Professional Documents
Culture Documents
Mammary Gland
Uploaded by
daitya99Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Mammary Gland
Uploaded by
daitya99Copyright:
Available Formats
Mammary gland
From Wikipedia, the free encyclopedia Jump to: navigation, search This article may require copy editing for grammar, style, cohesion, tone, or spelling. You can assist by editing it. (December 2012)
Mammary gland in a human female
Cross section of the breast of a human female
Dissection of a lactating breast. 1 Fat 2 Lactiferous duct/lobule 3 Lobule 4 Connective tissue 5 Sinus of lactiferous duct 6 Lactiferous duct Latin Gray's Artery Vein glandula mammaria subject #271 1267 Internal thoracic artery Lateral thoracic artery [1] Internal thoracic vein
Vein
Axillary vein [1] Supraclavicular nerves Intercostal nerves[2]
(lateral and medial branches)
Nerve
Lymph
Pectoral axillary lymph nodes [1] Mesoderm
(blood vessels and connective tissue)
Precursor
Ectoderm[3]
(cellular elements)
M eSH
A01.236.249
A mammary gland is an organ in female mammals that produces milk to feed young offspring. Mammals get their name from the word "mammary." In humans, the mammary glands are situated in the breasts. In ruminants such as cows, goats, and deer, the mammary glands are contained in the udders. The mammary glands of mammals having more than two breasts, such as dogs and cats, are sometimes called dugs.
Contents
1 Humans 1.1 Histology 1.2 Structure 1.3 Development and hormonal control 1.4 Breast cancer 2 Other mammals 3 Evolution 4 Gallery 5 See also 6 Notes 7 References 8 External links
Humans
Main article: Breast
Histology
A mammary gland is a specific type of apocrine gland specialized for manufacture of colostrum when giving birth. Mammary glands can be identified as apocrine because they exhibit striking "decapitation" secretion. Many sources assert that mammary glands are modified sweat glands.[4][5][6] Some authors dispute that and argue instead that they are sebaceous glands.[4]
Structure
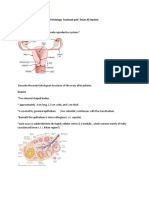
The basic components of a mature mammary gland are the alveoli (hollow cavities, a few millimeters large) lined with milk-secreting cuboidal cells and surrounded by myoepithelial cells. These alveoli join to form groups known as lobules. Each lobule has a lactiferous duct that drains into openings in the nipple. The myoepithelial cells contract under the stimulation of oxytocin, excreting the milk secreted by alveolar units into the lobule lumen toward the nipple. As the infant
begins to suck, the oxytocin-mediated "let down reflex" ensues and the mother's milk is secreted not sucked from the gland into the baby's mouth. All the milk-secreting tissue leading to a single lactiferous duct is called a "simple mammary gland"; in a "complex mammary gland" all the simple mammary glands serve one nipple. Humans normally have two complex mammary glands, one in each breast, and each complex mammary gland consists of 1020 simple glands. The presence of more than two nipples is known as polythelia and the presence of more than two complex mammary glands as polymastia. Maintaining the correct polarized morphology of the lactiferous duct tree requires another essential component mammary epithelial cells extracellular matrix (ECM) which, together with adipocytes, fibroblast, inflammatory cells, and others, constitute mammary stroma. [7] Mammary epithelial ECM mainly contains myoepithelial basement membrane and the connective tissue. They not only help to support mammary basic structure, but also serve as a communicating bridge between mammary epithelia and their local and global environment throughout this organ's development.[8][9]
Development and hormonal control
Further information: Mammary gland development Mammary glands develop during different growth cycles. They exist in both sexes during embryonic stage, forming only a rudimentary duct tree at birth. In this stage, mammary gland development depends on systemic (and maternal) hormones,[7] but is also under the (local) regulation of paracrine communication between neighboring epithelial and mesenchymal cells by parathyroid hormone-related protein(PTHrP).[10] This locally secreted factor gives rise to a series of outside-in and inside-out positive feedback between these two types of cells, so that mammary bud epithelial cells can proliferate and sprout down into the mesenchymal layer until they reach the fat pad to begin the first round of branching.[7] At the same time, the embryonic mesenchymal cells around the epithelial bud receive secreting factors activated by PTHrP, such as BMP4. These mesenchymal cells can transform into a dense, mammary-specific mesenchyme, which later develop into connective tissue with fibrous threads, forming blood vessels and the lymph system.