Professional Documents
Culture Documents
CBE 3233 MassTransfer
Uploaded by
Mike FordealsOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CBE 3233 MassTransfer
Uploaded by
Mike FordealsCopyright:
Available Formats
Qing Song Spring 2013
Lecture 3 Mass Transfer
1
Mass transfer by ordinary molecular diffusion
occurs because of a concentration gradient
Net movement of a species in a mixture from one
location to another.
2
Applications of Mass Transfer
Operation Extract
Phase
Raffinate
Phase
Distillation
Gas
adsorption/dehumidification
Membrane separations
Adsorption
Liquid extraction
Leaching
Crystallization
Drying
Vapor
Gas
Gas or liquid
Gas or liquid
Extract
Liquid
Mother liquor
Gas (usually
air)
Liquid
Liquid
Gas or liquid
Solid
Raffinate
Solid
Crystals
Wet solid
Transfer of material from one homogeneous phase to another
Driving force for transfer is a concentration difference
3
Absorption: solute transfer through the gas to the gas-
liquid interface, across the interface, and into the liquid
Distillation: separate a liquid mixture by vaporization into
individual components.
A solute gas is absorbed from an inert gas into a liquid in
which the solute is more or less soluble.
Washing of ammonia from mixture of NH
3
and air,
Remove of CO
2
and H
2
S from natural gas.
The low boiler (more volatile) diffuses through the liquid
phase to the interface and away from the interface into
the vapor. The high boiler (less volatile) diffuses in the
reverse direction.
Example: ethanol and water, crude oil into gasoline,
kerosene, fuel oil, and lubricating stock.
4
Mechanisms of Mass Transfer
Molecular diffusion: random, spontaneous,
Eddy (turbulent) diffusion by random,
macroscopic fluid motion
Both molecular and eddy diffusion may
involve the movement of different species in
opposing directions.
Bulk flow of the mixture in a direction
parallel to the direction of diffusion.
5
Molar concentration:
Mole fraction:
(liquids, solid (gases)
RT
p
V
n
M
c
A A
A
A
A
= = =
6
RT
p
V
n
M
c
i i
i
i
i
= = =
c
c
x
i
i
=
c
c
x
A
A
=
c
c
y
i
i
=
c
c
y
A
A
=
molar average velocity,
c
c
n
i
i i
=
=
1
M
v
v
velocity of a particular species relative to molar average
velocity is the diffusion velocity
M i iD
v v v =
Molar flux
i i i
c N v =
With respect to a fixed reference frame
Molar flux
With respect to a molar average velocity v
M
( )
M i i i
c J v v =
7
Flux: A vector quantity denoting amount of a particular
Species that passes per given time through a unit area
normal to the vector, given by Ficks First Law, for basic
molecular diffusion
Molar flux
or, in the z-direction,
For a general relation in a non-isothermal, isobaric
system,
A AB A
c D V = J
dz
dc
D J
A
AB z A
=
,
dz
dy
cD J
A
AB z A
=
,
Theory of Diffusion
dz
dw
D j
A
AB A
=
Mass flux
8
i i
c DV = J
dz
dc
D J
i
z i
=
,
dz
dy
cD J
i
z i
=
,
dz
dw
D j
i
i
=
Units of Above Parameters
J
i
: molar flux of component i, kgmol/m
2
.h (kmol/m
2
.h) or lb
mol/ft
2
.h
D: diffusivity m
2
/h
C
i
: mole concentration kgmol/m
3
, kmol/m
3
, or lbmol/ft
3
Z: distance in direction of diffusion, m or ft
i
: mass density of component i, kg/m
3
, or lb/ft
3
x
i
: mole fraction of i
w
i
: mass fraction of i
9
10
Units of Above Parameters for Binary System
J
A
: molar flux of component i, kgmol/m
2
.h (kmol/m
2
.h) or lb
mol/ft
2
.h
D
AB
: diffusivity m
2
/h
C
A
: mole concentration kgmol/m
3
, kmol/m
3
, or lbmol/ft
3
Z: distance in direction of diffusion, m or ft
A
: mass density of component A, kg/m
3
, or lb/ft
3
x
A
: mole fraction of A
w
A
: mass fraction of A
Similarities between Mass , Heat and Momentum Transfer
Mass
Heat
Momentum
dz
dc
D J
A
AB z A
=
,
Ficks first law
dz
dT
k q =
Fouriers law
( )
dz
v d
x
zx
t =
Newtowns law
11
Since mass is transferred by two means:
concentration differences
and convection differences from density
differences
12
( )
M i i M i i i M i i i
c N c c c J v v v v v = = =
M i
i
M i i i
c
dz
dc
D c J N v v + = + =
Rearranging:
Diffusion flux Convective flux
= =
= =
=
+ = + = + =
n
i
i
n
i
i
i i i
n
i
i
i
i
n
i
i
n
i
i i
i i i
N
c
c
J c
c
c
J
c
c
c J N
1 1
1 1
1
v
v
=
+ =
n
i
i i i i
N x J N
1
13
Flux Relationships for Binary System
dz
dx
cD J
A
AB A
=
( ) ( )
B A A
A
AB B A A A A
N N x
dz
dx
cD N N x J N + + = + + =
Summary of Molar and Mass fluxes
14
J
A
, and N
A
are equivalent statement of the
Fick rate equation. Any one of these
equations is adequate to describe molecular
diffusion.
