Professional Documents
Culture Documents
Zumdahl and Zumdahl (2003) Chemistry, 6 Ed
Uploaded by
partho143Original Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Zumdahl and Zumdahl (2003) Chemistry, 6 Ed
Uploaded by
partho143Copyright:
Available Formats
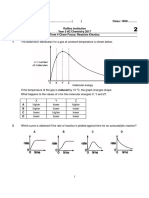
CHM 101 Project #3 Sinex
Name___________________ Reaction Dynamics: The Energetics
Lets consider a simple reaction using the molecules in the box approach and the energy involved in chemical bonds. Are bonds breaking or forming for the boxes below?
The example above illustrates the dissociation of a diatomic molecule into atoms and the process requires energy to be added. energy + Cl2 (g) 2Cl (g) The energy needed to break a mole of Cl-Cl bonds is the bond energy. The bond energies for diatomic halogen molecules with single bonds are given in the table below. Draw a Lewis dot structure of one of the halogens to verify the single bond. Molecules F2 Cl2 Br2 I2 Bond Energy 154 kJ/mole 239 193 149
Zumdahl and Zumdahl (2003) Chemistry, 6th ed.
Which halogen molecule is easiest to dissociate? Why?
If you placed 2.0 moles of fluorine atoms in a container, you would find, after a period of time, 1.0 mole of F2 molecules. Would energy be required or released in this reverse reaction, where bonds are formed? Explain. 1
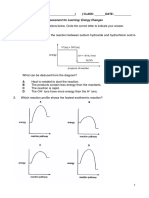
When energy is absorbed in a process or reaction, it is referred to as endothermic process or reaction. While when energy is released, it is an exothermic process or reaction. We can use average bond energies to estimate the amount of energy absorbed or released in a reaction. Process Bond breaking Bond forming Endothermic or exothermic
Is the dimerization of NO2, endothermic or exothermic? Why? 2NO2 (g) N2O4 (g)
+
Draw a Lewis dot structure of N2O4. What type of nitrogen-nitrogen bond forms?
Now lets consider a more involved reaction as given below. With the help of Lewis dot structures, what types of bonds and how many are broken and formed? List them and using your textbook, look up their bond energies. Add up the total energy for the bonds broken and compare to the total for the bonds formed. H2 (g) + Cl2 (g) 2HCl (g) Which side of the reaction (reactants or products) involves the larger amount of energy?
Is this reaction endothermic or exothermic? Why?
Is the synthesis of ammonia from its elements an endothermic or exothermic reaction? With the help of Lewis dot structures, what types of bonds and how many are broken and formed? List them and using your textbook, look up their bond energies. N2 (g) + 3H2 (g) 2NH3 (g)
This is a simple exercise in bookkeeping all the bonds broken and formed. The heat of reaction, H, is calculated by the equation below, where BE is the bond energy.
H = BE (bonds broken) BE (bonds formed)
What is the sign of H for an endothermic reaction? What is the sign of H for an exothermic reaction? Go to http://academic.pgcc.edu/~ssinex/chm101.html and find the Bond Energy Calculator, an interactive Excel spreadsheet that will do the calculations with the bookkeeping input and energies. Remember this is estimating H. Using Lewis dot structures to determine bond type (single, double, or triple) and average bond energies from your textbook, calculate the value of H and determine if the following reactions are endothermic or exothermic. CH4 (g) + 2O2 (g) CO2 (g) + 2H2O (g)
H2O (g)
2H (g) + O (g)
CH4 (g) +
2Cl2 (g) + 2F2 (g) CF2Cl2 (g) + 2HF (g) + 2HCl (g) 3
Explain these two general observations: 1. Acid-base neutralization reactions tend to be exothermic. H+ + OH- H2O
2.
Weak acid dissociation reactions, such as HCN shown below, tend to be endothermic. HCN H+ + CN-
Consider the gas-phase reaction for the addition of H2 to ethene, C2H4, given below. Do all the bonds need to be broken and then formed? Describe the actual bond breaking and forming needed for this reaction. H C C
C2H4 H2C= CH2
H2 H-H
C2H6 H3C- CH3
This reaction is the basis for hydrogenation (adding H2 across double bonds) of vegetable oils (liquids) to form Crisco vegetable shortening and margarines (solids).
