Professional Documents
Culture Documents
Error in Adjustment
Uploaded by
Oasica NawziOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Error in Adjustment
Uploaded by
Oasica NawziCopyright:
Available Formats
Reading: Analysis of Errors
Revised 3/6/12
ANALYSIS OF ERRORS
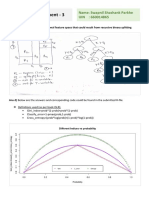
Precision and Accuracy Two terms are commonly associated with any discussion of error: "precision" and "accuracy". Precision refers to the reproducibility of a measurement while accuracy is a measure of the closeness to true value. The concepts of precision and accuracy are demonstrated by the series of targets below. If the center of the target is the "true value", then A is neither precise nor accurate. Target B is very precise (reproducible) but not accurate. The average of target C's marks give an accurate result but precision is poor. Target D demonstrates both precision and accuracy - which is the goal in lab.
All experiments, no matter how meticulously planned and executed, have some degree of error or uncertainty. In general chemistry lab, you should learn how to identify, correct, or evaluate sources of error in an experiment and how to express the accuracy and precision of measurements when collecting data or reporting results.
Errors Three general types of errors occur in lab measurements: random error, systematic error, and gross errors. Random (or indeterminate) errors are caused by uncontrollable fluctuations in variables that affect experimental results. For example, air fluctuations occurring as students open and close lab doors cause changes in pressure readings. A sufficient number of measurements result in evenly distributed data scattered around an average value or mean. This positive and negative 1
Reading: Analysis of Errors
Revised 3/6/12
scattering of data is characteristic of random errors. The estimated standard deviation (the error range for a data set) is often reported with measurements because random errors are difficult to eliminate. Also, a "best-fit line" is drawn through graphed data in order to "smooth out" random error. Systematic (or determinate) errors are instrumental, methodological, or personal mistakes causing "lopsided" data, which is consistently deviated in one direction from the true value. Examples of systematic errors: an instrumental error results when a spectrometer drifts away from calibrated settings; a methodological error is created by using the wrong indicator for an acid-base titration; and, a personal error occurs when an experimenter records only even numbers for the last digit of buret volumes. Systematic errors can be identified and eliminated after careful inspection of the experimental methods, cross-calibration of instruments, or examination of techniques. Gross errors are caused by experimenter carelessness or equipment failure. These "outliers" are so far above or below the true value that they are usually discarded when assessing data. The "Q-Test" (discussed later) is a systematic way to determine if a data point should be discarded.
Precision of a Set of Measurements A data set of repetitive measurements is often expressed as a single representative number called the mean or average. The mean ( x ) is the sum of individual measurements (xi) divided by the number of measurements (N). (1)
x =
xi
N
(mean)
Precision (reproducibility) is indicated by the deviation from the mean ( x xi), the difference between the individual experimental value and the mean value. The average deviation ( x ) is used when a data set contains less than 5 repetitive measurements. Widely scattered data results in a large average deviation indicating poor precision. A small average deviation indicates data points clustered closely around the mean and good precision.
Reading: Analysis of Errors
Revised 3/6/12
(2)
(x) = +
| x xi|
N
(average deviation)
The relative average deviation is the average deviation divided by the average and then expressed as a percentage: ( x )/ x * 100. For data sets with more than 5 measurements, the estimated standard deviation (s), is used to express the precision of the measurements. The number of degrees of freedom (N1) is the total number of measurements minus one. Estimated standard deviation is used frequently in lab when students pool their data. (3) s =
(xi x )2
N1
(estimated standard deviation)
Example: Student A recorded the volume of a gas at 1.0 atm and 23C in experiments 1-4. Student B recorded the volume of a gas at 1.0 atm and 23C in experiments 5-8. Student A Student B Expt # Volume (L) Expt # Volume (L) 1 2 3 4 26.05 26.18 26.30 26.20 5 6 7 8 26.02 26.27 26.17 26.22
Reading: Analysis of Errors Precision of Student As Data: Mean Volume: v = 26.05 + 26.18 + 26.30 + 26.20 = 26.18 Liters 4
Revised 3/6/12
Average Deviation: ( x ) = 0.13 + 0.00 + 0.12 + 0.02 = 0.068 4 (Note: For the average deviation the absolute value of (vi v ) is used.) Relative Average Deviation: %( x ) = 0.068/26.18 L x 100 = 0.26% Precision of All Data: Mean Volume: v = 26.05 + 26.18 + 26.30 + 26.20 + 26.02 + 26.27 + 26.17 + 26.22 = 26.18 L 8 Estimated Standard Deviation: s =
0.0169 + 0.000 + 0.0144 + 0.0004 +0.0256 + 0.0081 + 0.0001 + 0.0016 = +0.10 81
Note the difference between the average deviation used for the small set of data and the estimated standard deviation used for the larger set of data.
