Professional Documents
Culture Documents
Regularsunscreenuse PDF
Uploaded by
El MarcelokoOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Regularsunscreenuse PDF
Uploaded by
El MarcelokoCopyright:
Available Formats
ORIGINAL ARTICLE
See related commentary on pg 2745
Regular Sunscreen Use Is a Cost-Effective Approach to Skin Cancer Prevention in Subtropical Settings
Louisa G. Gordon1,3, Paul A. Scuffham2,3, Jolieke C. van der Pols1, Penelope McBride1, Gail M. Williams4 ` le C. Green1,3 and Ade
In many developed countries, total costs to health systems for cutaneous basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs) are among the highest of all cancers, yet the investment value of preventive measures remains unknown. Using primary data from a randomized controlled trial, we estimated the costeffectiveness of a skin cancer prevention initiative based on regular sunscreen use. Compared with usual practice (discretionary use), the sunscreen intervention cost an additional US$106,449 (2007) to prevent 11 BCCs, 24 SCCs, and 838 actinic keratoses among 812 residents over 5 years. These health outcomes required an annual average investment of US$0.74 per person and saved the Australian government a total of US$88,203 in healthcare costs over the same period. Such community-based interventions promoting regular sunscreen use among Caucasians in subtropical settings can prevent skin cancer and related skin tumors in practical ways and with great cost efficiency.
Journal of Investigative Dermatology (2009) 129, 27662771; doi:10.1038/jid.2009.141; published online 18 June 2009
INTRODUCTION In populations with large percentages of people of European descent, basal cell carcinomas (BCCs) and squamous cell carcinomas (SCCs) of the skin impose a substantial burden in human and economic terms. Because of the negligible mortality compared with other cancers, their high incidence rates and attendant costs tend to be overlooked. In the United States alone, Medicare spends US$13 billion each year on skin cancer treatment. Added to this are the costs of associated skin tumors such as actinic keratoses (AKs), which are highly prevalentaround 625% in the United Kingdom and the United States (Holmes et al., 2007) and 4060% in Australia (Darlington et al., 2003; Holmes et al., 2007)and among the strongest predictors of skin cancer (Darlington et al., 2003). Given that UV radiation is the major environmental cause of skin cancers (International Agency for Cancer Research, 1992), they are preventable by sun-protective behaviors. In combination with other primary sun-avoidance measures, such as shade and sun-protective clothing, the use of broad1
Department of Cancer and Population Health Studies, Queensland Institute of Medical Research, Brisbane, Queensland, Australia; 2School of Medicine, Griffith University, Brisbane, Queensland, Australia; 3Griffith Medical Research College, A Joint Program of Griffith University and the Queensland Institute of Medical Research, QIMR, Herston, Queensland, Australia and 4 Department of Epidemiology and Biostatistics, School of Population Health, University of Queensland, Brisbane, Queensland, Australia Correspondence: Dr Louisa G. Gordon, Cancer and Population Health Studies, Queensland Institute of Medical Research, PO Royal Brisbane, 300 Herston Road, Herston, Brisbane, Queensland 4029, Australia. E-mail: Louisa.Gordon@qimr.edu.au Abbreviations: AK, actinic keratosis; BCC, basal cell carcinoma; SCC, squamous cell carcinoma Received 13 November 2008; revised 5 March 2009; accepted 20 April 2009; published online 18 June 2009
spectrum sunscreens is an effective adjunct measure. The safety and efficacy of sunscreens in protecting human skin from squamous tumors have been established (Thompson et al., 1993; Green et al., 1999). Because of their central role in skin cancer prevention, sunscreens are unanimously recommended by health authorities in the United States (US Preventive Services Task Force, 2001), the United Kingdom (National Radiation Protection Board UK, 2008), and Australia (Cancer Council Australia and Australasian College of Dermatologists, 2007). Using data from a community-based randomized controlled trial (Green et al., 1999), we evaluated actual use of health-care resources, costs, and health outcomes of BCC and SCC prevention in order to address the question of whether an intervention for skin cancer prevention that promotes the daily application of sunscreen among Caucasians in a sunny environment is a sound economic investment. From a societal perspective, a cost-effectiveness analysis was undertaken using direct health-outcomes data obtained when individuals from a Queensland community took part in a single randomized controlled trial (Green et al., 1999) over a 5-year period. RESULTS Of the 1,621 residents enrolled, 1,383 (85%) remained active participants at the end of the trial. Participants who withdrew were younger on average than ongoing participants (47 vs. 49 years, respectively) but otherwise similar (Green et al., 1999). From January 1993 to December 1997 inclusive, 155 (10%) participants developed 277 new histologically confirmed skin cancers. The occurrence of 11 fewer BCCs and 24 fewer SCCs among the participants in the daily-sunscreen arm compared with those in the usual-practice arm (Table 1) was
& 2009 The Society for Investigative Dermatology
2766 Journal of Investigative Dermatology (2009), Volume 129
Cost-Effectiveness of Long-Term Sunscreen Use
LG Gordon et al.
