Professional Documents
Culture Documents
Ie071094p PDF
Uploaded by
Er Mayur PatilOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ie071094p PDF
Uploaded by
Er Mayur PatilCopyright:
Available Formats
Ind. Eng. Chem. Res.
2008, 47, 889-899
889
Feasibility of Reactive Distillation for Fischer-Tropsch Synthesis
S. Srinivas, Ranjan K. Malik, and Sanjay M. Mahajani*
Department of Chemical Engineering, Indian Institute of Technology, Powai, Mumbai - 400076, India
Fischer-Tropsch synthesis (FTS) is an area that is receiving revived interest worldwide as a technology alternative to produce transportation fuels as well as chemicals from syngas. SASOL and Shell are two of the major players who operate FT reactors on a commercial scale. To have a balance between gasoline and diesel production, one needs to have both the low temperature (LTFT) and high-temperature (HTFT) processes operating in parallel. Heat-removal from the exothermic FT reactions was the main driver in the development of conventional FT reactors (fixed-bed, fluidized bed, or slurry type). However, the focus has recently shifted toward the product distribution as well. Reactive distillation (RD) is a proven reactive separation method that can enhance yields as well as improve product selectivity in multiple reactant/product systems. This paper aims to check if FTS is feasible in RD from a theoretical viewpoint. In-built thermodynamic procedures and power-law kinetics of Aspen Plus, along with a simplified kinetic model that predicts product distribution, were used in performing the simulations. Simulation results of the conventional reactors are compared with RD, and it is seen that the performance of RD is at par or better than the conventional reactors in terms of conversion, yield, and product distribution. Within the RD mode for FTS, results of some of the alternate column configurations are presented. The results indicate that FTS can be a potential candidate to be implemented using RD.
1. Introduction Fischer-Tropsch synthesis (FTS) is one of the technology alternatives being used by SASOL, South Africa, since the past five decades1 and by Shell, Malaysia, since the past decade2 to obtain liquid fuels from syngas. The synthesis gas feed can come from coal or residual oil gasification, methane reforming, or from biological wastes. With the crude reserves dwindling at a fast pace, FTS is being seen as a viable alternative to convert coal or stranded gas reserves into liquid transportation fuels.3 The FT products vary depending on the syngas feed composition, the type of reactor and catalyst employed, and the operating conditions like temperature, pressure, gas velocities used, etc. FT product selectivity and yield are important to make the overall process economically viable. Exhaustive review articles on the development of Fischer-Tropsch reactors are published in the literature by few authors including Schulz,3 Davis,4 and Sie and Krishna.5 Both gas-phase and liquid-phase reactors are currently in use commercially for the FTS process. However, the selectivity is either toward wax (for the LTFT process) or gasoline (for HTFT). In some cases, a combination of both the reactors is used to meet the flexibility in market demand. The LTFT reactors are either fixed-bed-type or slurry-type, whereas fluidized bed reactors, fixed or circulating, are used for the HTFT process. The main design criterion that led to the advances in these conventional reactors is the removal of heat from the exothermic FT reactions. The relative merits and demerits of each of these reactors can be found in the reviews listed earlier. However, a large number of downstream operations are needed for both the HTFT and the LTFT processes before the final product is ready. The focus in FT has now shifted to the product distribution, with the aim of maximizing the yield of gasoline, diesel, or chemicals. Most of these efforts are on the catalyst level,6-7 with a few researchers trying to alter the product distribution on a macro scale by altering the reactor or process design.8
* To whom correspondence should be addressed. Tel: (022) 2576 7246. Fax: (022) 2572 6895. E-mail: sanjaym@che.iitb.ac.in.
In this work, we investigate FTS in a RD framework and the potential advantages that it can offer. The major factors that influence the performance in RD with respect to the FT reactions are elucidated. Different column configurations possible in RD are simulated using Aspen Plus, and the observations are explained on the basis of the major factors considered. 2. Reactive Distillation (RD) for FTS RD is an emerging technology that is used for commercial production of chemicals like MTBE, etc. and is looked upon as a tool to enhance selectivity.9 RD when applied to FTS is expected to offer the following major advantages: 1. Utilization of exothermic heat of the FT reactions in maintaining saturation conditions in the column and enabling separation, thereby operating as a multifunctional reactor. 2. Having side draws to get multiple products from a single column and options of varying parameters like reflux ratio, number of reactive stages, catalyst loading, etc. to alter these product yields and compositions. 3. Lower catalyst deactivation because of the presence of a liquid pool on each tray and the continuous washing of the catalyst by the reflux. Catalyst life may also go up due to the absence of hot spots or zones. 4. Process intensification through RD helps in reducing the plant size due to combination of reactions and fractionation in a single vessel, which otherwise need separate units in the conventional FT process. Overall, single operation may replace many downstream steps used in conventional FT processes to get the same products with a possible enhancement of selectivity and better flexibility. Few attempts to apply RD to FTS have been reported (York et al.10), but there is not much literature published on the same. Nemphos et al.11 in their patent describe the production of methanol using syngas in RD, which demonstrates that CO and H2 in spite of being noncondensables can be handled well in a reactive distillation column. FT reactions in RD would be in the liquid phase, and the reaction kinetics is expected to be similar to that of the slurry phase reactor. VLE and reaction kinetics are the
10.1021/ie071094p CCC: $40.75 2008 American Chemical Society Published on Web 01/15/2008
890
Ind. Eng. Chem. Res., Vol. 47, No. 3, 2008
two main inputs needed for RD, both of which are complicated for the FT system. Also, RD is feasible only if the phase equilibrium and reaction conditions match each other, which in a way is the main aim of this study. The phase equilibrium would also influence the solubility of the reactants in the liquid phase and hence the reaction rates. FTS in RD is different from other RD systems that are in practice due to the following: a. The feed is a gaseous stream to the column, whereas other RD systems have at least one liquid-phase reactant entering the column. b. Heat removal from each of the reactive stages needs to be considered for the FT reactions in order to reduce the condenser load as well as to make it viable for heat integration to generate steam, etc. c. The FT reactions are both series-parallel in nature, in addition to the polymerization characteristics exhibited by them and the highly nonlinear kinetics. d. The presence of a large number of components - paraffins and olefins from C1 to C50+, oxygenates like alcohols and acids and noncondensables like CO, H2, and CO2, all of which contribute to a wide variation in the boiling points of the FT reaction mixture. e. The presence of water as a separate liquid phase in addition to the noncondensables needs a gas-vapor-liquid-liquid equilibrium (GVLLE) model rather than a simple VLE model, if one desires a rigorous model considering all of the possible FT components. 3. Kinetics Whereas there are many kinetic models available in the literature that predict the FT and water gas shift (WGS) reaction rates, there are few that predict the overall rate as well as the product distribution. Wang et al.12 have proposed one such model for a Fe-Cu-K catalyst in a fixed-bed reactor study. They also point out the differences in their model vis-a-vis the ` models of Lox and Froment13 and van der Laan.14 The kinetic parameters used in this work have slightly modified values from the model of Wang et al.,12 keeping in view the liquid-phase reactions considered in this study. To keep the model simple and usable with the Aspen Plus power-law formulation23 (Aspen Plus user manual), the chain growth parameter R is given a value of 0.8 and has been clubbed with the pre-exponential factor value. Only paraffin and olefin formation reactions are considered. WGS reaction and olefin readsorption reactions are neglected in this work.