Error Propagation When performing a math operation using measurements with standard deviations, the magnitude of the resultant standard deviation is usually increased. In other words, the error is "propagated". The following examples demonstrate error propagation for addition/subtraction and multiplication/division. To calculate the resultant standard deviation use the formulas below where A, B, and C represent experimental measurements and a, b, and c are the respective standard deviations for each measurement:
Reading: Analysis of Errors
Revised 3/6/12
(4)
(A a) (B b) (C c) = (A B C)
a2 + b2 + c2
(addition / subtraction) Example:
(2.0 0.2) (1.0 0.1) + (3.0 0.3) = 4.0 ? = (2.0 1.0 + 3.0)
(5)
0.22 + 0.12 +0.32 = 4.0 0.4
(A a) x (B b) (C c)
AB C
AB C
b 2 a 2 c + + B A C
(multiplication / division) Example:
(2.0 0.2) x (1.0 0.1) (3.0 0.3)
2.0 x 1.0 2.0 x 1.0 3.0 3.0
2.0 x 1.0 ? 3.0
2
0.2 2 0.1 2 0.3 + + 2.0 1.0 3.0
= 0.67 0.12
Accuracy of a Result The accuracy of a result can be quantified by calculating the percent error. The percent error can only be found if the true value is known. Although the percent error is usually expressed as an absolute value, it can be given a negative or positive sign if the researcher wishes to indicate the direction of error from true value. (6)
% Error = (true value - experimental value) x 100 true value
(percent error)
Reading: Analysis of Errors
Revised 3/6/12
Assume the true value for the gas volume was 26.04 liters in the previous example. Then the error in the measurements is 0.54%
% Error = (26.04 - 26.18) x 100 = 0.54% 26.04
The Q-Test for Rejecting Data As mentioned previously, "outliers" are data measurements occurring from gross errors. Their value deviates significantly from the mean. The Q-Test can be used to determine whether an individual measurement should be rejected or retained. The quantity Q is the absolute difference between the questioned measurement (xq) and the next closest measurement (xn) divided by the spread (), the difference between the largest and smallest measurement, of the entire set of data. (7)
Q =
(xq xn)
If Q is greater than the values listed below for a particular confidence level, the measurement should be rejected. If Q is less than the values in Table 1, the measurement should be retained. (Note: A "confidence level" is the percent probability a measurement will fall into a range around the mean (x).) The Q-Test for Rejecting Data Confidence Levels 90% 96% 0.94 0.98 0.76 0.85 0.64 0.73 0.56 0.64 0.51 0.59 0.47 0.54 0.44 0.51 0.41 0.48
Number of Observations 3 4 5 6 7 8 9 >9
99% 0.99 0.93 0.82 0.74 0.68 0.63 0.60 0.57
Assume the student measuring the gas volumes in the previous example collected a ninth measurement equal to 27.58 liters. The Q-value for this measurement is 0.82.