Table 1. Incidence of histologically confirmed BCCs and SCCs on the head, neck, arms, and hands, from 1993 to 1997, by randomized sunscreen treatment group
Persons affected with skin cancer
BCC SCC
Daily sunscreen group
59 23
Discretionary sunscreen group
57 34
Total
116 57
Difference (persons with fewer cancers)
2 11
Rate ratio (95% CI)1
1.04 (0.721.49) 0.68 (0.401.15)
Total
79
76
1552
Counts of skin cancers BCC SCC 94 27 105 51 199 78
Difference (cancers prevented) 11 24 0.87 (0.561.34) 0.50 (0.270.92)3
Total
121
156
277
35
BCC, basal cell carcinoma; CI, confidence intervals; SCC, squamous cell carcinomas. 1 Rate ratios compared counts of skin cancers in the intervention group with those in the usual practice group. 2 18 persons affected with cancer had both BCC and SCC, and are not double-counted here. 3 Difference was statistically significant, P=0.03.
Table 2. Number of prevalent actinic keratoses (AKs) prevented, attributable to daily sunscreen use, and estimated cost-savings (US$ 2007) for avoided medical treatment
Management2
Rate ratio (95% CI) 19941992 0.78 (0.640.96)1 19961994 0.94 (0.751.19)1 Participants in daily sunscreen group 806 797 Estimated no. AKs prevented 50% of AKs from daily sunscreen use1 treated 806 166 403 83 Physician visits ($) 27,315 5,618 Cryotherapy ($) 31,438 6,466 Topical cream ($) 2,306 474 Total cost saving ($) 61,059 12,558
Total
838
486
32,933
37,904
2,781
73,617
CI, confidence interval. 1 On the basis of Darlington et al. (2003), which compares rates of prevalent AKs on sunscreen applied sites in the intervention group relative to the usual practice group. These results found a 24% reduction during 2-year period 19921994 or an equivalent of 1 AK prevented per person, 5% reduction during 19941996 or an equivalent of 0.208 prevented per person. Models were adjusted for age, sex, smoking, eye color, hair color, sunburn propensity, previous history of skin cancer, and occupational exposure. 2 Treatment estimates; number of AKs treated 50% (authors assumption), average physician visits per AK=2.87 (Streeton et al., 2006), estimated 95% cryotherapy & 5% topical creams (Rosen and Studniberg, 2003). Medicare items and unit costs are listed in the supplementary file.
attributed to sunscreen intervention. Similarly, on the basis of intention-to-treat analyses, an estimated 838 AKs were prevented in the daily-sunscreen arm from 1992 to 1996 (Darlington et al., 2003) (Table 2). From a societal perspective, the net total cost over 5 years for the daily-sunscreen intervention group was US$329,149 versus $222,700 for the usual-practice group (Table 3). The cost for the intervention group was partially offset by reduced medical care costs of $88,203 for avoided SCC, BCC, and AK (assuming 50% of AKs were treated). These net costs translated into $405 versus $275 per person for the intervention and usual-practice groups, respectively. From the perspective of the Australian government (which funds
the vast majority of health care in such cases), the net cost over 5 years was $50,103 for the daily-sunscreen group versus $138,306 for the usual-practice group; thus, cost savings, fewer skin cancers, and fewer AKs were attributed to the regular use of sunscreen. Combining skin cancer and cost outcomes, the incremental cost per skin cancer prevented over 5 years was US$3,041 from a societal viewpoint, but it represented a savings to the government (Table 3). This equated to an investment of $3.72 per person over 5 years (or $0.74 annually). Cost-effectiveness ratios were sensitive to the inclusion of conservative cost estimates for AKs and to the proportion of AKs treated (50% in the base analysis). If 100%
www.jidonline.org 2767
LG Gordon et al.