[11] A basement membrane, mainly containing laminin and collagen, formed afterward by differentiated myoepithelial cells, keeps the polarity of this primary duct tree. Lactiferous duct development occurs in females in response to circulating hormones. First development is frequently seen during pre- and postnatal stages, and later during puberty. Estrogen promotes branching differentiation,[12] whereas in males testosterone inhibits it. A mature duct tree reaching the limit of the fat pad of the mammary gland comes into being by bifurcation of duct terminal end buds (TEB), secondary branches sprouting from primary ducts[8][13] and proper duct lumen formation. These processes are tightly modulated by components of mammary epithelial ECM interacting with systemic hormones and local secreting factors. However, for each mechanism the epithelial cells' "niche" can be delicately unique with different membrane receptor profiles and basement membrane thickness from specific branching area to area, so as to regulate cell growth or differentiation sub-locally.[14] Important players include beta-1 integrin, epidermal growth factor receptor (EGFR), laminin-1/5, collagen-IV, matrix metalloproteinase(MMPs), heparan sulfate proteoglycans, and others. Elevated circulating level of growth hormone and estrogen get to multipotent cap cells on TEB tips through a thin, leaky layer of basement membrane. These hormones promote specific gene expression. Hence cap cells can differentiate into myoepithelial and luminal (duct) epithelial cells, and the increased amount of activated MMPs can degrade surrounding ECM helping duct buds to reach further in the fat pads.[15][16] On the other hand, basement membrane along the mature mammary ducts is thicker, with strong adhesion to epithelial cells via binding to integrin and non-integrin receptors. When side branches develop, it is a much more pushing-forward working process including extending through myoepithelial cells, degrading basement membrane and then invading into a periductal layer of fibrous stromal tissue.[8] Degraded basement membrane fragments (laminin-5) roles to lead the way of mammary epithelial
cells migration.[17] Whereas, laminin-1 interacts with non-integrin receptor dystroglycan negatively regulates this side branching process in case of cancer.[18] These complex "Yin-yang" balancing crosstalks between mammary ECM and epithelial cells "instruct" healthy mammary gland development until adult. Secretory alveoli develop mainly in pregnancy, when rising levels of prolactin, estrogen, and progesterone cause further branching, together with an increase in adipose tissue and a richer blood flow. In gestation, serum progesterone remains at a stably high concentration so signaling through its receptor is continuously activated. As one of the transcribed genes, Wnts secreted from mammary epithelial cells act paracrinely to induce more neighboring cells' branching.[19][20] When the lactiferous duct tree is almost ready, "leaves" alveoli are differentiated from luminal epithelial cells and added at the end of each branch. In late pregnancy and for the first few days after giving birth, colostrum is secreted. Milk secretion (lactation) begins a few days later due to reduction in circulating progesterone and the presence of another important hormone prolactin, which mediates further alveologenesis, milk protein production, and regulates osmotic balance and tight junction function. Laminin and collagen in myoepithelial basement membrane interacting with beta-1 integrin on epithelial surface again, is essential in this process.[21][22] Their binding ensures correct placement of prolactin receptors on the basal lateral side of alveoli cells and directional secretion of milk into lactiferous ducts.[21][22] Suckling of the baby causes release of the hormone oxytocin, which stimulates contraction of the myoepithelial cells. In this combined control from ECM and systemic hormones, milk secretion can be reciprocally amplified so as to provide enough nutrition for the baby. During weaning, decreased prolactin, missing mechanical stimulation (baby suckling), and changes in osmotic balance caused by milk stasis and leaking of tight junctions cause cessation of milk production. In some species there is complete or partial involution of alveolar structures after weaning, in humans there is only partial involution and the level of involution in humans appears to be highly individual. In some other species (such as cows), all alveoli and secretory duct structures collapse by programmed cell death (apoptosis) and autophagy for lack of growth promoting factors either from the ECM or circulating hormones.[23][24] At the same time, apoptosis of blood capillary endothelial cells speeds up the regression of lactation ductal beds. Shrinkage of the mammary duct tree and ECM remodeling by various proteinase is under the control of somatostatin and other growth inhibiting hormones and local factors.[25] This major structural change leads loose fat tissue to fill the empty space afterward. But a functional lactiferous duct tree can be formed again when a female is pregnant again.
Breast cancer
Tumorigenesis in mammary glands can be induced biochemically by abnormal expression level of circulating hormones or local ECM components,[26] or from a mechanical change in the tension of mammary stroma.[27] Under either of the two circumstances, mammary epithelial cells would grow out of control and eventually result in cancer. Almost all instances of breast cancer originate in the lobules or ducts of the mammary glands.