Reading: Analysis of Errors Q-value:
Q = 27.58 26.30 27.58 26.05 = 0.82
Revised 3/6/12
The limiting value is 0.60 for rejecting data at the 99% confidence level for 9 measurements. Since 0.82 is greater than 0.60, the student should reject the measurement. * A shortcut can be used to test a suspect measurement: Calculate the mean and average or standarad deviation without using the suspect measurement and reject the suspect measurement if its deviation from the mean is greater than four times the average or standard deviation. Example: The mean and standard deviation of the original eight gas volume measurements
is 26.18 0.10. Multiplying 0.10 by 4 gives 0.40. The deviation of the suspect measurement is 27.58 26.18 = 1.40 which is much greater than 0.40. Again, the student should reject the measurement. Although a set of measurements may have very small standard deviations (high precision), the calculated results can be inaccurate because of systematic error. At the same time, experimental measurements with large standard deviations (poor precision) from random errors can still give an averaged result close to true value. A good experimenter sets his goals on both precision and accuracy when making measurements. Instructor Reviewed Information on the Web: Please report any links that are no longer active. Measurement Good Practice Guide: A Beginners Guide to Uncertainty of Measurement: http://www.wmo.int/pages/prog/gcos/documents/gruanmanuals/UK_NPL/mgpg11.pdf
You might also like
- Error in MeasurementDocument8 pagesError in Measurementvipsdgr8No ratings yet
- Testing of Survey Network AdjustmentsDocument73 pagesTesting of Survey Network AdjustmentsGiora RozmarinNo ratings yet
- CHM 421 - ToPIC 3 - StatisticsDocument58 pagesCHM 421 - ToPIC 3 - StatisticsthemfyNo ratings yet
- Chen10011 NotesDocument58 pagesChen10011 NotesTalha TanweerNo ratings yet
- ERROR ANALYSIS Lab Precision & AccuracyDocument3 pagesERROR ANALYSIS Lab Precision & AccuracyMourougapragash SubramanianNo ratings yet
- Exp1 Measurements and Uncertainty Phy102LDocument11 pagesExp1 Measurements and Uncertainty Phy102LMohammad TahirNo ratings yet
- Evaluation of Analytical DataDocument58 pagesEvaluation of Analytical DataJoyce Mariele RomeroNo ratings yet
- ANALYSIS OF EXPERIMENTAL UNCERTAINTIESDocument5 pagesANALYSIS OF EXPERIMENTAL UNCERTAINTIESghera_gheraNo ratings yet
- Stat QuizDocument79 pagesStat QuizJanice BulaoNo ratings yet
- Basic Statistics Concepts for Environmental Data AnalysisDocument7 pagesBasic Statistics Concepts for Environmental Data AnalysisAsini Gavinya SirinimalNo ratings yet
- Error UncertaintyDocument6 pagesError UncertaintyAdron LimNo ratings yet
- Lecture 2Document26 pagesLecture 2Moamen MohamedNo ratings yet
- Statistics For Analytical ChemistryDocument49 pagesStatistics For Analytical ChemistryDragana Srdic100% (2)
- Basic Statistical ToolsDocument43 pagesBasic Statistical ToolsHaytham Janoub HamoudaNo ratings yet
- 6 Basic Statistical ToolsDocument30 pages6 Basic Statistical ToolsRose Ann MendigorinNo ratings yet
- Measurement and Data Processing: I. Uncertainties and Errors in Measurement and ResultsDocument6 pagesMeasurement and Data Processing: I. Uncertainties and Errors in Measurement and ResultsKip 2No ratings yet
- Statistics: Error (Chpt. 5)Document16 pagesStatistics: Error (Chpt. 5)Amit SahaNo ratings yet
- HerramientasBasicas FAODocument86 pagesHerramientasBasicas FAODenys Rivera GuevaraNo ratings yet
- 2.1 Data AnalysisDocument8 pages2.1 Data AnalysisLei YinNo ratings yet
- Section 03: Data Handling and Spreadsheets in Analytical ChemistryDocument45 pagesSection 03: Data Handling and Spreadsheets in Analytical ChemistryUMAIR ASHFAQNo ratings yet
- Chauvenet CriterionDocument2 pagesChauvenet CriterionBhavesh ChauhanNo ratings yet
- BSChem-Statistics in Chemical Analysis PDFDocument6 pagesBSChem-Statistics in Chemical Analysis PDFKENT BENEDICT PERALESNo ratings yet