Cost-Effectiveness of Long-Term Sunscreen Use
Table 3. Costs and skin cancers prevented in the randomized daily and discretionary sunscreen treatment groups, from 1992 to 1996, from the government and societal perspectives (US$ 2007)
Cost type1
(A) Provider costs (salaries, office, consumables, sunscreen) (B) Imputed costs for voluntary contributions (unpaid staff (dermatologists, other staff, rental)) (C) Participant costs (time to attend skin exam, apply sunscreen, visit doctor, out-of-pocket costs for doctors) (D) Government-provided health care costs for treatment of skin cancers and AKs Net government costs (D) Net societal costs (A+B+C+D) Incremental costsgovernment viewpoint Incremental costssocietal viewpoint Total skin cancers Skin cancers prevented Incremental cost per skin cancer prevented (over 5 years)government viewpoint Incremental cost per skin cancer prevented (over 5 years)societal viewpoint
Daily sunscreen use (n=812)
$145,632 $53,032 $80,382 $50,103 $50,103 $329,149 Cost saving $106,449 121 35 Cost saving $3,041
Discretionary sunscreen use (n=809)
$84,394 $138,306 $138,306 $222,700
156
AK, actinic keratoses. 1 Detailed information on resource measurement, unit costs, quantities, valuation, and analysis details are available in the supplementary file.
of the AKs prevented by sunscreen use had otherwise been treated, the incremental annual cost per cancer prevented would have dropped to $1.15 per person from $3.72; if 25% of prevented AKs had been treated, the ratio would have risen to $5.02 per person (Table 4). On the other hand, in all sensitivity analyses, the governments cost-saving position was preserved. The cost-effectiveness ratios changed marginally when base ratios were recalculated using upper and lower 95% confidence interval limits of bootstrapped mean estimates for participant sunscreen application time and purchases as well as the time and expense of physician visits (Table 4). The results of the probabilistic sensitivity analyses, based on 5,000 simulated incremental cost and incremental effect pairings, showed a mean incremental cost-effectiveness ratio of $3.72 within a wide 95% confidence interval range (from cost saving to $29.52 per person: 99% resulted in better outcomes (cancers prevented) and 8.6% were cost saving) (Figure 1). The wide interval was driven by the large possible variation in medical costs. DISCUSSION This economic evaluation of an intervention promoting sunscreen as a means of preventing skin cancer showed that the intervention saved the government money but imposed a small cost on society as a whole (health provider, governments, and participants) for these improved health outcomes. If health providers, governments, and individuals were collectively willing to pay and contribute to preventing skin cancer, this sunscreen initiative would cost society an outlay of US$3,041 per skin cancer prevented over a 5-year period, or $0.74 per person annually. Given that this investment is a fraction of what individuals in developed nations alone pay for cosmetic skin-care products, this expense seems
2768 Journal of Investigative Dermatology (2009), Volume 129
extremely worthwhile. For governments in a publicly funded health system that incur the cost of skin cancer medicine, this intervention yielded considerable cost savings within 5 years under a range of cost scenarios. Our cost-effectiveness ratios compare favorably with those for other primary prevention initiatives currently approved in Australia, even considering the upper 95% confidence limit of $29.52 per person (rather than the mean of $3.72) from our probabilistic sensitivity analyses. For example, the estimated cost of Gardasil (CSL, Sydney, Australia), the human papillomavirus vaccine for preventing cervical cancer, began at around US$360 per dose (Department of Health and Ageing PBAC, 2006), and for the Rotarix vaccine (GlaxoSmithKline, Boronia, Australia) against rotavirus gastroenteritis, the cost was around US$72 per dose (Department of Health and Ageing PBAC, 2006). Furthermore, there are an estimated 137,600 new cases and 410 deaths from SCC each year in Australia (Australian Institute of Health and Welfare and Cancer Australia, 2008), and if, through the use of sunscreen, a quarter of all new cases could be prevented over 5 years (Green et al., 1999), then up to 19 deaths could potentially be prevented each year with regular sunscreen use in Australia. High proportions of all skin cancers in the United Kingdom and Australia and around 50% in the United States are managed by primary care physicians in office-based settings. This explains the relatively low cost of managing individual tumors, estimated at a mean US$500 ($487) per skin cancer episode (Chen et al., 2001); when managed in hospital or ambulatory surgical rooms, these costs can be up to 10 times higher (Chen et al., 2006). Although skin cancers treated outside primary care were not included, one in five persons with skin cancer in our sample would need to have been treated as an inpatient to exceed the upper confidence
Cost-Effectiveness of Long-Term Sunscreen Use
LG Gordon et al.