FT reactions, R dictates the probability of a given alkyl species to either terminate and form an olefin/paraffin or propagate to the next higher alkyl species. R is dependent on the operating conditions as well as the nature of the catalyst. The ASF polymerization equation15 is given in (4), and a linear relationship is usually seen between log(wn/n) and n having slope log R in the FT product distribution, with some deviations.16
log(wn/n) ) n log R + log [(1 - R)2/R]
(4)
Paraffins: nCO + (2n + 1) H2 f CnH2n + 2 + nH2O, n ) 1 to 30 (1) Olefins: nCO + 2nH2 f CnH2n + nH2O, n ) 2 to 20 (2)
wn is the weight fraction of the product with carbon number n. All of the reaction rates in (3) are based on the catalyst weight. The rate constants are found using the Arrhenius relation. R is influenced by temperature, pressure, feed gas composition, and the catalyst composition.1 For example, the R value increases with an increase in basicity of the iron catalyst, and the increase is in the order of Li < Na < K < Rb. At a higher operating temperature, the product distribution shifts toward more hydrogenated and lower carbon number products, viz., R values are lowered. The effect of pressure and feed gas composition on R is not so straight forward, owing to the formation of CO2 and H2O during the reaction. These compete with CO and H2 for adsorption on the catalyst surface and alter the chain growth probability in a more complicated manner. The power-law formulation, which is relatively simple to work with, is used in this work as only a first step to check the feasibility of FT in RD. Whereas such formulations are reported in the literature, they are mainly used to describe the rate of consumption of CO and/or H2 but not the entire product spectrum. The power-law expressions also provided a good match between the theoretical and experimentally observed values for the conversion of the reactants. The exponent values (of a and b) associated with the equation depend on the catalyst type and composition, and we have assigned unity to each of them as a representative value. To capture the chain growth characteristic of the FT process, R is assigned a value of 0.8 and clubbed into the pre-exponential factor so that the product distribution is also obtained using the power law. The model of Wang et al.,12 however, calculates R as a function of temperature and pressure. As noted earlier, the presence of water would alter the reaction rate and affect the product distribution by altering the chain growth probability. Because we considered a fixed value for R, this possibility does not exist. Also, neglecting the water inhibition effect now justified as the presence of the extra term in the denominator would only have lowered the rates of all of the reactions to a similar extent, thereby keeping the product distribution the same. The temperature dependency of R would have given a different chain growth probability on each stage in RD. Because we have fixed R, this will not be realized in our study and is pointed out as one of the limitations. 4. Phase Equilibrium Models Caldwell and van Vuuren17 were the first to note the importance of VLE in the FT process and derived a criterion for the prediction of the maximum operating temperature for slurry systems based on ASF distribution. Experiments by Ray and Farley18 and Satterfield et al.19 have also shown that an FT wax composition shift causes changes in the conversion and hydrodynamics of the reactor. Solubility of the reactants is also important for liquid-phase synthesis. van Vuuren et al.20 reported an increase in solubility of H2 and CO with increase in temperature, and a decrease in solubility for all of the other components (H2O, CH4, C2H4, C2H6, and C3H6) as temperature
The number of available components in the in-built Aspen Plus component data bank limited the carbon numbers of paraffins and olefins, which are considered to be 30 and 20 respectively. The kinetic expression used is of the power-law form, without any inhibition term in the denominator and is empirical.
b Ri ) kiCa CH2, CO
a ) 1, b ) 1
(3)
Equation 3 above is for liquid-phase reactions. ki in (3) includes the chain-growth factor to take care of the ASF-like (AndersonSchulz-Flory) distribution present in FT products. During the
Ind. Eng. Chem. Res., Vol. 47, No. 3, 2008 891
Table 1. Kinetic Parameters Used in the Simulations Liquid Phase k0 kmol/(sec. kg cat.(kmol/m3)2) methane ethane propane ethylene propylene 1.73 106 1.27 105 1.01 105 2.66 106 2.13 106 E kJ/kmol 9.29 104 8.70 104 8.70 104 1.11 105 1.11 105
rises. No significant effect of pressure on solubilities was observed by them. The contradiction observed between the experiment and the theory was attributed to the molecular structure and the degree of saturation of the waxes used in their experiments. Marano and Holder21 have reported the characterization of FT liquids for VLE calculations, and their model is being used by investigators working with slurry systems (Ahon et al.22). Their model is an unsymmetric formulation using the Peng-Robinson equation of state (PR-EOS) with modified Huron-Vidal mixing rules. Asymmetric behavior correlations were developed by them, and these were used to predict the activity and fugacity coefficients. In our work, we have used the in-built thermodynamic properties and calculations of Aspen Plus23 by choosing the PRMHV2 option, which has the same EOS and mixing rules as those in the model of Marano and Holder.21 The UNIFAC model is used by the simulator while calculating the liquid phase activity coefficients with this property option. CO, H2, H2O, CH4, C2H6, C3H8, C2H4, and C3H6 are declared as Henry components in the simulation. The simulations were performed with the following components CO, H2, H2O, paraffins from C1 to C30, and olefins from C2 to C20. True components are used in all of the simulations as the components involved are pure components. 5. Simulation Modules and Conditions While doing the rigorous calculations involved in FTS using RD, a few of the difficulties encountered are: 1. Generation of a good initial guess to help the solution converge, which is made more difficult with the number of components involved and their wide-boiling range. 2. Inability to specify a B/F or D/F ratio as an operating condition for the RD column, owing to the gaseous feed entering the column. 3. The unsymmetric formulation involving the solution of a cubic EOS and an activity coefficient model in addition to presence of Henrys components makes the convergence of bubble-point and dew-point algorithms tedious and difficult. With the existing algorithms robust enough to handle multicomponent mixtures in cases like crude distillation columns and proven usefulness of the RadFrac module for RD simulations,24 Aspen Plus is chosen to perform the rigorous computations involved in RD for FTS. All of the simulations were performed with the components, kinetics, and the VLE model mentioned in the earlier sections using Aspen Plus. In all of the simulations, vapor-liquid freewater calculations are enabled. The feed flow-rate to all of the simulations was 1000 mol/hr of syngas (CO+H2) at 250 C and 25 atm, with three different H2/CO ratios: 2, 1, and 0.92. Kinetic parameters for methane, ethane, and ethylene used in the simulations are listed in Table 1. The others can be obtained by the multiplication of those for ethane and ethylene by 0.8, each time the carbon number increases (e.g., of propane and propylene in Table 1).