Molar fluxes, J
A
and N
A
, are used to describe
mass-transfer operations in which chemical
reactions are involved. They are often used to
describe the mass transfer in diffusion cells
for measuring diffusion coefficients.
Molecular Diffusion
15
Unit area
N
A
N
B
N
A
is positive N
B
is negative
Net flux=N=N
A
+N
B
A(Water) B(Ethanol)
16
Equimolar Counter Diffusion (EMD)
N x J N
A A A
+ =
( )
B A
A A
AB A
N N
c
c
dz
dc
D N + + =
( )
B A
B B
BA B
N N
c
c
dz
dc
D N + + =
( )
( )
dz
dc
D
dz
dc
D
c
c c
N N N N
B
BA
A
AB
B A
B A B A
+
+ = +
similarly,
Adding them together,
dz
dc
D
dz
dc
D
B
BA
A
AB
=
B A
J J = 0 = + =
B A
N N N
17
In the Absence of Bulk flow
dz
dx
cD J N
A
AB A A
= =
dz
dx
cD J N
B
AB B B
= =
(3-16)
(3-17)
Total concentration c, T, P are constants,
z=z
1
, x
A
=x
A1
, z=z
2
, x
A
=x
A2
A
AB
A
dx dz
cD
N
=
} }
=
A
A
x
x
A
z
z
AB
A
dx dz
cD
N
1 1
18
( )
1 1 A A
AB
A
x x z z
cD
N
=
Integrate between z
1
and z
Equimolar Counter Diffusion (EMD)
( )
1
1
A A
AB
A
x x
z z
cD
J
=
( )
B B
AB
B
x x
z z
cD
J
=
1
1
(3-18)
(3-19)
19
Unimolecular Diffusion (UMD)
N=N
A
, N
B
=0
Only one component such as A is being transferred,
Example: ammonia (NH
3
) (A) & air (B) absorbed by water
A A
A
AB A
N x
dz
dx
cD N + =
(3-26)
dz
dx
cD N x
A
AB A A
= ) 1 (
( ) dz
dx
x
cD
N
A
A
AB
A
=
1
(3-27)
Integrating eq.(3-27)
At quasi-steady-state conditions and constant molar activity
( )
( )
( )
) 1 ln(
1
1
1
1
1
1
1
A
x
x
A
AB
A
A
x
x
A
AB
A
A
x
x
A
AB
z
z
x d
N
cD
x
x d
N
cD
x
dx
N
cD
dz
A
A
A
A
A
A
=
=
} } } }
|
|
.
|
\
|
= =
1
1
1
1
ln ) 1 ( ln
1
A
A
A
AB
x
x
A
A
AB
x
x
N
cD
x
N
cD
z z
A
A
(3-31)
20
( )
AB
A
A
A
cD
z z N
x
x
1
1
1
1
ln
=
|
|
.
|
\
|
( )
AB
A
cD
z z N
A
A
e
x
x
1
1
1
1
=
( )
( )
(
=
AB
A
cD
z z N
A A
e x x
1
1
1 1
Unimolecular Diffusion (UMD)
(3-32)
|
|
.
|
\
|
=
1
2
1 2
1
1
ln
A
A AB
A
x
x
z z
cD
N ( )
( ) ( )
( )
( )
( )
( )
(
=
(
=
1
2
2 1
1
2
1 2
1
1
ln
1
1
ln
1 1
1
A
A
A A
A
A
A A
LM A
x
x
x x
x
x
x x
x
z=z
2
, eq.(3-21) becomes
( )
( )
LM A
A A AB
A
x
x x
z z
cD
N
=
1
2 1
1 2
21
A c A
c k N A =
A
C
Solute A
C
A1
Rate equation
Steady state diffusion of solute A
through a membrane as shown in
figure. After the solute diffuses
through the membrane, it is swept
from the external surface by a gas
stream. A mass transfer coefficient
for transfer of component A into the
free stream is defined in terms of
diffusion at the interface by
( ) ( )
|
.
|
\
|
c
c
=
=
A A
z
A
A A
A
c
c c
z
c
D
c c
J
k
1
0
1
k
c
: mass transfer coefficients
22
Equimolar Counter Diffusion (EMD)
( )
o
o
A A
AB
A
c c
D
J =
1
J
A
=-J
B
( )
1
1
A A
AB
A
x x
z z
cD
J
=
o
AB
c
D
k =
'
N
A
=-N
B
( )
1
1
A A
AB
A
x x
z z
cD
N
=
( )
o
o
A A
AB
A
c c
D
N =
1
( )
o A A c A
c c k N =
1
'
For liquids ( ) ( )
o o A A L A A c A
c c k c c k N = =
1
'
1
'
( ) ( )
o o A A x A A L A
x x k x x ck N = =
1
'
1
' And
For gases ( ) ( )
o o A A G A A
c
A
p p k p p
RT
k
N = =
1
'
1
'
RT
P
V
n
c
t
= =
t A A
P y p =
Dalton's law
( ) ) (
1
'
1
'
o o A A y A A G t A
y y k y y k P N = =
nRT V P
t
=
23
Unimolecular Diffusion (UMD)
N
A
=constant, N
B
=0
( )
( )
( )
LM
A
A A AB
LM A
A A AB
A
c
c
c c D
x
x x
z z
cD
N
|
.