Incremental cost (mean per person) US$
Table 4. Results of one-way sensitivity analyses1 on key costs: societal perspective (US$ 2007)
Incremental costeffectiveness ratio (mean per person)
Base analysis Costs involved in skin cancer misdiagnoses (positive predictive value) 0.60 0.80 Medical costs ($)
2
$1,500 $1,200 $900 $600 $300 $0 $300 $600 $900 $1,200 $1,500 20 0 20 40 60 80 100 Incremental cancers prevented
3.72 3.62 3.79 3.81 0.33 3.79 3.70 3.77 3.66 3.52 3.93 3.77 3.70 6.31 5.02 1.15
Low High3
Time to visit a GP ($)
Low High
Time to apply sunscreen ($)
Low High
Sunscreen purchases ($)
Low High
Out-of-pockets for GP visit ($)
Low High
Figure 1. Results of probabilistic sensitivity analyses with 95% confidence ellipse. Incremental cost cost of daily application minus cost of discretionary application (mean per person); incremental cancers prevented cancers in the discretionary group minus those in the daily-sunscreen group. The 95% confidence ellipse is based on 5,000 Monte Carlo simulations with binomial distributions assigned for skin cancers prevented, gamma distributions for cost variables, and normal distribution for positive predictor value estimates.
Proportion of AKs treated
0% 25% 100%
GP, general practitioner. Low and high estimates are the lower and upper 95% confidence intervals from bootstrapped mean results with the exception of sunscreen purchases that were tested over a 20% higher and lower cost. 2 Includes the scenario of higher accuracy and less resource use in experienced or specialist doctors. 3 Includes the scenario of higher costs of skin cancers treated in hospitals.
1
limit of medical costs in our sensitivity analysis on the basis of hospital costs for skin procedures. Thus, our medical costs are likely to be underestimated not only because of excluded inpatient costs but also because of our omission of the costs of treatment morbidity (NHMRC National Health and Medical Research Council, 2003), as well as because of additional costs of other common conditions avoidable through sunscreen use, such as photoaging (Stern, 2004). Our study used primary data from a rigorous intervention study (Green et al., 1999; Darlington et al., 2003) with a usual practice comparator along with high-quality clinical data collected over the study period. Although 15% of the participants were not active for the duration, their health outcomes would not have materially changed the results, given their similarity to the overall sample (Green et al., 1999)). On the other hand, it was not possible to precisely measure total AKs because of the extremely high prevalence rates coupled with high rates of spontaneous regression (Frost et al., 2000). However, their importance as strong predictors of skin cancer risk is well known, especially SCC (Marks et al., 1988; Frost et al., 1994). The paucity of evidence about AK treatment patterns led to our conservative estimation in
costs, although sensitivity testing showed their notable potential to alter cost-effectiveness ratios. Even with the exclusion of AK costs, however, the sunscreen intervention remained cost saving to the government. Our AK cost estimates emphasize the high level of health resources that AKs consume when they are managed like skin cancers (for example, with cryotherapy, topical creams, and periodic clinical visits). We conclude that year-round regular sunscreen use for the prevention of skin cancers produces cost savings for healthcare providers in Australia. Given the growing burden of costs for skin cancer treatment in the Northern Hemisphere, it seems likely that the fair-skinned populations of the United States and Europe could also benefit from cost savings were sunscreen to be regularly used during summer or during leisure time spent in subtropical climates. MATERIALS AND METHODS
The Nambour Skin Cancer Prevention Trial investigated the effectiveness of daily application of sunscreen (the intervention) versus the usual discretionary use of sunscreen (usual practice) (Green et al., 1999). The trial was conducted from 1992 to 1996 among a random sample of the residents of the township of Nambour, Queensland. Full details of methods (Green et al., 1994), a participant flow chart, and key findings (Green et al., 1994, 1999) have previously been published. A total of 812 participants randomized to the sunscreen intervention were supplied with a water-resistant, broad-spectrum sunscreen with a sun protection factor of 15 . They were asked to apply it daily to the head, neck, arms, and hands, and application habits were reinforced by study nurses via a quarterly contact. Ethical approval was obtained from the Human Ethics Committee of the Queensland Institute of Medical Research, and informed written consent was provided by all
www.jidonline.org 2769
LG Gordon et al.