Figure 1. (a) Schematic of the slurry reactor used in FT simulations. (b) Schematic of the RD configuration suggested for FTS.
The RCSTR module is used to simulate the performance of the FT slurry reactor (schematic shown in part a of Figure 1). This is taken to be an isothermal reactor operating at 25 atm and 250 C, having a volume of 24.53 liters with 4 kg of catalyst. Two streams exit the reactor - a vapor stream and the other having liquid and free water. The voidage of 0.4 is specified with catalyst volume considered in the rate/residence time calculations. In addition to the syngas feed, a second feed stream containing solvent is also fed continuously to ensure slurry conditions in the reactor. The solvent fed is the C30 paraffin at a rate of 100 mol/hr, the basis of which is explained in the next section. Reactions are in the liquid phase and are defined on the basis of concentration and mole fraction basis. We note that the FT slurry reactor is different from a normal CSTR because of having two exit streams that are in equilibrium. The FT slurry reactor can be considered to be a reactive flash. The RadFrac module is used to simulate the performance of reactive distillation (schematic shown in part b of Figure 1). This module is based upon a rigorous equilibrium stage model for solving the material and energy balance, phase equilibrium, and summation equations (MESH). A total of six stages with four reactive stages, a nonreactive reboiler, and a nonreactive condenser are present. For the sake of simplicity of analysis and convergence, the number of stages is taken as six. The condenser is specified to be of partial vapor-liquid type, having both a vapor and a liquid withdrawal. The reboiler is specified to be of the kettle type. Free-water calculations are enabled only in the condenser and not on the trays. There is a liquid withdrawal from the column bottom, thus giving a total of three
892
Ind. Eng. Chem. Res., Vol. 47, No. 3, 2008
Table 2. Temperature Profile for Equal and Distributed Catalyst Loading Temperature (C) stage 1 2 3 4 5 6 diff. cat. wt. 35 231 282 311 333 395 equal cat. wt. 35 229 281 315 346 390
exit streams from the RD column. The feed being in the vapor phase is fed below the last stage of the column. The column is operated at 25 atm and at a condenser temperature of 35 C. Whereas the pressure considered is equal to that of conventional FT reactors, the condenser temperature is taken to be in the range specified by York et al.10 in their patent. To have a reasonable extent of the reaction at every stage, the catalyst loading on the reactive stages from top to bottom is 1.9, 1.8, 0.25, and 0.05 kg, giving a total of 4 kg of catalyst hold-up. The reason for the choice of such a loading is elaborated in the next section. Reactions are in the liquid phase and are defined on the basis of concentration and mole fraction basis. The reflux ratio and Reboiler duty are varied to obtain convergence during the simulation runs. Though paraffins until C30 are considered in the simulations, C29 is the last paraffin presented in the analysis of the results, as C30 was used as a solvent in the slurry reactor simulations. Henceforth, CSTR relates to the slurry reactor, RD to the reactive distillation process of FT, and feed ratio to ratio of H2/CO in the feed. Each carbon number corresponds to the sum of the contributions from both paraffin and olefin. 6. Results and Discussion Parameters Defining Performance in RD. The major parameters that influence the performance of RD for FTS are the temperature of both of the stages and the overhead condenser and the solubility of the reactants. These in turn are dependent on operating parameters like reflux ratio, boil-up rate, distribution of catalyst weight on the reactive stages, side heat duties or side-product withdrawal and their rates, etc. The interaction among these parameters is not simple and makes FTS in RD a challenging task, both from a practical as well as a theoretical stand-point. The following sections discuss the simulation results, taking into consideration the two major parameters stated, with emphasis laid on conversion of the reactants, and yield and distribution of the FT products. Solvent Flow Rate. The solvent flow rate in the CSTR simulations was varied between 0.00001 to 1000 l/hr, with the concentration of C30 paraffin at 1 mol/L. With an increase in the solvent flow rate, the conversion of either reactant increased. The increase in conversion was not considerable beyond a certain solvent flow rate. Keeping this in view, a basis of 100 l/hr for the solvent flow rate is chosen in the CSTR simulations. Nemphos et al.11 suggest the use of n-octane as an inert solvent in the production of methanol by RD to maintain a sufficiently high liquid load in the rectifying section of the column. A similar option can be tried for FTS with one of the heavy paraffins, say C30, as the solvent, which is not studied in this work. Catalyst Distribution on the Reactive Stages. Simulations were performed with equal catalyst loading of 1 kg on each of the four reactive stages and with the distributed loading specified earlier. The conversion of CO and H2 and the temperature profile remained the same in either case (Table 2). Light gas (C1-C4), Naphtha (C5-C7), gasoline (C8-C12), diesel (C13-C18), and
Figure 2. Effect of catalyst distribution on the extent of the reaction on the stages (feed above the last stage of the column with a total of 4 kg catalyst).