You might also like
- Beginners PianoDocument25 pagesBeginners Pianopartho143100% (1)
- Solving SudokuDocument11 pagesSolving SudokuSantos100% (2)
- HMIWebDisplayBuildingGuide EngDocument181 pagesHMIWebDisplayBuildingGuide Engpartho143100% (2)
- CHEMICAL EQUATIONS Final VersionDocument33 pagesCHEMICAL EQUATIONS Final VersionFrancis Kirby BrutasNo ratings yet
- Heat Energy Is Absorbed And: Energy Cannot Be Created or Destroyed But Can Be Converted From One Form To AnotherDocument12 pagesHeat Energy Is Absorbed And: Energy Cannot Be Created or Destroyed But Can Be Converted From One Form To AnotherAeyyjayyNo ratings yet
- 21 Types of Chemical Reactions-SDocument6 pages21 Types of Chemical Reactions-SMichael BensonNo ratings yet
- Enthalpy ChangesDocument17 pagesEnthalpy ChangesDoc_Croc100% (1)
- PCI Board Review HandoutsDocument8 pagesPCI Board Review HandoutsChrissy LayugNo ratings yet
- Stoichiometry 1Document52 pagesStoichiometry 1Mero Miro100% (1)
- Chem 1 Week 3 Chemical Equations CompilerDocument11 pagesChem 1 Week 3 Chemical Equations CompilerMelcorr MontesclarosNo ratings yet
- 023 BP RefiningDocument27 pages023 BP Refiningpartho143No ratings yet
- XRY-1A Oxygen Bomb CalorimeterDocument16 pagesXRY-1A Oxygen Bomb CalorimeterFrank.J83% (6)
- Aakash Study PlannerDocument26 pagesAakash Study PlannerAaditya RavalNo ratings yet
- Electrical Generation and Distribution On Cargo ShipDocument10 pagesElectrical Generation and Distribution On Cargo ShipDavid Ella Inalegwu100% (2)
- Enthalpy Changes NotesDocument20 pagesEnthalpy Changes NotesIGCSE 2k21No ratings yet
- Practice Makes Perfect in Chemistry: Oxidation-ReductionFrom EverandPractice Makes Perfect in Chemistry: Oxidation-ReductionRating: 5 out of 5 stars5/5 (1)
- Guide To OPCDocument9 pagesGuide To OPCpartho143No ratings yet
- Unit 2 - Chemical ReactionsDocument9 pagesUnit 2 - Chemical ReactionsNobukhosi NdlovuNo ratings yet
- CFD Analysis of Flow in After BurnerDocument10 pagesCFD Analysis of Flow in After BurnermortezaastroNo ratings yet
- Notes and Topical Mcqs and Structured Questions From Caie Past PapersDocument15 pagesNotes and Topical Mcqs and Structured Questions From Caie Past PapersHamza KhalidNo ratings yet
- Types of Chemical ReactionsDocument7 pagesTypes of Chemical ReactionsAirene PalerNo ratings yet
- 5.3 Bond Enthalpy: IB SL Chemistry Mrs. Page 2015-2016Document26 pages5.3 Bond Enthalpy: IB SL Chemistry Mrs. Page 2015-2016Patrick AbidraNo ratings yet
- Introduction to Physical Organic Chemistry Thermodynamics and KineticsDocument11 pagesIntroduction to Physical Organic Chemistry Thermodynamics and KineticsAcidri Abdulkarim100% (1)
- Chemistry Form 5 Chapter 1Document19 pagesChemistry Form 5 Chapter 1rhythm_no1No ratings yet
- Energy & Speed of RexDocument22 pagesEnergy & Speed of Rexcook n bakesNo ratings yet
- Breaking and forming bonds determines reaction enthalpyDocument4 pagesBreaking and forming bonds determines reaction enthalpyEdvards GrīgsNo ratings yet
- Section 3 EnergeticsDocument47 pagesSection 3 Energeticsapi-3734333No ratings yet
- Bond EnthalpyDocument10 pagesBond EnthalpyPartha SenguptaNo ratings yet
- AS Chem CH 1.2 Hess LawDocument21 pagesAS Chem CH 1.2 Hess LawRaymond Chan100% (1)
- 2017 Y5 T4 Chem Focus - KineticsDocument4 pages2017 Y5 T4 Chem Focus - KineticsxmxmxmxmxmNo ratings yet
- A Energetics Notes Chem Unit 1 - (New)Document8 pagesA Energetics Notes Chem Unit 1 - (New)Khaila SimmondNo ratings yet