Cost-Effectiveness of Long-Term Sunscreen Use
participants. The study was conducted according to the Declaration of Helsinki Principles.
Skin cancer ascertainment
All participants received a full skin examination by a dermatologist unaware of treatment allocation, at the start (1992), midpoint (1994), and completion (1996) of the trial. Interim skin cancers were ascertained by self-report and/or physicians notifications, with medical record verification. Only histologically confirmed skin cancers on the head, neck, arms, and hands (sunscreen sites under the protocol) were included in our analysis. Cancers diagnosed during 1992 were excluded a priori, as no beneficial effect of sunscreen was expected in year 1, given the latent period of skin tumors in humans; similarly, cancers diagnosed during 1997 were included to account for the latent effects of the sunscreen intervention immediately after trial cessation.
Estimation of resources and costs
Because a wider societal perspective was taken, the assessed costs included those incurred by the program provider, community supporters, the participants, and the Australian government (through Medicare Australia, which bears the direct costs of treatment). The trial was conducted within the context of a large, publicly funded research program. Research-driven costs were identified and excluded from the resources for the preventive intervention. Specific details of measurement, valuation methods, and sources used to quantify resource use are available online in a Supplementary file. We also accounted for the avoided costs of AKs, the skin-cancerrelated secondary outcome of the sunscreen intervention (Darlington et al., 2003), using published Nambour Trial outcome data for AKs (Darlington et al., 2003). Because it is not feasible to track treatment pathways for individual AKs and the precise proportion of AKs treated (as opposed to monitored) in Australian primary care seems unknown and could vary greatly, we assumed an average treatment rate of AKs of 50% and tested this assumption in sensitivity analyses. Meanwhile, usual modes of AK treatment were ascertained by searching all published Australian studies reporting primary care management of actinic skin tumors (Raasch, 1999; NHMRC National Health and Medical Research Council, 2003; Rosen and Studniberg, 2003; Streeton et al., 2006). Medical services were valued using Medicare fees, which represent the cost to the Australian government that is reimbursed to physicians through Medicare Australia. Using diagnosis dates, we also accounted for cost efficiencies relating to physician visits, treatment, and pathology when a person seemed to have had several cancers treated simultaneously (Richmond-Sinclair et al., 2008). In addition, adjustments to medical costs were made to account for the percentage of benign lesions that would have been treated provisionally as cancers. These adjustments were made on the basis of a positive predictive value of 70% (Youl et al., 2007) and were further tested by varying the positive predictive value between 60 and 80% in analyses.
arm was carried out using Poisson regression. For AKs, ratios of successive counts of prevalent AKs indicated the rate of change in AK acquisition in each sunscreen treatment arm (Darlington et al., 2003). Statistically significant differences were tested using negative binomial regression. Tests were two-sided and results were considered statistically significant when Po0.05. Resources were identified and measured for the various time periods, and year-2007 Australian dollars were applied. Costs are presented in year-2007 US dollars (converted at AU$ 1 US$ 0.72, using Organization for Economic Cooperation and Development purchasing-power parities). The analysis did not involve future costs or benefits (because it was retrospective, with the costs and outcomes already known), and therefore discounting was not applied. Individual-level data relating to sunscreen purchase and application time, physician-visit time and expense, and other medical costs were right skewed; we therefore obtained bootstrapped mean costs per participant using 1,000 bootstrapped samples and the bias-corrected approach (Briggs, 2001). Walds test was used to detect significant group differences with 95% confidence intervals. The measure of benefit for this economic evaluation was skin cancers prevented. Final cost and health outcome data were combined into incremental costeffectiveness ratios, interpreted as the additional cost for preventing each new skin cancer in the intervention group. To address the uncertainty in our estimations, one-way and probabilistic sensitivity analyses were undertaken to test the stability of the base results when individual parameter values were varied (Briggs, 2001). In the probabilistic sensitivity analyses, all cost variables, the probability of skin cancer, and positive predictor values were simultaneously analyzed using 5,000 Monte Carlo simulations to assess the variation to the base results. After the actual patient-level data distributions, gamma distributions were assigned for cost variables, binomial distributions for skin cancer counts, and normal distributions for positive predictor estimates (Youl et al., 2007) across the two groups. Data were analyzed using STATA/SE V9 (StatCorp., College Station, Texas) and TreeAge Pro 2008 (TreeAge Software Inc, Williamstown, MA).
