Professional Documents
Culture Documents
Contents of Supplement 78
Uploaded by
Ghali_the_monsterOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Contents of Supplement 78
Uploaded by
Ghali_the_monsterCopyright:
Available Formats
EUROPEAN PHARMACOPOEIA 7.
Contents of Supplement 7.8
CONTENTS OF SUPPLEMENT 7.8
A vertical line in the margin indicates where part of a text has been revised or corrected. A horizontal line in the margin indicates where part of a text has been deleted. However, these indications, which are not necessarily exhaustive, are given for information and do not form an official part of the texts. Editorial changes are not indicated. Individual copies of texts will not be supplied. Subscribers to the current version (printed or electronic) of the European Pharmacopoeia have access to an archive version of all previous editions of the European Pharmacopoeia.
NEW TEXTS
The texts below appear for the first time in the European Pharmacopoeia. They will be implemented on 1 July 2013 at the latest. GENERAL CHAPTERS 2.6.33. Residual pertussis toxin and irreversibility of pertussis toxoid MONOGRAPHS Radiopharmaceutical preparations and starting materials for radiopharmaceutical preparations Gallium (68Ga) chloride solution for radiolabelling (2464) Herbal drugs and herbal drug preparations Blackcurrant leaf (2528) Monographs Atovaquone (2192) Oxcarbazepine (2577) Starch, hydroxypropyl, pregelatinised (2645)
REVISED TEXTS
The texts below have been technically revised since their last publication. They will be implemented on 1 July 2013. GENERAL CHAPTERS 2.2.20. Potentiometric titration Monographs Alteplase for injection (1170) Anti-T lymphocyte immunoglobulin for human use, animal 4. Reagents (new, revised, corrected) (1928) Betahistine mesilate (1071) MONOGRAPHS Bromocriptine mesilate (0596) Vaccines for human use Cellulose acetate butyrate (1406) Diphtheria, tetanus and pertussis (acellular, component) vaccine (adsorbed) (1931) Chlorhexidine digluconate solution (0658) Diphtheria, tetanus, pertussis (acellular, component) and Codergocrine mesilate (2060) haemophilus type b conjugate vaccine (adsorbed) (1932) Deferoxamine mesilate (0896) Diphtheria, tetanus, pertussis (acellular, component) and Dihydroergocristine mesilate (1416) hepatitis B (rDNA) vaccine (adsorbed) (1933) Dihydroergotamine mesilate (0551) Diphtheria, tetanus, pertussis (acellular, component) and Dimeticone (0138) poliomyelitis (inactivated) vaccine (adsorbed) (1934) Diphtheria, tetanus, pertussis (acellular, component) and Docusate sodium (1418) poliomyelitis (inactivated) vaccine (adsorbed, reduced antigen(s) Doxazosin mesilate (2125) content) (2329) Glucagon, human (1635) Diphtheria, tetanus, pertussis (acellular, component), hepatitis B Haemofiltration and haemodiafiltration, solutions for (0861) (rDNA), poliomyelitis (inactivated) and haemophilus type b conjugate vaccine (adsorbed) (2067) Human coagulation factor VIII (0275) Diphtheria, tetanus, pertussis (acellular, component), Human coagulation factor XI (1644) poliomyelitis (inactivated) and haemophilus type b conjugate Human plasma (pooled and treated for virus inactivation) (1646) vaccine (adsorbed) (2065) Human von Willebrand factor (2298) Pertussis vaccine (acellular, component, adsorbed) (1356) Hydroxypropylbetadex (1804) Pertussis vaccine (acellular, co-purified, adsorbed) (1595) Isoleucine (0770) Herbal drugs and herbal drug preparations Isoprenaline hydrochloride (1332) Bearberry leaf (1054) Leucine (0771) Buckwheat herb (2184) Lysine hydrochloride (0930) Digitalis leaf (0117) Magaldrate (1539) Juniper (1532) Molsidomine (1701) Juniper oil (1832) Nimesulide (1548) Knotgrass (1885) Pefloxacin mesilate dihydrate (1460) St. Johns wort (1438) xliii
Contents of Supplement 7.8
EUROPEAN PHARMACOPOEIA 7.8
Pergolide mesilate (1555) Phentolamine mesilate (1138) Poly(vinyl acetate) (1962) Poly(vinyl alcohol) (1961)
Rocuronium bromide (1764) Saquinavir mesilate (2267) Tamoxifen citrate (1046) Vecuronium bromide (1769)
CORRECTED TEXTS
The texts below have been corrected and are republished in their entirety. These corrections are to be taken into account from the publication date of Supplement 7.8 (1 January 2013). GENERAL CHAPTERS 5.8. Pharmacopoeial harmonisation MONOGRAPHS Vaccines for veterinary use Equine influenza vaccine (inactivated) (0249) Herbal drugs and herbal drug preparations Long pepper (2453) Orientvine stem (2450) Pepper (2477) Monographs Bleomycin sulfate (0976) Carprofen for veterinary use (2201) Chlortetracycline hydrochloride (0173)
DELETED TEXTS
The following text is deleted as of 1 April 2013. MONOGRAPHS Monographs Diflunisal (0818)