waxes (C19+) are the components considered. The net product flows, including liquid and vapor, on a mass basis in either case were also the same for each of the carbon numbers. The stagewise composition profiles also remained the same in either case with no significant changes. Figure 2 compares the conversion of reactants in both the cases. For the equal catalyst case, we find that the whole reaction occurs only on the reactive stage nearest to the feed. The distributed catalyst loading case has reasonable reaction extents on three reactive stages (3, 4, and 5). Thus, we find that a reactive stage with 1 kg catalyst at 346 C and three reactive stages with a 2.1 kg catalyst but at relatively lower temperatures (282, 311, and 333 C) give the same performance. This can be attributed to both the higher catalyst loading and higher temperature on the stage with the largest reaction extent in the former case compared to the latter. There yet exists a possibility that equal and distributed catalyst loading cases may show a difference in the product distribution. So, considering a total of 0.4 kg of catalyst now, another set of simulations were performed with following catalyst loading on the four reactive stages: 0.1 kg on each and 0.05, 0.15, 0.15, 0.05 kg from top to bottom, with all of the other conditions remaining same. The distributed catalyst loading case now gave a higher average temperature for the reactive stages as well as a higher conversion for both of the reactants. Further, the diesel fraction on the stages is more in case of nonuniform catalyst loading, as seen in Figure 3. Hence, catalyst loading can be varied prudently on the reactive stages to alter the temperature profile and extent of the reaction for FTS in RD. Conversion. Table 3 compares the conversion of CO and H2 at different feed ratios of the reactants in each of the reactor types. Comparison of the CSTR and RD shows that conversion of both of the reactants becomes close to each other as the H2/ CO feed ratio decreases. In the case of RD, the range of temperatures on the four reactive stages from top to bottom are 207-213, 239-248, 253-265, and 272-286 C, respectively. The average temperatures for the reactive stages in order of decreasing feed ratios are 245, 243, and 253 C, respectively, which are all close to the operating temperature of 250 C specified for CSTR simulations. Owing to the similarity in the temperatures and the net catalyst loading in the case of CSTR and RD, the reactant conversions are close to each other. Lower catalyst loading and higher temperature on the bottom reactive stage in RD compensate the effects of each other and it is viceversa for the top stages. Thus, conversion in CSTR and RD are close to each other for all of the cases considered. There is, however, some mismatch observed at the higher feed ratio of 2.03. Simulations performed with a different catalyst loading (1.9 kg on stage 4 and 2.1 kg on stage 5) at a different reflux
Ind. Eng. Chem. Res., Vol. 47, No. 3, 2008 893
Figure 3. Effect of catalyst distribution on component fractions on the stages (total of 0.4 kg catalyst). Table 3. Comparison of the Performance of Different Reactors H2/CO Ratio conversion (%) CSTR RD CO 79.6 65.06 2.03 H2 87.79 71.74 CO 42.31 39.16 1.00 H2 94.76 87.67 CO 39.11 38.44 0.92 H2 94.88 93.24
Table 4. Comparison of Total Product Yields for Different Reactors H2/CO Ratio 2.03 Net Product kg/hr CSTR RD 3.8 3.64 1.00 Net Product kg/hr 3.06 3.32 0.92 Net Product kg/hr 2.94 3.38
ratio with all of the other parameters remaining the same gave a higher conversion for the higher feed ratio case for RD (CO ) 75% and H2 ) 82%), which are closer to the conversions reported for CSTR. The parameters chosen are also not the optimum, and there is a scope of improving the conversion and product distribution by varying the parameters. Hence, we conclude that the mismatch is owing to the operating parameters chosen for the RD simulation, like the catalyst loading and reflux ratio. Net Product Yields. Table 4 presents the total product yields from each of the reactors on a mass basis. At the higher feed ratio, the net product rate (including liquid and vapor) follows the same trend as the conversion of the reactants in Table 3. At lower feed ratios, the net product rate is higher for RD. This shows that at similar conversion levels for low feed ratios, RD may be advantageous over the CSTR. Such a case is possible when there are more lighter compounds formed, which consume more H2 per mole of CO, as is observed in the case of RD (Figure 5). Figures 4 and 5 illustrate the net product rate for the two reactor types at different feed ratios in terms of mole and mass basis, respectively. The ASF distribution exhibited by the FT products is also seen in Figure 4. Figure 6 shows the product distribution between the vapor and liquid phases for the simulation case of CSTR at a feed ratio of 1. Similar trends were observed for all of the simulations of CSTR and RD. The crossover carbon number in the case of CSTR and RD was 9 and 7 respectively, irrespective of the feed ratios. In short, the product distributions seen in two cases for both CSTR and RD are different and are discussed in detail in the next section. Product Distribution. The net product composition, including liquid and vapor, on a mass basis for different feed ratios is shown in Figure 7. With the decrease in the feed ratio, RD shows
higher selectivity toward naphtha, gasoline, and diesel fractions as compared to CSTR, whereas selectivity toward waxes and light gases is similar for all of the reactor types. The performance of RD is comparable to that of the CSTR at the high feed ratio as well. Hence, it can be concluded that at similar levels of conversion in RD and CSTR, the selectivity toward gasoline and diesel is more in the case of RD. This can be attributed to the greater formation of components from C3 to C15 on a mass basis (Figure 5), some of which form a part of the gasoline and diesel fractions. This is in turn because of the distributed catalyst loading and temperature on the reactive stages in RD. The liquid-phase H2/CO ratios and absolute solubilities of reactants in the case of RD (average over reactive trays) are higher than those in the CSTR, and the difference increases with a decrease in the feed ratio. Availability of more H2 per mole of CO in the liquid-phase promotes the formation of relatively lighter compounds in RD, in addition to the favorable conditions of temperature. Water present in the vapor phase is higher in the case of CSTR compared to RD and would reduce the boiling points of the mixture further. The outcome would be a higher molecular weight of the solvent on reactive stages in RD compared to CSTR, as the lowering in partial pressure due to more water vapor in CSTR will cause lighter compounds to go to the vapor phase. The higher molecular weight of the solvent in RD thus permits greater solubility of the reactants at similar temperatures encountered in the CSTR and gives better conversion toward lighter products. Liquid-Phase H2/CO Ratios. Table 5 shows the liquid-phase H2/CO ratios in the reactors for different feed ratios. The ratio in the case of RD is the average of ratios on the reactive stages. The ratios are higher in the case of RD compared to that in CSTR, and the difference is more marked at lower feed ratios. Figure 8 is a representative profile of the H2/CO ratio in the RD column for both of the phases. With a decrease in the feed ratio, the gap between the profiles in either phase decreases. The H2/CO ratio increases with the increase in temperature as we go down the column for all of the feed ratios. From the earlier discussion on catalyst distribution effects and product distribution, we conclude that the liquid-phase H2/CO ratio is an important parameter in determining the performance of the reactor and needs to be considered together with absolute solubilities of reactants or temperature. Mutually Compensating Effects in RD. Figure 9 gives a measure of the extent of the reaction on each of the reactive stages as well as shows the temperature profile in the column.
894
Ind. Eng. Chem. Res., Vol. 47, No. 3, 2008
Figure 4. Net product rate on the molar basis for a feed ratio of 2.03.
Figure 6. Product distribution in CSTR for a feed ratio of 1. Table 5. H2/CO Ratios in Liquid Phase in the Reactors Feed H2/CO Ratio CSTR RD 2.03 1.15 1.73 1.00 0.0877 0.542 0.92 0.075 0.425
Figure 5. Net product rate on the mass basis for different feed ratios.
It can be seen that the temperature increases as we move from the top to the bottom of the column in the direction of the increasing molecular weight of the liquid present on each of the trays. Also, the H2/CO ratio in the liquid phase followed a trend similar to the temperature. But, there is no direct relation between the extent of the reaction and the tray temperature or the H2/CO ratio because the extent of reaction is higher on stages 3 and 2, which have a relatively lower temperature and H2/CO ratio compared to those of stages 4 and 5. This trend is followed
in the case of lower feed ratios as well. Hence, we check if absolute solubilities of individual reactants are a reason for this observation. It is known that solubilities of both H2 and CO increase with temperature as well as the solvent molecular weight. Figure 10 shows the individual liquid-phase concentrations of H2, CO, and water on each of the stages in RD for different feed ratios. Whereas the CO concentration first decreases and then increases, H2 concentration increases as we move in the direction of increasing temperature. The amount of water present in the liquid phase decreases as we go down the column. One may now argue that the higher catalyst loading present on the top stages (1.9 and 1.8 kg respectively) compared to the lower stages (0.25 and 0.05 kg respectively) is the cause of the greater extent of the reaction. Considering stage 2 (1.9 kg catalyst at 210 C and H2/CO ratio of 1.38) and stage 4 (0.25 kg catalyst at 259 C and H2/CO ratio of 1.89), we would expect a higher rate of reaction on stage 2 as per this argument. But, Figure 9 shows an almost similar extent of the reaction on either stage. Thus, the contributions from temperature, reactant solubilities, and catalyst loading compensate each other in such a manner that the extent of the reaction on stages 2 and 4 are close to each other. Water Removed from the Reactors. The amount of water removed from the reactors for each of the feed ratios on a mass basis is shown in Table 6. The total amount of water formed in
Ind. Eng. Chem. Res., Vol. 47, No. 3, 2008 895
Figure 7. Net product compositions at different feed ratios.
