New Model of The Universe Final TranslationDocument22 pagesEnrique Alvarez Vita Ensayo New Model of The Universe Final TranslationLuis Alberto Suarez100% (1)
- Components of Gas Turbine Power PlantDocument53 pagesComponents of Gas Turbine Power PlantRakesh ReddyNo ratings yet
- Principle of Operation of RCCBDocument5 pagesPrinciple of Operation of RCCBchamara kumarasingheNo ratings yet
- Energy Transition Model 2030 - Impact of Sustainable Lifestyle Without Usage of Natural GasDocument7 pagesEnergy Transition Model 2030 - Impact of Sustainable Lifestyle Without Usage of Natural GasStefan BruinsNo ratings yet
- Generator ProtectionDocument10 pagesGenerator ProtectionSriram ramsNo ratings yet
- HPP Ashta Project LeafletDocument4 pagesHPP Ashta Project LeafletsalicurriNo ratings yet
- Types of Faults in Power SystemDocument6 pagesTypes of Faults in Power Systemcollege safariNo ratings yet
- Laporan Gangguan Engine 2 AVR Diode Protection TripDocument3 pagesLaporan Gangguan Engine 2 AVR Diode Protection TripGIDEON SEBASTIAN TODING TIRANDANo ratings yet
- of HXEDocument31 pagesof HXEetayhailuNo ratings yet
- Internal Energy and The Ideal GasDocument9 pagesInternal Energy and The Ideal GasfagroupandahmadsonsNo ratings yet
- Exploring Energy Solutions for US RegionsDocument6 pagesExploring Energy Solutions for US RegionsAparnaBoddapatiNo ratings yet