Other mammals
The constantly protruding breasts of the adult human female, unusually large relative to body size, are a unique evolutionary development whose purpose is not yet fully known (see breasts); other mammals tend to have less conspicuous mammary glands that protrude only while actually filling with milk. The number and positioning of complex and simple mammary glands varies widely in different mammals. The nipples and glands can occur anywhere along the two milk lines, two roughly-parallel lines along the ventral aspect of the body. In general most mammals develop mammary glands in pairs along these lines, with a number approximating the number of young typically birthed at a time. The number of nipples varies from 2 (in most primates) to 18 (in pigs).
The Virginia Opossum has 13, one of the few mammals with an odd number. [28][29] The following table lists the number and position of glands normally found in a range of mammals: Species[30] Goat, sheep, horse guinea pig Cattle Cat Anterior Intermediate Posterior Total (thoracic) (abdominal) (inguinal) 0 0 0 2 2 0 2 6 0 2 4 4 2 or 4 4 4 6 0 2 4 8 8 or 10 10 12 18 2
0 2 4 Dog[31] Mouse 6 Rat 6 Pig 6 proboscideans, primates 2
Male mammals typically have rudimentary mammary glands and nipples, with a few exceptions: male mice do not have nipples, and male horses lack nipples and mammary glands.[citation needed ] The male Dayak fruit bat has lactating mammary glands;[32] male lactation occurs infrequently in some species, including humans. Mammary glands are true protein factories, and several labs have constructed transgenic animals, mainly goats and cows, to produce proteins for pharmaceutical use.[33] Complex glycoproteins such as monoclonal antibodies or antithrombin cannot be produced by genetically engineered bacteria, and the production in live mammals is much cheaper than the use of mammalian cell cultures.
Evolution
The mammary gland is difficult to explain with an evolutionary world-view. The reason for this is because mammary glands are typically required by mammals to feed their young. Which evolved first - the ability for a mammal to give birth, or the ability for a mammal to feed it's newly born young? Nevertheless, there are many theories on how mammary glands evolved, for example, it is believed that the mammary gland is a transformed sweat gland, more closely related to apocrine sweat glands.[34] Since mammary glands do not fossilize well, supporting such theories is difficult. Many of the current theories are based on comparisons between lines of living mammals- monotremes, marsupials and eutherians. One theory proposes that mammary glands evolved from glands that were used to keep the eggs of early mammals moist[35][36] and free from infection[37][38] (monotremes still lay eggs). Other theories propose that early secretions were used directly by hatched young,[39] or that the secretions were used by young to help them orient to their mothers.[40] Lactation is assumed to have developed long before the evolution of the mammary gland and mammals; see evolution of lactation.
Gallery
Cattle
Cat
Pig
Sheep
Goat
Elephant
Human
You might also like
- ACFrOgCu W0g9MxVK8YkEvfsqyGhjSstpPm3j2uJhpy4G69TRnw18zCsRC69oQlKhanJWV 6usc VqeS933XuRqaxNoOkxjXkvFwDAm6It15dFc0KjwYag EzHDH6St2KFBAy0eOIbDC WNeC9lpDocument18 pagesACFrOgCu W0g9MxVK8YkEvfsqyGhjSstpPm3j2uJhpy4G69TRnw18zCsRC69oQlKhanJWV 6usc VqeS933XuRqaxNoOkxjXkvFwDAm6It15dFc0KjwYag EzHDH6St2KFBAy0eOIbDC WNeC9lpUrvashi KhadeNo ratings yet
- Information Networks in The Mammary Gland: Lothar Hennighausen and Gertraud W. RobinsonDocument11 pagesInformation Networks in The Mammary Gland: Lothar Hennighausen and Gertraud W. RobinsonSueños De LunaNo ratings yet