each case falls in line with the conversions obtained in Table 3. The proportion of water removed as a liquid is maximum in the case of RD at all of the feed ratios, owing to the overhead free-water condenser and is almost completely removed. Water removal from the vapor stream of CSTR needs an additional heat exchanger followed by a decanter, whereas the condenser is an integral part of the RD column. 7. Other Possible RD configurations The RD column configuration used here is one of many possible alternatives. Other potential choices could be a RD column with:
a. Side draws to remove multiple products from different stages. b. Side heat duties to remove the heat of reaction from the reactive stages. c. Partial vapor condenser having a free-water liquid withdrawal but refluxing the entire hydrocarbon liquid. d. Alternating reactive and nonreactive stages with similar/ different catalyst loading. e. Different types of catalysts loaded in different sections of the column, for example placing an acid catalyst on stages where the C9-C14 fraction is high, to increase the yield of the branched isomers and hence the gasoline octane number, etc. f. Side-reactor configuration (SRC): The performance of RD is often compared with a distillation column coupled to a side reactor25-26 or an RD column integrated with a side-reactor.27 The potential of such a configuration has been evaluated for the production of MTBE, isobutene,25 methyl acetate,26 and ETBE27 by different authors. Withdrawing a liquid stream from the distillation column and sending it to a side reactor under such a configuration may not be a proper choice for FTS in RD. This is because either reactant in FT is in the vapor phase and has limited solubility in the liquid, which is in sharp contrast to other systems studied previously25-27 where the reactants are in liquid phase. However, once the feasibility of RD for FTS is well-established, further investigations on the side-reactor configuration can be done, and its potential needs to be studied with olefin readsorption included in the kinetics. One should also note that SRC is looked upon as an attractive option from a practical viewpoint, whereas the prime motive of this study is to check for the preliminary feasibility only, and column hardware aspects, economics, etc. are not considered. A combination of the choices listed earlier is also possible but would make the design and analysis more complicated. Simulation results for the first two alternatives are presented here. Effect of Side Duties to Remove Reaction Heat. For the CSTR, heat that arises from the heat of reaction is removed from the reactor to maintain isothermal conditions. In the case of RD, the heat load is the net difference in enthalpies of incoming and outgoing streams as well as the heat supplied/ removed from the reboiler/condenser. It is reported that steam generation from the exothermic heat in conventional reactors contributes partly to the economy of the process. The same is possible in RD if we have side duties for removing heat from each of the reactive stages. In such a case, we have heat sources at relatively higher temperatures that can be used for steam generation. Simulations were performed to study this alternative.
Figure 8. Stage-wise profile in RD for a H2/CO ratio of 2.03.
896
Ind. Eng. Chem. Res., Vol. 47, No. 3, 2008
Figure 9. Temperature profile and the extent of the reaction on the stages in RD for a feed ratio of 2.03.
Figure 10. Liquid-phase concentration in RD for Henry components (inorganic) at different feed ratios.
The heat duties for the reactive stages were 0.4, 3.5, 5.1, and 2.6 kW from top to bottom in the simulation. These numbers were arrived at by estimating the amount of heat released on each stage as per the reaction extent. We note that there is no
direct relation between the stage temperature and reaction heat to be removed. The following conclusions were arrived at: a. Conversion of both of the reactants is similar with and without side heat duties, and so is the net product rate on a mass basis. This is because of the compensating effects of temperature (which is higher on reactive stages when there are no side duties) and the H2/CO ratio (which is lower on reactive stages for the case of no side duties) as can be seen in Tables 7 and 8. b. Overall heat removed is nearly equal in both of the cases. When there are side heat removals, the condenser duty comes down. As expected, this heat is now available at a higher temperature and can be utilized more efficiently. c. There is no significant alteration either in the product distribution or on the stage-wise composition of various fractions like gasoline, diesel, etc. with additional heat removal from the reactive trays (Figure 11). These conclusions are true irrespective of whether water is removed as a vapor or as a liquid from the overhead condenser. Effect of Side Product Withdrawals. Figure 12 is a representative composition profile on the stages in RD for a feed ratio of 0.92. The profiles at other feed ratios are qualitatively similar. We observe that each of the components shows a maximum on one of the stages and then a decrease as seen in case of naphtha and gasoline. With more numbers of stages, the diesel fraction is also expected to show a similar trend. Waxes being the heaviest are expected to be maximum on the bottom tray of the column. Overall, the profiles suggest that having a side draw would be beneficial in removing a product selectively, for example gasoline from stage 4. Such an arrangement is not possible in the CSTR, and, hence, RD would offer the advantage of reduction in capital cost as foreseen earlier. In other words, the inherent characteristic of the distillation column to fractionate the product may be exploited advantageously to reduce the load on the downstream processing of the products. Simulations with a larger number of stages (9) and two side product withdrawals (from stage 4, reactive; and stage 7, nonreactive) were performed. The liquid withdrawal rate from stage 7 was fixed at 0.1 kg/hr, and that from stage 4 was varied as 0.1, 0.2, and 0.3 kg/hr. The following conclusions were arrived at from the simulation results: a. The conversion of both of the reactants remains unchanged by altering the side-stream rates. There is a slight increase in
Ind. Eng. Chem. Res., Vol. 47, No. 3, 2008 897
Figure 11. Effect of side heat duties on the component fractions in RD.