CONFLICT OF INTEREST
al Research, August 2006June AG had an unrelated project funded by LOre 2009. The other authors state no conflict of interest.
ACKNOWLEDGMENTS
We thank all participants and volunteers in the Nambour Trial for their loyalty to this research program. Margaret Oziemski (dermatologist) gave valuable clinical advice, Lee Casey (QIMR) assisted with retrieving key project records, and Ross Cosmetics and Woolworths supplied the sunscreen (AuScreen). We also thank Terri Jackson for providing critical feedback on an earlier draft of the paper. This analysis was funded through National Health and Medical Research Council (NHMRC) grant 442976. Louisa Gordon is funded through an NHMRC Public Health Fellowship.
SUPPLEMENTARY MATERIAL Supplementary material is linked to the online version of the paper at http:// www.nature.com/jid
Analysis
Using intention-to-treat analyses, the numbers of BCCs and SCCs in the intervention and usual-practice arms were compared and tested for statistically significant group differences using negative binomial regression, and a comparison of persons affected with cancer in each
2770 Journal of Investigative Dermatology (2009), Volume 129
REFERENCES
Australian Institute of Health and Welfare, Cancer Australia (2008) Nonmelanoma skin cancer, general practice consultations, hospitalisation and mortality. In: Cancer Series No. 43. Canberra: Australian Institute of Health and Welfare
Cost-Effectiveness of Long-Term Sunscreen Use
LG Gordon et al.
Briggs A (2001) Handling uncertainty in economic evaluation and presenting the results. In: Economic Evaluation in Health Care: Merging Theory with Practice (Drummond MF, McGuire A, eds), Oxford: Oxford University Press, 172214 Cancer Council Australia, Australasian College of Dermatologists (2007) Position Statement: Screening and early detection of skin cancer. (Update 10/07, http://www.cancer.org.au/policy/positionstatements/ sunsmart/screeningandearlydetectionofskincancer.htm ) Chen JG, Fleischer AB Jr, Smith ED, Kancler C, Goldman ND, Williford PM et al. (2001) Cost of nonmelanoma skin cancer treatment in the United States. Dermatol Surg 27:10358 Chen JG, Yelverton CB, Polisetty SS, Housman TS, Williford PM, Teuschler HV et al. (2006) Treatment patterns and cost of nonmelanoma skin cancer management. Dermatol Surg 32:126671 Darlington S, Williams G, Neale R, Frost C, Green A (2003) A randomized controlled trial to assess sunscreen application and beta carotene supplementation in the prevention of solar keratoses. Arch Dermatol 139:4515 Department of Health and Ageing PBAC (2006) Public Summary Document Quadrivalent Human Papillomavirus (Types 6, 11, 16, 18) recombinant vaccine, injection, 0.5 ml, Gardasil Human Rotavirus Vaccine, lyophilised powder & solvent for oral administration, one ml dose, Rotarix. (Update 03/06, http://www.health.gov.au/internet/main/publishing.nsf/Content/ ) Frost C, Williams G, Green A (2000) High incidence and regression rates of solar keratoses in a Queensland community. J Invest Dermatol 115:2737 Frost CA, Green AC, Marks R, Rennie G, Selwood TS (1994) Epidemiology of solar keratoses. Br J Dermatol 131:45564 Green A, Battistutta D, Hart V, Leslie D, Marks G, Williams G et al. (1994) The Nambour skin cancer and actinic eye disease prevention trial: design and baseline characteristics of participants. Control Clin Trials 15:51222 Green A, Williams G, Neale R, Hart V, Leslie D, Parsons P et al. (1999) Daily sunscreen application and betacarotene supplementation in prevention of basal-cell and squamous-cell carcinomas of the skin: a randomised controlled trial. Lancet 354:7239 [erratum The Lancet 1999; 1354:1038]