|
\
|
=
1
1
1 1
1
o o
o
o
( )
( )
(
|
.
|
\
|
|
.
|
\
|
=
|
.
|
\
|
c c
c c
c
c
c
c
c
c
A
A
A A
LM
A
1
1
1
1
ln
1 1
1
o
o
( )
LM
A
AB
c
c c
D
k
=
1 o
( )
( )
( )
o o A A
LM
A
c
A A c A
c c
c c
k
c c k N
= =
1
'
1
1
24
( ) ( )
o o A A L A A c A
c c k c c k N = =
1 1
( ) ( )
o o A A xL A A L A
x x k x x ck N = =
1 1
For liquids
or
Unimolecular Diffusion (UMD)
Introducing ideal gas law and make use of dalton's
law
( ) ( ) ( )
AG A y A A G t A A G A
y y k y y k P p p k N = = =
1 1 1 o o
or
( )
( )
( )
( )
o o A A
LM
B
G t
A A
LM
B
c t
A
p p
p
k P
p p
p RT
k P
N = =
1
'
1
'
Units of Mass Transfer Coefficients
25
EMD UMD
Gases:
Units
o
AB
c
D
k =
'
RT
D
k
AB
G
o
=
'
RT
D P
k
AB t
y
o
=
'
Liquids:
o
AB
L
D
k =
'
o
AB
x
cD
k =
'
( )
LM
B
AB t
c
P
D P
k
o
=
( )
LM B
AB t
G
p RT
D P
k
o
=
( )
M B
AB
L
x
D
k
o
=
( )
LM
B
AB t
y
p RT
D P
k
o
2
=
mole
(time)(area)(mol/vol)
mole
(time)(area)(pressure)
mole
(time)(area)(mol fra.)
mole
(time)(area)(mol/vol)
mole
(time)(area)(mol fra.)
( )
LM B
AB
x
x
cD
k
o
=
26
Conversion between Mass Transfer Coefficients
( ) ( )
LM
B L
LM
B x L L x
x Ck x k k
M
Ck k = = = =
' ' '
( ) ( )
LM
B
LM
B
x C C =
( )
( )
P
k P
k P k Pk
RT
Pk
y
LM
B
G
LM
B y G
c
= = = =
' '
'
Gases:
Liquids:
Example 1
27
28
Film Theory
Resistance to the diffusion is equivalent to that in a
stagnant film of a certain thickness
A thin boundary layer
near the interface where
the fluid is in laminar flow
All resistance in the film
Transport or Mass transfer
is by molecular diffusion
29
Penetration Theory
Diffusion of a short distance
Unsteady-state molecular transport
Mass flux at interface of gas and liquid is:
Surface elements will be renewed by
eddies from the turbulent core
Instantaneous mass transfer, with solute
penetrating into eddy after exposure to
surface
( )
Ab Ai
AB
A
c c
t
D
N =
t
30
Penetration Theory
Total solute transferred is
with average mass transfer rate
With distribution in element ages at the
surface, rate of surface renewal is constant
and given a factor s, so mass transfer is
( )
2 / 1
exp
0
2
exp
|
|
.
|
\
|
=
}
t
t D
c c dt N
AB
Ab Ai
t
A
( )
2 / 1
exp
2
|
|
.
|
\
|
=
t
D
c c N
AB
Ab Ai A
t
( )
Ab Ai AB A
c c s D N =
31
Penetration Theory
32
For absorption, the solute may diffuse through a gas
phase and then diffuse through and be absorbed in an
adjacent and immiscible liquid phase.
The two phases are in direct contact with each other,
and the interfacial area between the phases is usually
not well defined.
A concentration gradient must exist to cause this mass
transfer through the resistances in each phase.
Mass Transfer Between Phases
Ammonia (NH
3
) (A) +air (B) absorbed by water (C)
33
distance from interface
N
A
y
AG
y
Ai
interface
gas-phase mixture
of A in gas G
liquid-phase solution
of A in liquid L.
x
Ai
x
AL
Where
y
AG
= concentration of A in the bulk gas phase
y
Ai
= concentration of gas A at the interface
x
Ai
=concentration of liquid A at the interface
x
AL
= concentration of A in the bulk liquid phase
Mass Transfer Between Phases
34 34
Mass Transfer Between Phases
( ) ( )
AL Ai x Ai AG y A
x x k y y k N = =
' '
Equimolar counterdiffusion (EMD)
Where
ky = Gas phase mass transfer coefficient (kg mol/s.m
2
.mol frac)
kx = Liquid phase mass transfer coefficient (kg mol/s.m
2
.mol frac)
Diffusion of A through stagnant or
nondiffusing B (UMD)
( ) ( )
AL Ai x Ai AG y A
x x k y y k N = =
( )
LM
A
y
y
y
k
k
=
1
'
( )
LM
A
x
x
x
k
k
=
1
'
Where
35
The interface composition (x
Ai and
y
Ai
) can be determined by drawing
the line PM with the slope (-k
x
/k
y
) intersecting the equilibrium line
Mass Transfer Between Phases
y
*
A
x
AL
~
x
*
A
~
y
AG
y
AG
y
Ai
y
*
A
x
AL
x
Ai
x
*
A
M
D
E
P
equilibrium
line
slope = m
y
slope = m
x
|
|
.