- Chemistry Spring Benchmark ReviewDocument13 pagesChemistry Spring Benchmark ReviewElyse GarciaNo ratings yet
- CHEMICAL REACTIONS Lecture NotesDocument4 pagesCHEMICAL REACTIONS Lecture NotesHarven Lim DinerosNo ratings yet
- Chapter 11: Thermochemistry - Heat and Chemical Changes Part 1 - Notes: Enthalpy and Bond EnergiesDocument11 pagesChapter 11: Thermochemistry - Heat and Chemical Changes Part 1 - Notes: Enthalpy and Bond EnergiesSarthakNo ratings yet
- CHEM1101 Worksheet 10: Enthalpy of Reaction ( ) Model 1: Endothermic and Exothermic ProcessesDocument4 pagesCHEM1101 Worksheet 10: Enthalpy of Reaction ( ) Model 1: Endothermic and Exothermic ProcessesJesus carbonoNo ratings yet
- POGIL Oxidation and Reduction-S-1Document6 pagesPOGIL Oxidation and Reduction-S-1demyeets64No ratings yet
- 16 Heats of Formation - SDocument5 pages16 Heats of Formation - Sapi-313691183100% (1)
- Chemistry 1: Quarter 4: Module 2 Types of Chemical ReactionsDocument3 pagesChemistry 1: Quarter 4: Module 2 Types of Chemical ReactionsRain AlmsNo ratings yet
- How Do We Represent The Chemical Reaction in A Way That Is Convenient and Easy To Understand?Document24 pagesHow Do We Represent The Chemical Reaction in A Way That Is Convenient and Easy To Understand?JeffreyNo ratings yet
- Chapter 5 Lecture NotesDocument64 pagesChapter 5 Lecture NotesLuke FaivreNo ratings yet
- 05 - en Lec 1 Key PointsDocument10 pages05 - en Lec 1 Key Points2022 BALAKRISHNAN ADHITHINo ratings yet
- Bond Energy NotesDocument6 pagesBond Energy NotesErlyn Sapita M. ParreñoNo ratings yet
- Module 018 - ElectrochemistryDocument10 pagesModule 018 - ElectrochemistryLeycoline AlmrenNo ratings yet
- Gen Chem 1 Module 3 Lesson 3Document7 pagesGen Chem 1 Module 3 Lesson 3hjNo ratings yet
- Calculate Energy Changes Using Bond EnthalpiesDocument12 pagesCalculate Energy Changes Using Bond EnthalpiesnivineNo ratings yet
- Answer ALL The Following Questions Below. Circle The Correct Letter To Indicate Your AnswerDocument4 pagesAnswer ALL The Following Questions Below. Circle The Correct Letter To Indicate Your AnswerTimothy HandokoNo ratings yet
- Energetics - CN - STDT4Document2 pagesEnergetics - CN - STDT4NkemziNo ratings yet
- FPISA0 Week 5Document45 pagesFPISA0 Week 5sassy2202018No ratings yet
- Chemical Reactions and HeatDocument37 pagesChemical Reactions and HeatDamir BalmassovNo ratings yet
- Chemical Bond Energies and Reaction Heat ChangesDocument0 pagesChemical Bond Energies and Reaction Heat ChangesAdnan ChowdhuryNo ratings yet
- EnergrticsDocument31 pagesEnergrticsnaeem mushtaqNo ratings yet
- Classification of Chemical ReactionsDocument7 pagesClassification of Chemical Reactionscalew17036No ratings yet
- Presentation 1Document14 pagesPresentation 1patel16122006No ratings yet
- HL SummDocument12 pagesHL SummWilliam AungkhantNo ratings yet
- Chem12 C1102 SWBSDocument5 pagesChem12 C1102 SWBSAhmad asaNo ratings yet
- IbchkineticsDocument16 pagesIbchkineticsapi-293306937No ratings yet
- Redox Reaction Is Related To Gain or Loss of Electrons PDFDocument4 pagesRedox Reaction Is Related To Gain or Loss of Electrons PDFClarence B. MacaraegNo ratings yet
- Engineering Chemistry Complete Lecture in One FileDocument66 pagesEngineering Chemistry Complete Lecture in One Filemeen19111087 KFUEITNo ratings yet
- Chapter 5 - Chemical EnergeticsDocument15 pagesChapter 5 - Chemical EnergeticsAdam BeyNo ratings yet