- HerramientasBasicas FAODocument86 pagesHerramientasBasicas FAOVíctor A. Huamaní LeónNo ratings yet
- Instructions for Error Analysis LabDocument21 pagesInstructions for Error Analysis LabM Arslan SaeedNo ratings yet
- Chapter 2Document57 pagesChapter 2WANNo ratings yet
- Chem 321 Lecture 6 - Calibration Methods: Student Learning ObjectivesDocument8 pagesChem 321 Lecture 6 - Calibration Methods: Student Learning ObjectivesCelrose FernandezNo ratings yet
- Instrument and Measurement: PresentationDocument22 pagesInstrument and Measurement: PresentationHopedejene DejeneNo ratings yet
- Error Types and Error PropagationDocument6 pagesError Types and Error PropagationDerioUnboundNo ratings yet
- Uncertainty and Error PropagationDocument12 pagesUncertainty and Error PropagationSamantha Marie RebolledoNo ratings yet
- Chapter 02Document40 pagesChapter 02徐郁真No ratings yet
- Statistical Analysis of Weight Variation in Coin SamplesDocument8 pagesStatistical Analysis of Weight Variation in Coin Sampleskristiaa_1No ratings yet
- Experiment 1 - Mass, Volume and GraphingDocument10 pagesExperiment 1 - Mass, Volume and GraphingThina Cruz Torres0% (1)
- 6 Basic Statistical Tools: There Are Lies, Damn Lies, and Statistics...... (Anon.)Document44 pages6 Basic Statistical Tools: There Are Lies, Damn Lies, and Statistics...... (Anon.)Bhagwati ShuklaNo ratings yet
- L-10 (SS) (Ia&c) ( (Ee) Nptel)Document12 pagesL-10 (SS) (Ia&c) ( (Ee) Nptel)Abdelraheem S. AlkuorNo ratings yet
- Lec Set 1 Data AnalysisDocument55 pagesLec Set 1 Data AnalysisSmarika KulshresthaNo ratings yet
- Statistical Approach in HematologyDocument33 pagesStatistical Approach in HematologycandiddreamsNo ratings yet
- Error and Uncertainty: General Statistical PrinciplesDocument8 pagesError and Uncertainty: General Statistical Principlesdéborah_rosalesNo ratings yet
- Ch.5 Errors During The Measuremen T ProcessDocument82 pagesCh.5 Errors During The Measuremen T ProcessD7ooM_612No ratings yet
- Unit 2-Evaluating Analytical DataDocument21 pagesUnit 2-Evaluating Analytical DataKhánh Vy NguyênNo ratings yet
- PHYS1815 ExperimentI Fall2014 En2Document19 pagesPHYS1815 ExperimentI Fall2014 En2BuddahManNo ratings yet
- 02 - ErrorsDocument31 pages02 - ErrorsRenzhel Mae CabalunaNo ratings yet
- Guide 6 basic toolsDocument30 pagesGuide 6 basic toolsdrs_mdu48No ratings yet
- Data Handling, Statistic and ErrorsDocument38 pagesData Handling, Statistic and ErrorsWanIntanNadiah67% (3)
- Errors ExperimentalDocument10 pagesErrors ExperimentaljoesuhreNo ratings yet
- Analytical ChemistryDocument16 pagesAnalytical ChemistryS JNo ratings yet
- chm202 Statistics PDFDocument24 pageschm202 Statistics PDFMohammad MouallemNo ratings yet
- WelcomeDocument19 pagesWelcomeSharath sharuNo ratings yet
- Improving Calibration Accuracy in Environmental LabsDocument7 pagesImproving Calibration Accuracy in Environmental LabssocoladenNo ratings yet
- Introduction To Error TheoryDocument10 pagesIntroduction To Error TheoryTrần HuyNo ratings yet
- CTEC233: Statistical Analysis of Measurement UncertaintiesDocument8 pagesCTEC233: Statistical Analysis of Measurement UncertaintiesjahmanNo ratings yet
- Harris' Chapter 4: StatisticsDocument22 pagesHarris' Chapter 4: StatisticskarolinebritoNo ratings yet
- Means and Standard Deviation (6)Document8 pagesMeans and Standard Deviation (6)Tishonna DouglasNo ratings yet
- Fizik LabDocument25 pagesFizik LabshamurniNo ratings yet
- Practical SkillsDocument35 pagesPractical SkillsNatasha GhoseNo ratings yet
- Practical Engineering, Process, and Reliability StatisticsFrom EverandPractical Engineering, Process, and Reliability StatisticsNo ratings yet
- Sample Size for Analytical Surveys, Using a Pretest-Posttest-Comparison-Group DesignFrom EverandSample Size for Analytical Surveys, Using a Pretest-Posttest-Comparison-Group DesignNo ratings yet