|
\
|
=
Ai A
Ai AG
y
x x
y y
m
*
|
|
.
|
\
|
=
AG Ai
A Ai
x
x x
y y
m
*
Ai AL
Ai AG
y
x
x x
y y
k
k
slope
= =
'
'
Fig.: Concentration driving force and interface concentrations in inter
phase mass transfer (equimolar counter diffusion EMD)
36
y
AG
y
Ai
y
*
A
x
AL
x
Ai
x
*
A
M
D
E
P
equilibrium
line
slope = m
y
slope = m
x
|
|
.
|
\
|
=
Ai A
Ai AG
y
x x
y y
m
*
|
|
.
|
\
|
=
AG Ai
A Ai
x
x x
y y
m
*
( )
( )
AL Ai
Ai AG
LM A y
LM A x
x x
y y
y k
x k
slope
=
1
1
'
'
Fig.: Concentration driving force and interface concentrations in inter
phase mass transfer (A diffusing through stagnant B).
Mass Transfer Between Phases
y
*
A
x
AL
~
x
*
A
~
y
AG
37
Overall mass-transfer coefficient
Similar to overall heat-transfer coefficient
Mass Transfer Between Phases
Unimolecular diffusion (UMD)
( ) ( )
AL A x A AG y A
x x K y y K N = =
* *
( ) ( )
AL Ai x Ai AG y A
x x k y y k N = =
Overall Mass Transfer Coefficient
38
( ) ( ) ( ) ( )
AL Ai x Ai AG A Ai Ai AG A AG
x x m y y y y y y y y + = + =
* *
m
x
is the slope of ME
x
A
x
y
A
y
A
k
N
m
k
N
K
N
+ =
x
x
y y
k
m
k K
1 1 1
+ =
( ) ( ) ( ) ( )
AL Ai Ai AG
y
AL Ai Ai A AL A
x x y y
m
x x x x x x + = + =
1
* *
x
A
y y
A
x
A
k
N
k m
N
K
N
+ =
x y y x
k k m K
1 1 1
+ =
Similarly
m
y
is the slope of DM
(3-242)
(3-243)
|
|
.
|
\
|
=
AG Ai
A Ai
x
x x
y y
m
*
|
|
.
|
\
|
=
Ai A
Ai AG
y
x x
y y
m
*
39
Mass Transfer Resistance
39
Resistance in gas phase
Total resistance in both phases
y
y
K
k
1
1
=
Resistance in liquid phase
Total resistance in both phases
x
x
K
k
1
1
=
Solute A is very soluble, m
x
is small
Solute A is insoluble, m
y
is large
40
Two Resistance Theory
Three resistance: the gas phase, the liquid phase, and
the interface.
The interface resistance is negligible and any
fluctuations in y
Ai
, and x
Ai
are small, y
Ai
and x
Ai
are
equilibrium values given by the systems equilibrium-
distribution curve
Individual Mass Transfer Coefficient
41
( )
i A g A G z A
p p k N
, , ,
=
( )
L A i A L z A
c c k N
, , ,
=
Combining both and rearrange to
i A L A
i A G A
G
L
c c
p p
k
k
, ,
, ,
=
42
Overall Mass Transfer Coefficient
( )
*
, A G A G A
p p K N =
i A i A
mc p
, ,
=
L A A
mc p
,
*
=
*
, A G A
mc p =
( ) ( )
z A
L A i A
z A
i A G A
G
N
c c m
N
p p
K
,
, ,
,
, ,
1
+
=
At low concentrations, we have linear
equilibrium relations
L G G
k
m
k K
+ =
1 1
Where : P
AG
the bulk composition in the gas phase
P
A
*
is the partial pressure of A in equilibrium with the bulk
concentration of C
AL
K
G
the overall mass transfer coefficient based on partial pressure
(time)(interfacial area)(pressure)
43 43
Overall Mass Transfer Coefficient
( )
L A A L A
c c K N
,
*
=
i A i A
mc p
, ,
=
L A A
mc p
,
*
=
*
, A G A
mc p =
At low concentrations, we have linear
equilibrium relations
L G L
k mk K
1 1 1
+ =
Where : C
A
*
the bulk composition in the liquid in equilibrium with
the bulk gas phase
CAL is the bulk concentration in the liquid
K
L
the overall mass transfer coefficient based on a liquid driving force
(time)(interfacial area)(mole A/volume)
( ) ( )
z A
L A i A
z A
i A G A
z A
AL A
L
N
c c
mN
p p
N
C C
K
,
, ,
,
, ,
,
*
1
+
=
44
*
A
P
*
A
C
A
P A
total
AG
P A
AL
P A
A
C A
AG
C A
AL
C A
total
Concentration Driving Force for the Two-resistance Theory
45
Ratio of resistances in individual phase to
total resistance
Ratio of resistances in individual phase to
total resistance
L
L
total A
film liquid A
K
k
C
C
/ 1
/ 1
,
,
=
A
A
G
G
total A
film gas A
K
k
P
P
/ 1
/ 1
,
,
=
A
A
Mass Transfer Resistance
46
Resistance in gas phase
Total resistance in both phases
G
G
K
k
1
1
=
Resistance in liquid phase
Total resistance in both phases
L
L
K
k
1
1
=
Solute A is very soluble, m
x
is small
Solute A is insoluble, m
y
is large
47
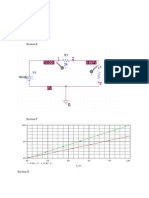
Wetted Wall Column (glass tube)
Header
Header
Water
Moist air
Moist air
Experimental Setup
Blower
Thomas
Meter
Wet and Dry Bulb
Temperature
Thermometer
Hygrometer
Area for mass transfer is defined
Film Theory
Resistance to the diffusion is equivalent to that in a
stagnant film of a certain thickness
Ai
c c =
0 = z
Ab
c c =
o = z
( )
Ab Ai
AB
A
c c
D
J =
o
o
AB
c
D
k =
at
at
is not directly measureable
contains all of the fluid
dynamics in the system and will
change with the Re
0 = z o = z
c
Ab
Interface
Thin layer
(stagnant)
able to calculate (Re)
from experimental data
48
49
The solute A is being absorbed from a gas mixture of A and B in a
wetted-wall tower with the liquid flowing as a film downward along the
wall. At certain point in the tower the bulk gas concentration
y
AG
= 0.380 mol fraction and the bulk liquid concentration is
x
AL
= 0.1. The tower is operating at 298 K and 1.013 x 10
5
Pa and the
equilibrium data are as follows:
x
A
y
A
0 0
0.05 0.022
0.1 0.052
0.15 0.087
0.2 0.131
0.25 0.187
0.3 0.265
0.35 0.385
Example : Interface Composition in Interphase Mass Transfer.