Figure 12. Stage-wise relative mass fraction of the components in RD for a feed ratio of 0.92. Table 6. Amount of Water Removed in the Reactors H2/CO Ratio water removed CSTR RD net kg/hr 4.73 3.86 2.03 liquid kg/hr 0.974 3.85 % liquid 20.59 99.74 net kg/hr 3.81 3.52 1.00 liquid kg/hr 0.574 3.51 % liquid 15.07 99.72 net kg/hr 3.66 3.6 0.92 liquid kg/hr 0.529 3.58 % liquid 14.45 99.44
Table 7. Effect of Side duties on the Water Concentration and Liquid-phase H2/CO Ratios in the Column Water mole frac. (liq.) stage no. 1 2 3 4 5 6 side duty 0.001 0.081 0.085 0.044 0.014 0.002 no side duty 0.001 0.056 0.046 0.024 0.009 0.001 H2/CO ratio (liq.) side duty 0.152 0.185 0.333 0.747 0.913 0.922 no side duty 0.142 0.173 0.353 0.732 0.911 0.919
Table 8. Effect of Side Duties on the Temperature Profiles in the Column Temperature (C) stage 1 2 3 4 5 6 side duty 35 187 218 227 258 355 no side duty 35 207 239 253 272 355
the conversion of the reactants in the case of the multiple product withdrawal compared to the base case when there are no side draws of products. b. The net mass flows in all of the cases are almost equal, with the base case (having no side draws) having a slightly lower net flow rate. This can be attributed to the slightly lower average temperature on the reactive stages for it (Table 9).
c. The temperature profiles in all of the cases remain similar until stage 4, from which the first draw is taken out. Temperatures on the stages below this stage, particularly on the nonreactive stages, increase with an increase of the withdrawal flow rate, owing to the changes in composition on each tray, which are pushed to a higher molecular weight and hence a higher bubble-point temperature. The side draws at a higher temperature provide heat integration options.
898
Ind. Eng. Chem. Res., Vol. 47, No. 3, 2008
Figure 14. Multiple steady states (MSS) for FTS in RD. Table 10. Mass Fractions of Gasoline and Diesel on the Stages with Side Draws Stage 4 side draw 0.1/0.1 0.2/0.1 0.3/0.1 gasoline mass frac. 66.01 65.71 65.01 diesel mass frac. 6.35 6.95 7.99 Stage 7 gasoline mass frac. 47.76 46.85 44.51 diesel mass frac. 31.76 32.65 33.91
Figure 13. Effect of multiple side draws on the component fractions on stages in RD. Table 9. Temperature Profiles in RD in the Case of Multiple Side Draws Temperature (C) stage 1 2 3 4 5 6 7 8 9 0/0 35 209 239 252 268 339 368 386 395 0.1/0.1 35 210 243 258 279 357 386 387 405 0.2/0.1 35 210 243 259 281 360 389 401 412 0.3/0.1 35 210 243 261 284 367 393 409 416
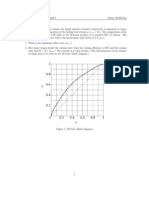
to the same. The decrease in the mass fraction of diesel below the second draw stage, that is, 7 could not be observed as the flow rate of this draw was not changed (Figure 13). An increase in the withdrawal rate of this draw would reduce the amount of diesel slipping out in the bottoms stream. Thus, there would be a draw from stage 4 rich in the gasoline fraction and a draw from stage 7 rich in the diesel fraction (Table 10). The overhead vapors would be rich in the light gases and LPG, the condenser liquid in naphtha, and the bottoms in the wax components. Thus, one can obtain streams rich in desirable fractions by the judicious choice of the location and rate of the side draws. 8. Multiple Solutions Despite the simplifications made to the kinetic model, and other assumptions taken, convergence in some of the simulations still proved difficult for a slight change in the parameter values like condenser temperature, etc. Jacobs and Krishna28 reported multiple steady states (MSS) for MTBE synthesis in RD for the first time. Many authors have later on proven the existence of MSS in RD for different systems, attributing it to the nonlinearity in kinetics, or VLE, or to the energy balances involved. Kienle and Marquardt29 have extensively reviewed the nonlinear dynamics studies done in RD. MSS for FT in slurry reactors is shown by Song et al.30 In the present work, the possibility of MSS in RD for FTS was investigated in one of the simulations by varying the condenser temperature from 1 to 45 C and by examining the temperature of the reactive stages. The variation in the forward and backward paths is different (Figure 14), indicating the presence of at least two steady states in the region of 14 to 37 C. Simulations performed near the region of the jumps, like at 14 C, indicated convergence problems. The cause of multiplicity can be the nonlinearity in reaction kinetics, VLE, energy balance, or a combi-
d. The extent of reaction is found to be almost similar on each reactive stage for all of the cases, owing to a close similarity in temperature profiles and H2/CO ratios on the reactive stages. e. There is no effect on the composition of the naphtha fraction on the stages, with an increase in the top side draw (from stage 4). And as expected, the mass fraction of gasoline on the lower stages (5 onward) decreases as the rate of withdrawal from stage 4 increases from 0.1 to 0.3 kg/hr. There is an increase in the mass fraction of diesel below stage 4, owing
Ind. Eng. Chem. Res., Vol. 47, No. 3, 2008 899
nation of these effects. Further investigation is necessary to get a better insight into the specific reasons for the existence of MSS for FT in RD, which was not the primary aim of this work. Nevertheless, one should be aware of such nonlinear dynamic effects, and carefully interpret the results of the steady-state simulation. 9. Conclusion To the best of the authors knowledge, this is the first attempt to evaluate the applicability of RD for a complex system like FTS through simulations. The preliminary simulations performed in this work show that it is feasible to carry out FTS in the reactive distillation mode. The performance of RD is at par or better than the conventional FT reactors, and the selectivity is shown to be improved in the case of RD. It is proven in this study that having multiple product draws from the column is advantageous and will help in reducing the downstream processing. Similarly, side heat duties do not hamper product selectivity and provide an option for heat integration in RD as well to generate steam, etc. Conventional FT reactors operate at 25 atm, which is not a good parameter to operate a distillation column because the component boiling points would be higher. However, the large amount of water formed in the FT reactions and its presence in the vapor phase in the column lowers the partial pressure of the hydrocarbons and makes separation possible. The results presented here are not optimal and may vary with the variation of parameters like reboiler duty, reflux ratio, catalyst loading on the stages, rate of side-product/heat removal, etc. Sensitivity analysis to this extent needs to be performed to get to the best possible set of operating parameters, keeping in mind the conversion of reactants, product yields, and selectivity toward a desired fraction. The two major parameters, that is, temperature and solubility, either counteract or support each other in deciding the performance of RD depending on other conditions. The presence of MSS needs further investigation to comprehend the steady-state simulation results in a better way. All of the reactions of FT are considered to be parallel in this study, which is not the actual case. Once the olefin readsorption mechanism is considered, the reactions are seriesparallel in nature, and a clever RD design may further enhance the selectivity, which needs to be studied. To add to this, the reactions are assumed to be first order in both of the reactants, which may not be true. The kinetic model of Wang et al.12 incorporates the chain-growth parameter, R, and the olefin readsorption factor, , into the kinetic expression, and the rate is a function of the carbon number. Each component would hence be having a different order with respect to the reactants as per its carbon number. RD may enhance the selectivity for parallel reactions if the rates are of a different order with respect to the reactants. The inclusion of the WGS reaction in FTS kinetics also needs to be studied because it is believed to maintain a balance between the reactants in the case of the COrich syngas feed. Literature Cited
(1) Dry, M. E. Fischer-Tropsch Process: 1950-2000. Catal. Today 2002, 71, 227-241. (2) Sie, S. T.; Senden, M. M. G.; van Wechem, H. M. H. Conversion of Natural Gas to Transportation Fuels via the Shell Middle Distillate Synthesis Process. Catal. Today 1991, 8, 371-394. (3) Schulz, H. Short History and Present Trends of FT Synthesis. Appl. Catal., A 1999, 186, 3-12. (4) Davis, B. H. Overview of Reactors for Liquid Phase FTS. Catal. Today 2002, 71, 249-300.