- Bond Energy Chemistry QuestionsDocument2 pagesBond Energy Chemistry QuestionsSaaid ShafiqueNo ratings yet
- Activity 5 Chemical Reactions and Balancing Chemical Equations IDocument6 pagesActivity 5 Chemical Reactions and Balancing Chemical Equations INivla Genesis100% (2)
- Inter Material Iindyearem Chemistry 04-01-03 Collision Theory and ConceptsDocument7 pagesInter Material Iindyearem Chemistry 04-01-03 Collision Theory and ConceptsAnnampadmaiah AnnamNo ratings yet
- Essential Cell Biology 4e Test BankDocument30 pagesEssential Cell Biology 4e Test Bankmeaganstephensonmdbapgcjfezt100% (45)
- Energy Changes in Chemical Reactions (ECCRDocument10 pagesEnergy Changes in Chemical Reactions (ECCRSamuel LiewNo ratings yet
- Part B Chemical Reactions and Stoichiometry Presentation-2013!10!25-1-Slide-per-pageDocument31 pagesPart B Chemical Reactions and Stoichiometry Presentation-2013!10!25-1-Slide-per-pageBoldie LutwigNo ratings yet
- Grade 10 Chemical ReactionDocument13 pagesGrade 10 Chemical ReactionSheendy Claire BeljotNo ratings yet
- Oes Something When Things Observed To Be Disorder: Learning Package WeekDocument2 pagesOes Something When Things Observed To Be Disorder: Learning Package WeekPatrick Casquejo AndalesNo ratings yet
- Gener AL Chemi Stry 1: Week 3Document11 pagesGener AL Chemi Stry 1: Week 3Faith AsdfNo ratings yet
- Ib Enthalpy KHDocument39 pagesIb Enthalpy KHSamer EhabNo ratings yet
- Project SynopsisDocument2 pagesProject SynopsisdhootshabNo ratings yet
- Space Motion of RocketsDocument14 pagesSpace Motion of Rocketspartho143No ratings yet
- Urinalysis Crystals GuideDocument4 pagesUrinalysis Crystals Guidepartho143No ratings yet
- TI-84CE Graphing CalculatorDocument4 pagesTI-84CE Graphing Calculatorpartho143No ratings yet
- VSFTPDDocument8 pagesVSFTPDjoshua551No ratings yet
- 2m Current ElectricityDocument20 pages2m Current ElectricityRohitt MathurNo ratings yet
- Bootable UsbDocument7 pagesBootable Usbapi-3810182100% (1)
- 1m ELECTROSTATICS PDFDocument82 pages1m ELECTROSTATICS PDFKeshav JoshiNo ratings yet
- How To Find Security HolesDocument7 pagesHow To Find Security Holesapi-3714226No ratings yet
- C General Chemistry PDFDocument3 pagesC General Chemistry PDFpartho143No ratings yet
- Managing Change in Oil Pipeline InfrastructureDocument2 pagesManaging Change in Oil Pipeline Infrastructurepartho143No ratings yet
- 04 SET MagnetismDocument5 pages04 SET Magnetismpartho143No ratings yet
- Form Object (Access)Document11 pagesForm Object (Access)partho143No ratings yet
- Ten Commandments of AccessDocument1 pageTen Commandments of Accesspartho143No ratings yet
- Job Application (Includes Both)Document3 pagesJob Application (Includes Both)partho143No ratings yet
- Add or Remove A Macro From CodeDocument2 pagesAdd or Remove A Macro From Codepartho143No ratings yet
- 01 ArrayDocument17 pages01 Arraypartho143No ratings yet
- Upload and Read Excel File in ASPDocument2 pagesUpload and Read Excel File in ASPpartho143No ratings yet
- Mind Map RubricDocument1 pageMind Map Rubricpartho143No ratings yet
- Class11 - Digestion and Absorption Assignment (2018-2019)Document1 pageClass11 - Digestion and Absorption Assignment (2018-2019)partho143No ratings yet
- Add, Edit, Delete and Run Access Queries With VBADocument5 pagesAdd, Edit, Delete and Run Access Queries With VBApartho143No ratings yet
- Textarea HTML TagDocument1 pageTextarea HTML Tagpartho143No ratings yet