50
The solute A diffuse through stagnant B in the gas
phase and then through a nondiffusing liquid.
Using correlations for dilute solutions in wetted-wall
towers, the film mass-transfer coefficient for A in the
gas phase is predicted as
k
y
= 1.465 x 10-3 kg mol A/s.m
2
mol frac. And for the
liquid phase as
k
x
= 1.967 x 10
-3
kg mol A/s.m
2
mol frac.
Calculate the interface concentrations yAi and xAi
and the flux NA.
Solution
First we plot the data
51
y
A
x
A
0
0
0.1
0.1
0.2
0.2
0.3
0.3
0.4
0.4
52
Now we need to find the point P on the graph
So :
Since the correlations are for dilute solutions, (1-y
A
)
iM
and (1-
x
A
)
iM
are approximately 1.0 and the coefficients are the same
as k
y
and k
x
.
Point P is plotted at y
AG
= 0.380 and x
AL
= 0.1.
For the first trial (1-y
A
)
iM
and (1-x
A
)
iM
are assumed as 1.0 and
the slope of line PM is,
A line through point P with a slope of 1.342 is plotted in the
figure intersecting the equilibrium line at M
1
, where
y
Ai
= 0.183 and x
Ai
= 0.247.
342 . 1
0 . 1 / 10 465 . 1
0 . 1 / 10 967 . 1
) 1 /(
) 1 /(
3
3
'
'
=
LM A y
LM A x
y k
x k
slope
53
For the second trial we use y
Ai
and x
Ai
from
the first trial to calculate the new slope.
Substituting into corresponding Eqs.,
)] 1 /( ) 1 ln[(
) 1 ( ) 1 (
) 1 (
AG Ai
AG Ai
LM A
y y
y y
y
=
715 . 0
)] 38 . 0 1 /( ) 183 . 0 1 ln[(
) 380 . 0 1 ( ) 183 . 0 1 (
=
=
)] 1 /( ) 1 ln[(
) 1 ( ) 1 (
) 1 (
Ai AL
Ai AL
LM A
x x
x x
x
=
825 . 0
)] 247 . 0 1 /( ) 1 . 0 1 ln[(
) 247 . 0 1 ( ) 1 . 0 1 (
=
=
54
Substituting into Eq. (10.4-9) to obtain the new slope,
A line through point P with a slope of 1.163 is
plotted and intersects the equilibrium line at M,
where y
Ai
= 0.197 and x
Ai
= 0.257. Using these new
values for the third trial, the following values are
calculated:
163 . 1
715 . 0 / 10 465 . 1
825 . 0 / 10 967 . 1
) 1 /(
) 1 /(
3
3
'
'
=
LM A y
LM A x
y k
x k
slope
709 . 0
)] 38 . 0 1 /( ) 197 . 0 1 ln[(
) 380 . 0 1 ( ) 197 . 0 1 (
) 1 ( =
=
LM A
y
820 . 0
)] 257 . 0 1 /( ) 1 . 0 1 ln[(
) 257 . 0 1 ( ) 1 . 0 1 (
) 1 ( =
=
LM A
x
Please notice the values
of x
Ai
and yAi didnt
change much from the
first trial and that means
we are on the right way
of solving the problem
(refining the answer)
55
This slope of 1.160 is essentially the same as the
slope of 1.163 for the second trial.
Hence, the final values are y
Ai
= 0.197 and x
Ai
=
0.257 and are shown as point M. To calculate the
flux,
Note that the flux N
A
through each phase is the
same as in other phase, which should be the case at
steady state.