(5) Sie, S. T.; Krishna, R. Selection of Advanced FT Reactors. Appl. Catal., A 1999, 186, 55-70. (6) Jalama, K.; Coville, N. J.; Hildebrandt, D.; Glasser, D.; Jewell, L. L. FTS over Co/TiO2: Effect of Ethanol Addition. Fuel 2007, 86, 73-80. (7) Tavasoli, A.; Khodadadi, A.; Mortazavi, Y.; Sadaghiani, K.; Ahangari, M. G. Lowering Methane and Increasing Distillates Yields in FTS by Using Promoted and Unpromoted Cobalt Catalysts in a Dual Bed Reactor. Fuel Process. Technol. 2006, 87, 641-647. (8) Sharifnia, S.; Mortazavi, Y.; Khodadadi, A. Enhancement of Distillate Selectivity in FTS on a Co/SiO2 Catalyst by Hydrogen Distribution along a Fixed-Bed Reactor. Fuel Process. Technol. 2005, 86, 1253-1264. (9) Sharma, M. M. and Mahajani, S. M., Industrial Applications of Reactive Distillation, In ReactiVe Distillation: Status and Future Directions; Sundmacher, K., Kienle, A., Eds.; Wiley VCH: Germany, 2003. (10) York, K. M.; Keller, A. E.; Wright, H.; Harrins, T. H. Catalytic Distillation Reactor. Patent No. WO 01/36066 A2, 2001. (11) Nemphos, S. P.; Groten, W. A.; Adams, J. R. Process for Production of Methanol. U.S. Patent No. 588605, 1999. (12) Wang, Y. N.; Ma, W. P; Lu, Y. J.; Yang, J.; Xu, Y. Y.; Xiang, H. W.; Li, Y. N.; Zhao, Y. L.; Zhang, B. J. Kinetics Modeling of FTS over an Industrial Fe-Cu-K Catalyst. Fuel 2003, 82, 195-213. (13) Lox, E. S.; Froment, G. F. Kinetics of the FT reaction on a Precipitated Promoted Iron Catalyst. 2. Kinetic Modeling. Ind. Eng. Chem. Res. 1993, 32, 71-82. (14) van der Laan, G. P. Ph.D. Thesis, University of Groningen, The Netherlands, 1999. (15) Rao, V. U. S.; Stiegel, G. J.; Cinquegrane, G. J.; Srivastava, R. D. Iron-Based Catalysts for Slurry-Phase Fischer-Tropsch Process: Technology Review. Fuel Process. Technol. 1992, 30, 83-107. (16) Schulz, H.; Claeys, M. Kinetic modeling of Fischer-Tropsch product distributions. Appl. Catal., A 1999, 186, 91-107. (17) Caldwell, L.; van Vuuren, D. S. On the Formation and Composition of the Liquid Phase in FT Reactors. Chem. Eng. Sci. 1986, 41, 89-96. (18) Ray, D. J.; Farley, R. Design and Operation of a Pilot-Scale Plant for Hydrocarbon Synthesis in the Slurry Phase. J. Inst. Pet. 1964, 50, 2746. (19) Satterfield, C. N.; Huff, C. N.; Stenger, H. G. Effect of Carbon Formation on Liquid Viscosity and Performance of FT Bubble Column Reactors. Ind. Eng. Chem. Process Des. DeV. 1981, 20, 666-670. (20) van Vuuren, D. S.; Hunter, J. R.; Heydenrych, M. D. The Solubility of Various Gases in FT Reactor Wax. Chem. Eng. Sci. 1988, 43, 12911296. (21) Marano, J. J.; Holder, G. D. Characterization of FT liquids for VLE Calculations. Fluid Phase Equilib. 1997, 138, 1-21. (22) Ahon, V. R.; Costa Jr., E. F.; Monteagudo, J. E. P.; Fontes, C. E.; Biscaia, E. C.; Lage, P. L. C. A Comprehensive Mathematical Model for the Fischer-Tropsch Synthesis in Well-Mixed Slurry Reactors. Chem. Eng. Sci. 2005, 60, 677-694. (23) Aspen Plus User Manual, Aspen Plus, version 13.2; Aspen Technologies Inc.: Cambridge, MA. (24) Taylor, R.; Krishna, R. Modeling Reactive Distillation. Chem. Eng. Sci. 2000, 55, 5183-5229. (25) Jakobsson, K.; Pyhalahti, A.; Pakkanen, S., Keskinen, K.; Aittamaa, J. Modelling of a Side Reactor Configuration Combining Reaction and Distillation. Chem. Eng. Sci. 2002, 57, 1521-1524. (26) Baur, R.; Krishna, R. Distillation Column with Reactive Pump Arounds: An Alternative to Reactive Distillation. Catal. Today 2003, 7980, 113-123. (27) Bisowarno, B. H.; Tian, Y. C.; Tade, M. O. Application of Side Reactors on ETBE Reactive Distillation. Chem.sEng. J. 2004, 99, 35-43. (28) Jacobs, R.; Krishna, R. Multiple Solutions in Reactive Distillation for MTBE Synthesis. Ind. Eng. Chem. Res. 1993, 32, 1706-1709. (29) Kienle, A. and Marquardt, W., Nonlinear Dynamics and Control of Reactive Distillation Processes, In ReactiVe Distillation: Status and Future Directions Sundmacher, K., Kienle, A., Eds.; Wiley VCH: Germany, 2003. (30) Song, H. S.; Ramkrishna, D.; Trinh, S.; Espinoza, R. L.; Wright, H. Multiplicity and Sensitivity Analysis of FT Bubble-Column Slurry Reactors: Plug-Flow Gas and Well-Mixed Slurry Model. Chem. Eng. Sci. 2003, 58, 2759-2766.