The following text is deleted as of 1 January 2013. MONOGRAPHS Monographs Protamine hydrochloride (0686)
The following texts are deleted as of 1 July 2012. MONOGRAPHS Monographs Chlorothiazide (0385) Dienestrol (0483) Emetine hydrochloride heptahydrate (0080) Etofylline (0492) Hexobarbital (0183) Histamine phosphate (0144) Iotalamic acid (0751) Methaqualone (0510) Methylatropine bromide (0511) Methylatropine nitrate (0512) Physostigmine sulfate (0684) Succinylsulfathiazole (0357) Sulfisomidine (0639) Tubocurarine chloride (0305)
The following text is deleted as of 1 April 2012. MONOGRAPHS Monographs Benfluorex hydrochloride (1601) xliv
You might also like
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5795)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1091)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Downfall of The German Shepherd: by Koos HassingDocument15 pagesThe Downfall of The German Shepherd: by Koos HassingMustafa Ehlizevak100% (1)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- How To Raise Coturnix QuailDocument45 pagesHow To Raise Coturnix QuailWally Roderick100% (2)
- Immunology of Transplant RejectionDocument8 pagesImmunology of Transplant Rejectionxplaind100% (1)
- Guruji 2Document176 pagesGuruji 2Cine Dada67% (6)
- TB Dots Ri Dan RJ Jan - Juli 2020Document6 pagesTB Dots Ri Dan RJ Jan - Juli 2020Dina MariyanaNo ratings yet
- Kuliah Colon DDS Reguler-Ganjil 2010 INDDocument125 pagesKuliah Colon DDS Reguler-Ganjil 2010 INDYartiSulistiaNingratNo ratings yet
- Aids Campaign ProposalDocument10 pagesAids Campaign Proposalapi-252337160No ratings yet
- Newsletter v6n1Document88 pagesNewsletter v6n1Transverse Myelitis AssociationNo ratings yet
- Grudgitis BMHegde PDFDocument2 pagesGrudgitis BMHegde PDFsunilreddymNo ratings yet
- Management of Conflict Species: LU3 - Wildlife Management in ParksDocument17 pagesManagement of Conflict Species: LU3 - Wildlife Management in ParksNeehaNo ratings yet
- Cockerspaniel Pra CarriersDocument28 pagesCockerspaniel Pra CarriersJo M. ChangNo ratings yet
- Classification of Angle Class III MalocclusionDocument11 pagesClassification of Angle Class III MalocclusionAnda TarasciucNo ratings yet
- Manual para La Utilización de Pluma para Estímulos Eléctricos.Document26 pagesManual para La Utilización de Pluma para Estímulos Eléctricos.carlosbuzo57No ratings yet
- Cell Structure and OrganizationDocument18 pagesCell Structure and OrganizationMuhammad SamhanNo ratings yet
- OB HandoutsDocument16 pagesOB HandoutsericNo ratings yet
- The Cantilever Fixed Partial Denture-A Literature Review: Forces and Stress DistributionDocument4 pagesThe Cantilever Fixed Partial Denture-A Literature Review: Forces and Stress DistributionMile RojanoNo ratings yet
- Subjective: Sto: DX: Fully Met: TheDocument2 pagesSubjective: Sto: DX: Fully Met: TheSoniaMarieBalanayNo ratings yet
- Planeshifted Guide To Innistrad - GM BinderDocument32 pagesPlaneshifted Guide To Innistrad - GM BinderGuide AstralNo ratings yet
- Jose R. Reyes Memorial Medical Center Department of Surgery: Clinical DatabaseDocument2 pagesJose R. Reyes Memorial Medical Center Department of Surgery: Clinical DatabaseMary Rose DomalantaNo ratings yet
- IGCSE-Revision-Booklet-Part-1-2018-2019 - (New-Spec)Document69 pagesIGCSE-Revision-Booklet-Part-1-2018-2019 - (New-Spec)MaryamNo ratings yet
- 160 174+Pendampingan+Asuhan+Keperawatan+Medikal+Bedah+Pada+Pasien+Dengan+Gangguan+Sistem+Integument+ (Snake+Bite) +Di+Ruang+Anggrek+RSUD+BanjarDocument15 pages160 174+Pendampingan+Asuhan+Keperawatan+Medikal+Bedah+Pada+Pasien+Dengan+Gangguan+Sistem+Integument+ (Snake+Bite) +Di+Ruang+Anggrek+RSUD+BanjarRafli TarNo ratings yet
- A Body, A Dog and A Fistful of ScatsDocument4 pagesA Body, A Dog and A Fistful of ScatsNoelle SpitzNo ratings yet
- Ygeia Medical Center - Manila, PhilippinesDocument3 pagesYgeia Medical Center - Manila, PhilippinesKratos de GuzmanNo ratings yet
- A Detailed Lesson Plan in Science 9 6asDocument15 pagesA Detailed Lesson Plan in Science 9 6askilatmarielleann.cte.cpcNo ratings yet
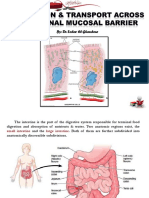
- Intestinal Mucosal Barrier & Highly Absorptive CellsDocument25 pagesIntestinal Mucosal Barrier & Highly Absorptive Cellsas qw100% (1)
- 1 s2.0 S0896627314001718 MainDocument18 pages1 s2.0 S0896627314001718 MainAhmet KayalıNo ratings yet
- ACT H Cracked Nipples FactSheet 2018-V1Document2 pagesACT H Cracked Nipples FactSheet 2018-V1dhea handyaraNo ratings yet
- Complete Denture ImpressionsDocument222 pagesComplete Denture ImpressionsAmar BimavarapuNo ratings yet
- G.KDocument201 pagesG.Kvsr359No ratings yet
- For Reaction Paper Per GroupDocument26 pagesFor Reaction Paper Per GroupvinzNo ratings yet