- This is The Statistics Handbook your Professor Doesn't Want you to See. So Easy, it's Practically Cheating...From EverandThis is The Statistics Handbook your Professor Doesn't Want you to See. So Easy, it's Practically Cheating...Rating: 4.5 out of 5 stars4.5/5 (6)
- MTH221 Probability & StatisticsDocument3 pagesMTH221 Probability & Statisticsraghav dhamaniNo ratings yet
- Forecasting For Economics and Business 1st Edition Gloria Gonzalez Rivera Solutions ManualDocument36 pagesForecasting For Economics and Business 1st Edition Gloria Gonzalez Rivera Solutions Manualbrownismkanacka9oir100% (26)
- AbstractDocument1 pageAbstractmonkeybike88No ratings yet
- Unit1 Dav PDFDocument166 pagesUnit1 Dav PDFRutvi DhameliyaNo ratings yet
- Chap 8 Multiple Regression Analysis The Problem of InferenceDocument79 pagesChap 8 Multiple Regression Analysis The Problem of InferenceSamina AhmeddinNo ratings yet
- Frequency Distributions and Graphs2Document8 pagesFrequency Distributions and Graphs2Ron InaganNo ratings yet
- Exercises 111Document19 pagesExercises 111LostorageAceNo ratings yet
- Stats Answer KeyDocument10 pagesStats Answer KeyJrlyn DaneNo ratings yet
- NTC Power Point TemplateDocument13 pagesNTC Power Point TemplateKevin EspirituNo ratings yet
- Random Process Fundamentals for Engineering StatsDocument29 pagesRandom Process Fundamentals for Engineering StatsGita PatilNo ratings yet
- Lecture 5 PDFDocument14 pagesLecture 5 PDFQuoc KhanhNo ratings yet
- Probability Theory: Sargur N. Srihari Srihari@cedar - Buffalo.eduDocument49 pagesProbability Theory: Sargur N. Srihari Srihari@cedar - Buffalo.eduasdfasdffdsaNo ratings yet
- Random WalkDocument8 pagesRandom WalkMeral OcakNo ratings yet
- 2015 HCI Prelim Paper 2 SolutionsDocument13 pages2015 HCI Prelim Paper 2 SolutionsnasyrahNo ratings yet
- Introduction To The Practice of Statistics: Section 6.1 Homework AnswersDocument3 pagesIntroduction To The Practice of Statistics: Section 6.1 Homework AnswersTuy AnNo ratings yet
- Roberson Et Al. (2007) Does The Measure of Dispersion Matter in Multilevel ResearchDocument25 pagesRoberson Et Al. (2007) Does The Measure of Dispersion Matter in Multilevel ResearchCrónicasNo ratings yet
- Chapter 08 - QuizDocument74 pagesChapter 08 - Quizhp50875% (4)
- ID Faktor Faktor Yang Berkontribusi Terhadap Perilaku Politik Dan Hasil Kerja KaryaDocument15 pagesID Faktor Faktor Yang Berkontribusi Terhadap Perilaku Politik Dan Hasil Kerja KaryaTri SupraptiNo ratings yet
- Probability Distributions: X X X X X XDocument14 pagesProbability Distributions: X X X X X XIshy HereNo ratings yet
- Lumpers vs. Splitters Intelligence in Children With Specific Learning DisordersDocument10 pagesLumpers vs. Splitters Intelligence in Children With Specific Learning DisordersSridewi Murni AgriyantiNo ratings yet
- Probability: Very Short Answer Type Questions (1 Mark)Document7 pagesProbability: Very Short Answer Type Questions (1 Mark)Jukie ChanNo ratings yet
- Presentation RegressionDocument12 pagesPresentation RegressionSachin GadhaveNo ratings yet
- Cheat Sheet PSMDocument3 pagesCheat Sheet PSMZundaNo ratings yet
- Data SPSS Kak Ela Persen InhibisiDocument11 pagesData SPSS Kak Ela Persen InhibisiAgustia AmlizaNo ratings yet
- AML Winter 2021 SolutionDocument6 pagesAML Winter 2021 SolutionAkshit PrajapatiNo ratings yet
- Section13 PDFDocument7 pagesSection13 PDFMASHIAT MUTMAINNAHNo ratings yet
- Markov Chain and Markov ProcessesDocument9 pagesMarkov Chain and Markov Processesdeary omarNo ratings yet
- STA 122 Instruction: Answer 10 Questions From Each of The Five Sections Time Allowed: 40 MinutesDocument40 pagesSTA 122 Instruction: Answer 10 Questions From Each of The Five Sections Time Allowed: 40 MinutesdamiNo ratings yet
- Asif Hanif: Analysis With Chi-SquareDocument14 pagesAsif Hanif: Analysis With Chi-SquareAsad ChaudharyNo ratings yet
- IDS 575 Assignment - 3: Name: Swapnil Shashank Parkhe UIN: 660014865Document7 pagesIDS 575 Assignment - 3: Name: Swapnil Shashank Parkhe UIN: 660014865Swapnil ParkheNo ratings yet