ReceiVed for reView August 10, 2007 ReVised manuscript receiVed October 2, 2007 Accepted October 8, 2007 IE071094P
You might also like
- Energy Efficient Solvents For CO Absorption From Flue Gas: Vapor Liquid Equilibrium and Pilot Plant StudyDocument26 pagesEnergy Efficient Solvents For CO Absorption From Flue Gas: Vapor Liquid Equilibrium and Pilot Plant StudyEr Mayur PatilNo ratings yet
- InTech-Transesterification in Supercritical Conditions PDFDocument23 pagesInTech-Transesterification in Supercritical Conditions PDFEr Mayur PatilNo ratings yet
- Velocity - Velocity - Pressure: Bernoulli's Principle (Demos)Document8 pagesVelocity - Velocity - Pressure: Bernoulli's Principle (Demos)suna06m6403No ratings yet
- Preparation of Ester Derivatives of Fatty Acids For GC AnalysisDocument27 pagesPreparation of Ester Derivatives of Fatty Acids For GC Analysism_m_hamedi3866No ratings yet
- Bioetanol PDFDocument304 pagesBioetanol PDFEr Mayur PatilNo ratings yet
- Doctor of Philosophy (Ph. D.) Programmes: 10.1 Courses of Doctoral StudiesDocument14 pagesDoctor of Philosophy (Ph. D.) Programmes: 10.1 Courses of Doctoral StudiesEr Mayur PatilNo ratings yet
- Application Form For PH DTech PHD SciandIntegratedPhDTech PDFDocument2 pagesApplication Form For PH DTech PHD SciandIntegratedPhDTech PDFEr Mayur PatilNo ratings yet
- 45 Icsia2011 H30024 PDFDocument5 pages45 Icsia2011 H30024 PDFEr Mayur PatilNo ratings yet
- Distillation Dynamics and Control Workbook 2006 PDFDocument18 pagesDistillation Dynamics and Control Workbook 2006 PDFEr Mayur PatilNo ratings yet
- Information BoucherDocument255 pagesInformation Boucherparchure123No ratings yet
- ANFIS Based Distillation Column Control: R. Sivakumar K. BaluDocument7 pagesANFIS Based Distillation Column Control: R. Sivakumar K. BaluEr Mayur PatilNo ratings yet
- Ms Exercise06Document4 pagesMs Exercise06Er Mayur PatilNo ratings yet
- Aaee16 PDFDocument8 pagesAaee16 PDFEr Mayur PatilNo ratings yet
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5795)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- LS-32001-BCR Bench Top Chemical ReactorsDocument5 pagesLS-32001-BCR Bench Top Chemical ReactorsAhmed AliNo ratings yet
- Introduction To Numerical Methods in Chemical Engineering. Pradeep Ahuja. PHI Learning, India, 2010Document299 pagesIntroduction To Numerical Methods in Chemical Engineering. Pradeep Ahuja. PHI Learning, India, 2010Anonymous 8mxTh1m60% (5)
- Difficulties in Scaling Up The Fluidized Bed ReactorDocument5 pagesDifficulties in Scaling Up The Fluidized Bed Reactorandry86No ratings yet
- Ah SHHHHDocument44 pagesAh SHHHHAjay RamaniNo ratings yet
- Levenspiel 1999 - Chemical Reaction EngineeringDocument4 pagesLevenspiel 1999 - Chemical Reaction EngineeringDrudervenNo ratings yet
- Aspen Example Test - EO v2018Document2 pagesAspen Example Test - EO v2018marij233No ratings yet
- Cre Exp 9 Lab Report (CSTR & PFR IN SERIES)Document12 pagesCre Exp 9 Lab Report (CSTR & PFR IN SERIES)sukhmaniNo ratings yet
- DEP 30.10.05.11-GEN Feb 2012 3D Model Review.Document41 pagesDEP 30.10.05.11-GEN Feb 2012 3D Model Review.lingarajavenkatesh100% (1)
- Bubble ColumnDocument34 pagesBubble ColumnihsanNo ratings yet
- Linear Control of Nonlinear ProcessesDocument23 pagesLinear Control of Nonlinear ProcessesSurya Budi WidagdoNo ratings yet
- Baku Higher Oil School: Chemical Engineering Department Design Project Report Final YearDocument50 pagesBaku Higher Oil School: Chemical Engineering Department Design Project Report Final Yearİlkin İbrahimliNo ratings yet
- Packed Bed Reactor Lab ReportDocument30 pagesPacked Bed Reactor Lab ReportAmy100% (1)
- Modified Differential Evolution (MDE) For Optimization of Non-Linear Chemical ProcessesDocument14 pagesModified Differential Evolution (MDE) For Optimization of Non-Linear Chemical ProcessesBAKRNo ratings yet
- Case Studies, Optimizations and Trials For PSDDocument5 pagesCase Studies, Optimizations and Trials For PSDIbrahim Nick DibalNo ratings yet
- Design of Acetone HYSYSDocument6 pagesDesign of Acetone HYSYSlockas222100% (1)
- Guerbet Process Hydroformylation Fermentation Reactants Reaction Type ReactionsDocument2 pagesGuerbet Process Hydroformylation Fermentation Reactants Reaction Type ReactionsfaranimohamedNo ratings yet
- The Properties of Cobalt Oxide Catalyst For Ammonia Oxidation Szalowki Et Al. Appl. Catal. 1998Document11 pagesThe Properties of Cobalt Oxide Catalyst For Ammonia Oxidation Szalowki Et Al. Appl. Catal. 1998juan davidNo ratings yet
- UreaDocument1 pageUreaHarsha100% (2)
- Liden 1988Document16 pagesLiden 1988Sajid Mohy Ul DinNo ratings yet
- Design For A High Temperature Shift ConverterDocument43 pagesDesign For A High Temperature Shift ConverterAaron GyamfiNo ratings yet
- Biological Treatment of WastewaterDocument64 pagesBiological Treatment of WastewaterDr. Akepati Sivarami Reddy100% (7)
- Mole Balances in Reacting Systems & Reactor Design EquationsDocument60 pagesMole Balances in Reacting Systems & Reactor Design EquationsMaryjean Almodiel InfornonNo ratings yet
- Dimethyl EtherDocument7 pagesDimethyl EtherAna Laura Sanchez100% (1)
- 01 Basic Concepts of Process Dynamics and ControlDocument13 pages01 Basic Concepts of Process Dynamics and Controldr. rickNo ratings yet
- PGM Catalyst Handbook USADocument93 pagesPGM Catalyst Handbook USAAFLAC ............No ratings yet
- Types of Phenol Manufacturing ProcessDocument4 pagesTypes of Phenol Manufacturing ProcessIsma AzraNo ratings yet
- B11Document5 pagesB11Amanda GrazieleNo ratings yet
- CHE 309: Chemical Reaction Engineering: Lecture-5Document14 pagesCHE 309: Chemical Reaction Engineering: Lecture-5Enauris MateoNo ratings yet
- Cjce 22656Document6 pagesCjce 22656Tua HalomoanNo ratings yet
- Applied Catalysis A: General: V. Balcaen, I. Sack, M. Olea, G.B. MarinDocument12 pagesApplied Catalysis A: General: V. Balcaen, I. Sack, M. Olea, G.B. MarinMayteNo ratings yet