Holmes C, Foley P, Freeman M, Chong AH (2007) Solar keratosis: epidemiology, pathogenesis, presentation and treatment. Australas J Dermatol 48:6774 International Agency for Cancer Research (1992) Solar and ultraviolet radiation. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, Vol. 55. Lyon, France Marks R, Rennie G, Selwood TS (1988) Malignant transformation of solar keratoses to squamous cell carcinoma. Lancet 1:7957 National Radiation Protection Board UK (2008) Health effects from ultraviolet radiation: Report of an Advisory Group on Non-Ionising Radiation 13/1 NHMRC National Health and Medical Research Council (2003) Clinical Practice Guidelines, Non-Melanoma Skin Cancer: Guidelines for Treatment and Management in Australia. Canberra: Commonwealth Government Raasch BA (1999) Suspicious skin lesions and their management. Aust Fam Physician 28:46671 Richmond-Sinclair NM, Pandeya N, Ware RS, Neale RE, Williams GM, van der Pols JC et al. (2008) Incidence of basal cell carcinoma multiplicity and detailed anatomic distribution: longitudinal study of an Australian population. J Invest Dermatol 31:3238 Rosen RH, Studniberg H (2003) Solar keratoses: analysis in a dermatological practice in Australia. Australas J Dermatol 44:349 Stern RS (2004) Clinical practice. Treatment of photoaging. N Engl J Med 350:152634 Streeton CL, Gospodarevskaya E, Harris AH (2006) How are solar keratoses treated by general practitioners in Australia? Int J Dermatol 45:2726 Thompson SC, Jolley D, Marks R (1993) Reduction of solar keratoses by regular sunscreen use. N Engl J Med 329:114751 US Preventive Services Task Force (2001) Screening for skin cancer, recommendations and rationale. Am J Prev Med 20:446 Youl PH, Baade PD, Janda M, Del Mar CB, Whiteman DC, Aitken JF (2007) Diagnosing skin cancer in primary care: how do mainstream general practitioners compare with primary care skin cancer clinic doctors? Med J Aust 187:21520
www.jidonline.org 2771
You might also like
- StantonTennRev07 PDFDocument28 pagesStantonTennRev07 PDFEl MarcelokoNo ratings yet
- Parsons Sistemas SocialesDocument12 pagesParsons Sistemas SocialesEl MarcelokoNo ratings yet
- Social Status & Group StructureDocument12 pagesSocial Status & Group StructureEl MarcelokoNo ratings yet
- Elepahnt Classroom PDFDocument10 pagesElepahnt Classroom PDFEl MarcelokoNo ratings yet
- Human Psychology Behavioural Ecology PDFDocument9 pagesHuman Psychology Behavioural Ecology PDFEl MarcelokoNo ratings yet
- British Empire Liberal MissionDocument21 pagesBritish Empire Liberal MissionEl MarcelokoNo ratings yet
- Marx South KoreaDocument8 pagesMarx South KoreaEl MarcelokoNo ratings yet
- Psychoanalysis and Social Critique PDFDocument16 pagesPsychoanalysis and Social Critique PDFEl MarcelokoNo ratings yet
- Inequality Outcomes Vs Inequality Opportunitie ChileDocument18 pagesInequality Outcomes Vs Inequality Opportunitie ChileEl MarcelokoNo ratings yet
- Xeno CentrismDocument5 pagesXeno CentrismEl MarcelokoNo ratings yet
- Psychology Psychiatry PDFDocument7 pagesPsychology Psychiatry PDFEl MarcelokoNo ratings yet
- Classroom Agression Disruptive PDFDocument8 pagesClassroom Agression Disruptive PDFEl MarcelokoNo ratings yet
- Skincancerpreventionyouth PDFDocument21 pagesSkincancerpreventionyouth PDFEl MarcelokoNo ratings yet
- Individualsentinglevelpredictor PDFDocument14 pagesIndividualsentinglevelpredictor PDFEl MarcelokoNo ratings yet
- Cambios Patron Enfermedad PDFDocument10 pagesCambios Patron Enfermedad PDFEl MarcelokoNo ratings yet
- 31parents PDFDocument6 pages31parents PDFEl MarcelokoNo ratings yet
- Menores Quemados PDFDocument12 pagesMenores Quemados PDFEl MarcelokoNo ratings yet
- 21psychologicalreview PDFDocument10 pages21psychologicalreview PDFEl MarcelokoNo ratings yet