160 . 1
709 . 0 / 10 465 . 1
820 . 0 / 10 967 . 1
) 1 /(
) 1 /(
3
3
'
'
=
LM A y
LM A x
y k
x k
slope
2 4
3
'
. / 10 78 . 3
) 197 . 0 380 . 0 (
709 . 0
10 967 . 1
) (
) 1 (
m s kgmol
y y
y
k
N
Ai AG
LM A
y
A
=
56
Fig.: Location of interface concentrations
for example.
y
AG
y
Ai
y
*
A
x
AL
x
Ai
x
*
A
M
D
E
P
M
1
0
0
0.1
0.1
0.2
0.2
0.3
0.3
0.4
0.4
Example 3
57
58
59
60
61
Example 4
62
63
64
65
66
67
68
Diffusion Coefficients
69
Diffusion can be considered by a mechanistic
approach in which a consideration of atom
movement is important or by a continuum approach
such as with Ficks first law, where no consideration
is given to the actual mechanism by which atom
transfer occurs.
Gases: molecules are far apart, intermolecular forces
can often be disregarded or considered only during
collisions.
A molecule is assumed to travel along a straight line
until it collides with another molecule, after which its
speed and direction are altered.
70
Diffusion Coefficients for Gases
The diffusion coefficient can be derived for
an idea gas by using the simplified discussion
presented by Sherwood et al (1975).
From kinetic theory, the diffusion coefficient
is assumed to be directly proportional to the
mean molecular velocity , and the mean
free path
U
U D
71
For an idea gas the motion of the molecule is assumed
to be totally random, with the mean free path , being
inversely proportional to both the average cross-
sectional area of the molecules, A, and the number
density, n, of all molecules in a specified volume.
The number density of an idea gas varies directly with
pressure and inversely with the temperature,
Diffusion Coefficients for Gases
PA
T
nA
1
Mean molecular velocity is related to the temperature
and molecular weight of the molecule by the
expression
PA
T
M
T
M
T
D
B A
AB
2
1
|
|
.
|
\
|
+
2
1
2
3
'
1 1
|
|
.
|
\
|
+ =
B A avg
AB
M M PA
T K
D
Aavg: average cross-sectional area
of both types of molecules
K a costant
Semi-empirical Equations
72
( ) ( ) | |
2
3 1 3 1
2 1
75 . 1
00143 . 0
B
V
A
V AB
BA AB
PM
T
D D
+
= =
Equation of Fuller, Schettler, and Giddings
D
AB
is in cm
2
/s
P: atm
T: K
V
=summation of atomic and structural diffusion
volumes from Table 3.1, which includes diffusion
volumes of simple molecules
You might also like
- Tutorial/HW Week #7Document49 pagesTutorial/HW Week #7saeedeh951No ratings yet
- Heat and Mass Transfer ResistancesDocument51 pagesHeat and Mass Transfer ResistancesSidNo ratings yet
- Analytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportFrom EverandAnalytical Modeling of Solute Transport in Groundwater: Using Models to Understand the Effect of Natural Processes on Contaminant Fate and TransportNo ratings yet
- Mass Transfer 1 CLB 20804Document54 pagesMass Transfer 1 CLB 20804KumaranNo ratings yet
- Masstransfer FundamentalsDocument49 pagesMasstransfer FundamentalsMarco SilvaNo ratings yet
- MT-I NotesDocument79 pagesMT-I NotesPrakhar AggarwalNo ratings yet
- Interactions between Electromagnetic Fields and Matter: Vieweg Tracts in Pure and Applied PhysicsFrom EverandInteractions between Electromagnetic Fields and Matter: Vieweg Tracts in Pure and Applied PhysicsNo ratings yet
- Module 3: Mass Transfer Coefficients: Lecture No. 1Document4 pagesModule 3: Mass Transfer Coefficients: Lecture No. 1walisyhNo ratings yet
- Week 7Document49 pagesWeek 7arfpowerNo ratings yet
- CN2125 Heat and Mass Transfer: Dept of Chemical and Biomolecular EngineeringDocument90 pagesCN2125 Heat and Mass Transfer: Dept of Chemical and Biomolecular EngineeringarfpowerNo ratings yet
- External Diffusion Effects On Heterogeneous Reactions: A. Sarath BabuDocument59 pagesExternal Diffusion Effects On Heterogeneous Reactions: A. Sarath BabuboiroyNo ratings yet
- Tutorial/HW Week #10: WWWR Chapter 28Document53 pagesTutorial/HW Week #10: WWWR Chapter 28Abubakkar SiddiqNo ratings yet
- 2c.chap27.UNSteady State Molecular Diffusion (Fall 2013)Document33 pages2c.chap27.UNSteady State Molecular Diffusion (Fall 2013)Ibrahim Loqman DaoudiNo ratings yet
- L - 21 External Mass Transfer Effects: Prof. K.K.Pant Department of Chemical Engineering IIT DelhiDocument57 pagesL - 21 External Mass Transfer Effects: Prof. K.K.Pant Department of Chemical Engineering IIT DelhiMehul VarshneyNo ratings yet
- Mass Transfer AssignmentDocument4 pagesMass Transfer AssignmentShanmugam GunasekaranNo ratings yet