- Molecular Mechanism of ImplantationDocument19 pagesMolecular Mechanism of ImplantationAbunu TilayeNo ratings yet
- Reproductive Biology and Technology in Animals PDFDocument126 pagesReproductive Biology and Technology in Animals PDFJuan CaroNo ratings yet
- Lactation PhysiologyDocument17 pagesLactation PhysiologysanathNo ratings yet
- Module 5Document10 pagesModule 5Phan MhiveNo ratings yet
- Breast Development PDFDocument26 pagesBreast Development PDFgiant nitaNo ratings yet
- EMBRYOLOGYDocument30 pagesEMBRYOLOGYMSc. PreviousNo ratings yet
- Buhimschi 2004 Endocrinology of LactationDocument17 pagesBuhimschi 2004 Endocrinology of LactationCarolina GómezNo ratings yet
- Oophorectomy &its Rolrole in Breast Cancer Risk ReductionDocument23 pagesOophorectomy &its Rolrole in Breast Cancer Risk ReductionNorhan Ahmed Ahmed Hassan El-MeniawyNo ratings yet
- Grimm 2020Document16 pagesGrimm 2020MD LarasatiNo ratings yet
- The Growing Fetus FertilizationDocument6 pagesThe Growing Fetus FertilizationJustJ ThingsNo ratings yet
- Implantation and Development of The PlacentaDocument8 pagesImplantation and Development of The PlacentaZulfi Nur Amrina RosyadaNo ratings yet
- Signalling Pathways Implicated in Early Mammary Gland Morphogenesis and Breast CancerDocument19 pagesSignalling Pathways Implicated in Early Mammary Gland Morphogenesis and Breast CancerhajimehikariNo ratings yet
- Fisiologia de La Secrecion Leche Materna Truchet2017Document45 pagesFisiologia de La Secrecion Leche Materna Truchet2017vanetoledoNo ratings yet
- Tutorial 4 Biology 2 Group 3 HN2Document3 pagesTutorial 4 Biology 2 Group 3 HN2Firdaus ZulkifliNo ratings yet
- CH 3Document8 pagesCH 3api-288494741No ratings yet
- GametogenesisDocument8 pagesGametogenesisSOPHIA ALESNANo ratings yet
- CVM Pres - IIDocument152 pagesCVM Pres - IIseraphim8No ratings yet
- 1.1c Implantation & Placental DevelopmentDocument5 pages1.1c Implantation & Placental DevelopmentBea Samonte100% (1)
- Department of Morphology: Penza State University Medical InstituteDocument18 pagesDepartment of Morphology: Penza State University Medical InstituteunnatiNo ratings yet
- Reproduction in AnimalsDocument12 pagesReproduction in AnimalsYuh moddaNo ratings yet
- Anatomy and Physiology GCSDocument7 pagesAnatomy and Physiology GCSSherry Ann FayeNo ratings yet
- Chapter - 3 Human ReproductionDocument3 pagesChapter - 3 Human ReproductionMr.Prashant SharmaNo ratings yet
- Normal PregnancyDocument65 pagesNormal PregnancyRhesie Joyce AguilarNo ratings yet
- Approach To Breast FeedingDocument13 pagesApproach To Breast FeedingsmokaNo ratings yet
- Obstetrics V10 Common Obstetric Conditions Chapter Pathology of The Placenta 1663285373Document11 pagesObstetrics V10 Common Obstetric Conditions Chapter Pathology of The Placenta 1663285373Nurliana NingsihNo ratings yet
- Mammary GlandDocument11 pagesMammary GlandPRITAM BHOIRNo ratings yet
- BlastulaDocument4 pagesBlastulaCyanDesNo ratings yet
- Why Is Placentation Abnormal in Preeclampsia?Document8 pagesWhy Is Placentation Abnormal in Preeclampsia?cecilliacynthiaNo ratings yet
- Postimplantation Development in MammalianDocument52 pagesPostimplantation Development in MammalianBiology BảoNo ratings yet
- 4 1Document265 pages4 1sillypoloNo ratings yet
- Fertilization Reflection PaperDocument2 pagesFertilization Reflection PaperCrisandro Allen Lazo100% (2)
- Abnormal ImplantationDocument3 pagesAbnormal ImplantationMuhammet Fatih CantepeNo ratings yet
- Last Minute Embryology: Human embryology made easy and digestible for medical and nursing studentsFrom EverandLast Minute Embryology: Human embryology made easy and digestible for medical and nursing studentsNo ratings yet
- Module 2 HandsoutDocument10 pagesModule 2 HandsoutErin SaavedraNo ratings yet