- NetBIOS HackingDocument10 pagesNetBIOS Hackingapi-3748467100% (1)
- Manual J Heat Load InstructionsDocument2 pagesManual J Heat Load Instructionspartho143No ratings yet
- 23 ProjectFinalDocument68 pages23 ProjectFinalpartho143No ratings yet
- Insulation Resistance Testing: Application NoteDocument8 pagesInsulation Resistance Testing: Application Notefernando_aragon2001No ratings yet
- Chapter 7 - The Superposition of Waves PDFDocument20 pagesChapter 7 - The Superposition of Waves PDFCcA BcggNo ratings yet
- SOLA2060 Introduction To Electronic Devices Semester 1, 2019Document43 pagesSOLA2060 Introduction To Electronic Devices Semester 1, 2019Marquee BrandNo ratings yet
- Material Engineering in CompositesDocument31 pagesMaterial Engineering in CompositesCaryl Alvarado SilangNo ratings yet
- Heiser - Temperature Charts For Induction and Constant Temperature HeatingDocument10 pagesHeiser - Temperature Charts For Induction and Constant Temperature Heatingsharkdude1134No ratings yet
- CRT Monitor PhysicsDocument2 pagesCRT Monitor PhysicsAbdullionNo ratings yet
- University of Cambridge International Examinations General Certificate of Education Advanced LevelDocument4 pagesUniversity of Cambridge International Examinations General Certificate of Education Advanced LevelHubbak KhanNo ratings yet
- Conceptual Physical Science: 5 EditionDocument65 pagesConceptual Physical Science: 5 EditionAldin HernandezNo ratings yet
- Crystal GrowthDocument6 pagesCrystal GrowthArunmaalaNo ratings yet
- PRELIM EXAM SOLUTIONSDocument8 pagesPRELIM EXAM SOLUTIONSamielynNo ratings yet
- TRANSCRIPTDocument3 pagesTRANSCRIPTCepi KusdianaNo ratings yet
- Maglev Wind Mill ReportDocument24 pagesMaglev Wind Mill ReportBalu MahendarNo ratings yet
- Hydraulics Practice Quiz #1Document2 pagesHydraulics Practice Quiz #1Matthew MlacedaNo ratings yet
- Career Endeavour Test Series1Document13 pagesCareer Endeavour Test Series1Gugan Raj100% (1)
- Thermodynamic Principles and Ideal Gas FunctionsDocument17 pagesThermodynamic Principles and Ideal Gas FunctionsLê Xuân NamNo ratings yet
- Chapter 6 ACKulkarniDocument43 pagesChapter 6 ACKulkarnipurijatinNo ratings yet
- AP Chemistry Syllabus 2014 2015Document7 pagesAP Chemistry Syllabus 2014 2015Basel OsmanNo ratings yet
- M2x2 2179enDocument8 pagesM2x2 2179enSerge RinaudoNo ratings yet
- 3.A - The First Law of Thermodynamics (Answer) - Physics LibreTextsDocument5 pages3.A - The First Law of Thermodynamics (Answer) - Physics LibreTextsFASIKAW GASHAWNo ratings yet
- Photoluminescence and Photocatalytic Activity of Spin Coated Ag+ Doped Anatase TiO2 Thin FilmsDocument14 pagesPhotoluminescence and Photocatalytic Activity of Spin Coated Ag+ Doped Anatase TiO2 Thin FilmsJasielRuizDesalesNo ratings yet
- fle, Tii,:v,' Jy ' TR Ijf, Q D, 4cto'r:.,ma, Ri:al' :1j::"""Document4 pagesfle, Tii,:v,' Jy ' TR Ijf, Q D, 4cto'r:.,ma, Ri:al' :1j::"""Ovidijus RučinskasNo ratings yet
- What Is Tertiary WindingDocument3 pagesWhat Is Tertiary Windingchirag2011No ratings yet
- Ruta Al Caos PDFDocument33 pagesRuta Al Caos PDFDiego VilchesNo ratings yet
- Ph501 Electrodynamics Problem Set 6: Princeton UniversityDocument46 pagesPh501 Electrodynamics Problem Set 6: Princeton UniversityEdgar RamirezNo ratings yet
- NMR Spectroscopy Integrals and MultiplicityDocument6 pagesNMR Spectroscopy Integrals and MultiplicitysupriyoNo ratings yet
- 05 - Beams and FramesDocument10 pages05 - Beams and FramesOn Fan ChowNo ratings yet