- Mass TransferDocument4 pagesMass TransferEddz Del Rosario RodriguezNo ratings yet
- Fixed Bed ReactorDocument43 pagesFixed Bed ReactorMaher Al-busaidi100% (2)
- X. Reactions x.1 Order of Reactions x.1.01 Zero Order ReactionsDocument28 pagesX. Reactions x.1 Order of Reactions x.1.01 Zero Order ReactionsJon Bisu DebnathNo ratings yet
- Diffusion Mass TransferDocument18 pagesDiffusion Mass TransferbhuniakanishkaNo ratings yet
- Chapter 2 DiffusionDocument13 pagesChapter 2 DiffusionjaiminNo ratings yet
- Fluid 11Document210 pagesFluid 11Omolafe Olawale SamuelNo ratings yet
- Adv Math ProjectDocument19 pagesAdv Math ProjectJeanne Kamille Evangelista PiniliNo ratings yet
- 07-Absorption For HAP and VOCcontrolDocument118 pages07-Absorption For HAP and VOCcontrolTakeshi Tanohuye TanohuyeNo ratings yet
- Chapter 3 ConSol PPT by E.cusslerDocument39 pagesChapter 3 ConSol PPT by E.cusslerheena_scottNo ratings yet
- Solution ThermoDocument9 pagesSolution ThermofarahanisiliasNo ratings yet
- Chapter11 Lecture Notes 1Document23 pagesChapter11 Lecture Notes 1fahadm12No ratings yet
- 2-Diffusivity Equation-Linear PDFDocument30 pages2-Diffusivity Equation-Linear PDFLoh Chun LiangNo ratings yet
- Conservation Laws: Control-Volume Approach: M Total Mass (KG) of A Within The System at Any TimeDocument8 pagesConservation Laws: Control-Volume Approach: M Total Mass (KG) of A Within The System at Any TimePortia ShilengeNo ratings yet
- Lecture 3-MassDocument16 pagesLecture 3-Mass501238196002No ratings yet
- Me 1303 Gas Dynamics and Jet Propulsion: Presented byDocument24 pagesMe 1303 Gas Dynamics and Jet Propulsion: Presented byArul SankaranNo ratings yet
- Introduction To Fluid DynamicsDocument76 pagesIntroduction To Fluid DynamicsRajrdbNo ratings yet
- General Features of Steady One Dimensional FlowDocument29 pagesGeneral Features of Steady One Dimensional FlowMSK65No ratings yet
- Chap1 1Document8 pagesChap1 1Ws LimNo ratings yet
- Gate 2006 PDFDocument21 pagesGate 2006 PDFVammsy Manikanta SaiNo ratings yet
- Fe Chemical EngineeringDocument5 pagesFe Chemical EngineeringJudith LugoNo ratings yet
- MM FormulaeDocument2 pagesMM FormulaeReddyvari VenugopalNo ratings yet
- Fundamental Notions of Viscous Fluid MechanicsDocument63 pagesFundamental Notions of Viscous Fluid Mechanicsbakri10101No ratings yet
- Models For Mass Transfer at A Fluid-Fluid Interface: Absorption, Distillation, Extraction, and StrippingDocument14 pagesModels For Mass Transfer at A Fluid-Fluid Interface: Absorption, Distillation, Extraction, and StrippingLexa Athena GadorNo ratings yet
- 1 Derivation of The Fugacity Coefficient For The Peng-Robinson ... - FETDocument13 pages1 Derivation of The Fugacity Coefficient For The Peng-Robinson ... - FETÁngel Lugo100% (2)
- HydrodynamicsDocument122 pagesHydrodynamicsIustin Cristian100% (2)
- MT 1Document34 pagesMT 1Vishal VnNo ratings yet
- MASS TRANSFER Coefficient and Inter Phase Mass TransferDocument41 pagesMASS TRANSFER Coefficient and Inter Phase Mass TransferSannala Prudhvi100% (1)
- MM301 2 Fluid Statics-UpdatedDocument32 pagesMM301 2 Fluid Statics-UpdatedoddomancanNo ratings yet
- Gas Dynamics and Jet Propulsion PDFDocument49 pagesGas Dynamics and Jet Propulsion PDFdass143143No ratings yet
- CDB 3033 Transport Phenomena: Ii. Diffusion Through A Stagnant Gas FilmDocument18 pagesCDB 3033 Transport Phenomena: Ii. Diffusion Through A Stagnant Gas Filmafiq_akashah1264No ratings yet
- Unit Operations Notes & EquationsDocument19 pagesUnit Operations Notes & Equationslucho_lemeitNo ratings yet
- Tugas PP Lanjut Shinta Leonita 0906635772Document5 pagesTugas PP Lanjut Shinta Leonita 0906635772HarryNo ratings yet
- Parallel Plate Transmission Line: Region Air The in - K, 0: Modes TM With Variation No, Variation AssumeDocument16 pagesParallel Plate Transmission Line: Region Air The in - K, 0: Modes TM With Variation No, Variation AssumejafunggolNo ratings yet
- Cre Ii - 28Document37 pagesCre Ii - 28Mehul VarshneyNo ratings yet
- Flory HuggsDocument18 pagesFlory HuggsCarlos OliveiraNo ratings yet
- Coriolis Acceleration Is FicticiousDocument5 pagesCoriolis Acceleration Is FicticiousJoseph StanovskyNo ratings yet
- Guide To Mass BalancesDocument5 pagesGuide To Mass BalancesAlieren KaraismailoğluNo ratings yet