- Gametogenesis and FertilizationDocument10 pagesGametogenesis and FertilizationJustus MutuaNo ratings yet
- Obstetrics: The Normal Menstrual CycleDocument4 pagesObstetrics: The Normal Menstrual Cycleapi-3829364No ratings yet
- UntitledDocument234 pagesUntitledMarco Antonio AlvarezNo ratings yet
- Eterinary Bstetrics: - Dr. S.BalasubramanianDocument413 pagesEterinary Bstetrics: - Dr. S.BalasubramanianprernaNo ratings yet
- Phases of Embryonic DevelopmentDocument9 pagesPhases of Embryonic DevelopmentIshu ChoudharyNo ratings yet
- Prenatal GrowthDocument56 pagesPrenatal GrowthnavjotsinghjassalNo ratings yet
- Wella S, VIRINA Bsn-I: Review Question Reproductive SystemDocument5 pagesWella S, VIRINA Bsn-I: Review Question Reproductive SystemEllee HadesNo ratings yet
- Histo FemDocument9 pagesHisto FemzaidNo ratings yet
- CH 5 Implantation, Placental Dev (Part1)Document36 pagesCH 5 Implantation, Placental Dev (Part1)Tengku Chairannisa PutriNo ratings yet
- Embryology GastrulationDocument9 pagesEmbryology GastrulationRivera Gómez América LucíaNo ratings yet
- Regulation of Blastocyst Formation.Document24 pagesRegulation of Blastocyst Formation.christopher LopezNo ratings yet
- Embryology of Genital System & Congenital Anomalies: Kenbon S. (MS) January 30,2021Document56 pagesEmbryology of Genital System & Congenital Anomalies: Kenbon S. (MS) January 30,2021gimNo ratings yet
- 1st, 2nd & 3rdweeksDocument9 pages1st, 2nd & 3rdweeksPristine KrNo ratings yet
- Reproduction (M, F) : Lecturer: Dr. R. Ahangari University of Central Florida, OrlandoDocument10 pagesReproduction (M, F) : Lecturer: Dr. R. Ahangari University of Central Florida, Orlandokuku411No ratings yet
- Chapter 29 - Development and Inheritance: ST ND RD STDocument5 pagesChapter 29 - Development and Inheritance: ST ND RD STtomorrow.today.yesterday .yesterdayNo ratings yet
- Preclinical Anatomy Review 2023: For USMLE Step 1 and COMLEX-USA Level 1From EverandPreclinical Anatomy Review 2023: For USMLE Step 1 and COMLEX-USA Level 1Rating: 5 out of 5 stars5/5 (2)
- Naim LBM 1-1Document38 pagesNaim LBM 1-1dimasNo ratings yet
- EmbryologyDocument101 pagesEmbryologytawandarukwavamadisonNo ratings yet
- Funcion Endocrina de La Placenta HumanaDocument30 pagesFuncion Endocrina de La Placenta HumanaSofia RossoNo ratings yet
- USMLE NotesDocument165 pagesUSMLE NotesHerliani HalimNo ratings yet
- Sexual Reproduction and Meiosis: Four Haploid CellsDocument10 pagesSexual Reproduction and Meiosis: Four Haploid CellsJohnieer Bassem MoferdNo ratings yet
- LAB EXERCISE 2 - OogenesisDocument4 pagesLAB EXERCISE 2 - OogenesisGerald Angelo DeguinioNo ratings yet
- Reproduction PPT 6 PHYSIOLOGY OF PREGNANCYDocument98 pagesReproduction PPT 6 PHYSIOLOGY OF PREGNANCYlisanames.23No ratings yet
- Review of Fetal DevelopmentDocument71 pagesReview of Fetal DevelopmentlisafelixNo ratings yet
- NarasimhaDocument11 pagesNarasimhadaitya99No ratings yet
- VamanaDocument3 pagesVamanadaitya99No ratings yet
- VarahaDocument5 pagesVarahadaitya99No ratings yet
- ValmikiDocument2 pagesValmikidaitya99No ratings yet
- BrahmaDocument5 pagesBrahmadaitya99No ratings yet
- NaradDocument2 pagesNaraddaitya99No ratings yet
- Old World MonkeyDocument6 pagesOld World Monkeydaitya99No ratings yet
- Kingdom of BhutanDocument21 pagesKingdom of Bhutandaitya99No ratings yet
- Mammal: MammalsDocument13 pagesMammal: Mammalsdaitya99No ratings yet
- PrimatesDocument15 pagesPrimatesdaitya99No ratings yet
- Republic of Fiji: Navigation Search Fiji (Disambiguation)Document18 pagesRepublic of Fiji: Navigation Search Fiji (Disambiguation)daitya99No ratings yet
- List of Fossil PrimatesDocument20 pagesList of Fossil Primatesdaitya99No ratings yet