- Chapter 8-Filtration (56 P)Document55 pagesChapter 8-Filtration (56 P)shardulkaviNo ratings yet
- Del OperatorDocument6 pagesDel OperatorSupriya Aggarwal100% (1)
- 6 Streeter Phelps S11Document20 pages6 Streeter Phelps S11Cristian Valenzuela RiveraNo ratings yet
- Condensation: - in The Continuum Regime, Diffusion Theory Is Used. at Steady StateDocument28 pagesCondensation: - in The Continuum Regime, Diffusion Theory Is Used. at Steady StateJorn DoeNo ratings yet
- Burner Ignition InstructionsDocument1 pageBurner Ignition InstructionsMike FordealsNo ratings yet
- ReadMe OverviewDocument2 pagesReadMe OverviewaksmdksaNo ratings yet
- Telecommunications GroundingDocument24 pagesTelecommunications GroundingMike FordealsNo ratings yet
- Sonnet SpreadsheetDocument2 pagesSonnet SpreadsheetMike FordealsNo ratings yet
- Sonnet XviiiDocument1 pageSonnet XviiiMike FordealsNo ratings yet
- Electronics1 HW 0Document2 pagesElectronics1 HW 0Mike FordealsNo ratings yet
- Force and Motion On An Incline: o Iy I Iy oDocument3 pagesForce and Motion On An Incline: o Iy I Iy oChetan B ArkasaliNo ratings yet
- Introduction To Time Wave Form AnalysisDocument19 pagesIntroduction To Time Wave Form Analysissaidha4568483No ratings yet
- 63996.indd - KVSApplDesign - CatalogDocument56 pages63996.indd - KVSApplDesign - CatalogTeu SoklengNo ratings yet
- Quantum ComputingDocument13 pagesQuantum ComputingSrikanthNo ratings yet
- Sem III OLD-1Document119 pagesSem III OLD-1sanket0% (1)
- FSI Manuscript Rev05Document9 pagesFSI Manuscript Rev05ADITYA SINGH PATELNo ratings yet
- Indian Standard: Application Guide For Voltage Transformers (Document16 pagesIndian Standard: Application Guide For Voltage Transformers (Pardeep KhosaNo ratings yet
- Albao Laboratory 3Document35 pagesAlbao Laboratory 3Shaun Patrick AlbaoNo ratings yet
- Springs and The Balance WheelDocument2 pagesSprings and The Balance WheelNiels Kjaer-PedersenNo ratings yet
- Shaft Calculation BaseDocument40 pagesShaft Calculation BaseObaciuIonel100% (1)
- Installation Operation & Maintenance of 33kV & 11kV SwitchgearDocument23 pagesInstallation Operation & Maintenance of 33kV & 11kV SwitchgearSaff MdNo ratings yet
- Arschs KK PDFDocument312 pagesArschs KK PDFilgarNo ratings yet
- 2022-23 Kat Level - I SyllabusDocument2 pages2022-23 Kat Level - I SyllabusAkshit MittalNo ratings yet
- Advanced Linear and Nonlinear Control DesignDocument15 pagesAdvanced Linear and Nonlinear Control Designdenise_meira_2100% (1)
- Elevated Water Tank Design SpreadsheetDocument12 pagesElevated Water Tank Design SpreadsheetRuben Dario Posada B100% (3)
- Geankoplis 2.6-4 2.7-4Document7 pagesGeankoplis 2.6-4 2.7-4BenePicarNo ratings yet
- LT 3 - Nuclear ChemDocument2 pagesLT 3 - Nuclear Chemaj eneriaNo ratings yet
- Power System Generation Transmission and Protection Series 2Document3 pagesPower System Generation Transmission and Protection Series 2maniNo ratings yet
- Dielectric Resonator Antenna Theory Anf FabricationDocument39 pagesDielectric Resonator Antenna Theory Anf FabricationjacnjillNo ratings yet
- Ekaterina Izgorodina Ionic LiquidsDocument6 pagesEkaterina Izgorodina Ionic LiquidsizabelaNo ratings yet
- Mri ArtifactsDocument59 pagesMri ArtifactsMarc Michael Dela CruzNo ratings yet
- 09 - Chapter 2 PDFDocument34 pages09 - Chapter 2 PDFrajkumarNo ratings yet
- Optics 27 YearsDocument15 pagesOptics 27 YearssmrutirekhaNo ratings yet
- Overview of Vertical Axis Wind Turbine VAWT Is OneDocument6 pagesOverview of Vertical Axis Wind Turbine VAWT Is OneAjeem DurraniNo ratings yet
- Frontiers in Relativistic Celestial MechDocument372 pagesFrontiers in Relativistic Celestial MechCarl MarksNo ratings yet
- Solutions - AIATS Medical-2020 (XII Studying) - Test-5 - (Code-A & B) - 22-12-2019 PDFDocument30 pagesSolutions - AIATS Medical-2020 (XII Studying) - Test-5 - (Code-A & B) - 22-12-2019 PDFShashank KashyapNo ratings yet
- Lewis Wolpert - The Unnatural Nature of Science PDFDocument201 pagesLewis Wolpert - The Unnatural Nature of Science PDFBekim Paci100% (2)
- Chemistry PPT - RadioactivityDocument23 pagesChemistry PPT - RadioactivityRishita SinghNo ratings yet
- Sensor Lect4Document23 pagesSensor Lect4morton1472No ratings yet
- Ch15 Differential Momentum BalanceDocument20 pagesCh15 Differential Momentum Balance89kkNo ratings yet