- Standard Assessment Form For Postgraduate Courses (Microbiology)Document15 pagesStandard Assessment Form For Postgraduate Courses (Microbiology)drunken monkeyNo ratings yet
- Suicide CaseDocument2 pagesSuicide Casedaitya99No ratings yet
- Ratna Shastra - The Mystery Hidden in GemstonesDocument35 pagesRatna Shastra - The Mystery Hidden in Gemstonesdaitya9957% (7)
- Marketing Study of Mango JuiceDocument18 pagesMarketing Study of Mango JuiceVijay ArapathNo ratings yet
- DR K.M.NAIR - GEOSCIENTIST EXEMPLARDocument4 pagesDR K.M.NAIR - GEOSCIENTIST EXEMPLARDrThrivikramji KythNo ratings yet
- Test On QuantifiersDocument1 pageTest On Quantifiersvassoula35No ratings yet
- 220hp Caterpillar 3306 Gardner Denver SSP Screw Compressor DrawingsDocument34 pages220hp Caterpillar 3306 Gardner Denver SSP Screw Compressor DrawingsJVMNo ratings yet
- Powerful Communication Tools For Successful Acupuncture PracticeDocument4 pagesPowerful Communication Tools For Successful Acupuncture Practicebinglei chenNo ratings yet
- Atlas of Feline Anatomy For VeterinariansDocument275 pagesAtlas of Feline Anatomy For VeterinariansДибензол Ксазепин100% (4)
- Chap 6 - Karen HorneyDocument95 pagesChap 6 - Karen HorneyDiana San JuanNo ratings yet
- ContinueDocument2 pagesContinueNeal ReppNo ratings yet
- Issue of HomosexualityDocument4 pagesIssue of HomosexualityT-2000No ratings yet
- Hodgkin LymphomaDocument44 pagesHodgkin LymphomaisnineNo ratings yet
- Pressure Classes: Ductile Iron PipeDocument4 pagesPressure Classes: Ductile Iron PipesmithNo ratings yet
- Index Medicus PDFDocument284 pagesIndex Medicus PDFVania Sitorus100% (1)
- TherabandDocument1 pageTherabandsuviacesoNo ratings yet
- Varioklav Steam Sterilizer 75 S - 135 S Technical SpecificationsDocument10 pagesVarioklav Steam Sterilizer 75 S - 135 S Technical Specificationssagor sagorNo ratings yet
- A Cook's Journey To Japan - Fish Tales and Rice Paddies 100 Homestyle Recipes From Japanese KitchensDocument306 pagesA Cook's Journey To Japan - Fish Tales and Rice Paddies 100 Homestyle Recipes From Japanese KitchensEthan F.100% (1)
- CFPB Discount Points Guidence PDFDocument3 pagesCFPB Discount Points Guidence PDFdzabranNo ratings yet
- ClistDocument14 pagesClistGuerraNo ratings yet
- Vaccination Schedule in Dogs and CatsDocument3 pagesVaccination Schedule in Dogs and CatsAKASH ANANDNo ratings yet
- Borelog CP.101Document1 pageBorelog CP.101radixkusumaNo ratings yet
- Intentions and Results ASFA and Incarcerated ParentsDocument10 pagesIntentions and Results ASFA and Incarcerated Parentsaflee123No ratings yet
- Cyber Safety PP Presentation For Class 11Document16 pagesCyber Safety PP Presentation For Class 11WAZ CHANNEL100% (1)
- Facts About Concussion and Brain Injury: Where To Get HelpDocument20 pagesFacts About Concussion and Brain Injury: Where To Get HelpJess GracaNo ratings yet
- Geography - Development (Rural - Urban Settlement)Document32 pagesGeography - Development (Rural - Urban Settlement)jasmine le rouxNo ratings yet
- Chi - Square Test: PG Students: DR Amit Gujarathi DR Naresh GillDocument32 pagesChi - Square Test: PG Students: DR Amit Gujarathi DR Naresh GillNaresh GillNo ratings yet
- Gay Costa Del Sol - 2010Document2 pagesGay Costa Del Sol - 2010gayinfospainNo ratings yet
- Cemco T80Document140 pagesCemco T80Eduardo Ariel Bernal100% (3)
- Social Style InventoryDocument12 pagesSocial Style InventoryMaheshwari JaniNo ratings yet
- 7 Fuel Failure in Water Reactors - Causes and MitigationDocument165 pages7 Fuel Failure in Water Reactors - Causes and MitigationLauri RubertiNo ratings yet
- Waste Heat Recovery UnitDocument15 pagesWaste Heat Recovery UnitEDUARDONo ratings yet
- Week5 6 2Document2 pagesWeek5 6 2SAMANIEGO BERMEO DAVID SEBASTIANNo ratings yet