- 34 PDFDocument10 pages34 PDFEl MarcelokoNo ratings yet
- Skincancrbrazil PDFDocument6 pagesSkincancrbrazil PDFEl MarcelokoNo ratings yet
- Selfeficcyintegration PDFDocument21 pagesSelfeficcyintegration PDFEl MarcelokoNo ratings yet
- 21improvesun PDFDocument10 pages21improvesun PDFEl MarcelokoNo ratings yet
- 42universitystudents PDFDocument7 pages42universitystudents PDFEl MarcelokoNo ratings yet
- Effects of A Multicomponent Intervention On Motivation and Sun Protection Behaviors Among Midwestern BeachgoersDocument5 pagesEffects of A Multicomponent Intervention On Motivation and Sun Protection Behaviors Among Midwestern BeachgoersEl MarcelokoNo ratings yet
- 36younadults PDFDocument6 pages36younadults PDFEl MarcelokoNo ratings yet
- 30trs PDFDocument9 pages30trs PDFEl MarcelokoNo ratings yet
- 35adolescence PDFDocument7 pages35adolescence PDFEl MarcelokoNo ratings yet
- Preventive Medicine: ArticleinfoDocument5 pagesPreventive Medicine: ArticleinfoEl MarcelokoNo ratings yet
- 25guidelenes PDFDocument5 pages25guidelenes PDFEl MarcelokoNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5783)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (72)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- RX Int Festival PlanGuideDocument8 pagesRX Int Festival PlanGuideIke1234567No ratings yet
- Levorphanol - The Forgotten Opioid PDFDocument6 pagesLevorphanol - The Forgotten Opioid PDFfchem11No ratings yet
- Bioactive Compounds in Phytomedicine BookDocument228 pagesBioactive Compounds in Phytomedicine BookAnil KumarNo ratings yet
- BONE Level 2-BDocument60 pagesBONE Level 2-Bjefri banjarnahorNo ratings yet
- Pharmacy Math CalculationsDocument8 pagesPharmacy Math CalculationsRahmawati KuswandiNo ratings yet
- Introduction To QBD FixDocument25 pagesIntroduction To QBD FixAdel ZilviaNo ratings yet
- Understanding the Immunogenic Properties of VirusesDocument41 pagesUnderstanding the Immunogenic Properties of VirusesMaruf Raza DarubagiNo ratings yet
- Annotated BibliographyDocument3 pagesAnnotated Bibliographyicemocha6910% (1)
- Lower Limb ProstheticsDocument28 pagesLower Limb ProstheticsSomu VictorNo ratings yet
- The Behavioral Medicine Treatment Planner - Douglas E. DeGood & Angela L. Crawford & Arthur E. JongsmaDocument283 pagesThe Behavioral Medicine Treatment Planner - Douglas E. DeGood & Angela L. Crawford & Arthur E. JongsmaSergio Mora88% (8)
- Nutrition Quiz: Build a Healthy DietDocument17 pagesNutrition Quiz: Build a Healthy DietJan Angelo OcadoNo ratings yet
- CPG 2013 - Prevention and Treatment of Venous ThromboembolismDocument170 pagesCPG 2013 - Prevention and Treatment of Venous ThromboembolismMia Mus100% (1)
- Ubd Stage 1-3Document24 pagesUbd Stage 1-3api-336167164No ratings yet
- Gordon Functional Health AssessmentDocument18 pagesGordon Functional Health Assessmentjokazel100% (1)
- Pes WesDocument12 pagesPes WesjotapintorNo ratings yet
- ECTDocument22 pagesECTRubz Bulquerin0% (1)
- A Comparison of Muscle Strength and Flexibility Between The Preferred and Non Preferred Leg in English Soccer PlayersDocument10 pagesA Comparison of Muscle Strength and Flexibility Between The Preferred and Non Preferred Leg in English Soccer PlayersMalikiNo ratings yet
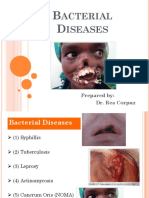
- Acterial Iseases: Prepared By: Dr. Rea CorpuzDocument63 pagesActerial Iseases: Prepared By: Dr. Rea CorpuzMedecine Dentaire100% (2)
- Safety Precautions When Driving: Seatbelt and SRS AirbagDocument7 pagesSafety Precautions When Driving: Seatbelt and SRS AirbagPeter InfanteNo ratings yet
- Pathophysiology Tia VS CvaDocument6 pagesPathophysiology Tia VS CvaRobby Nur Zam ZamNo ratings yet
- 133-Article Text-648-3-10-20190525 PDFDocument6 pages133-Article Text-648-3-10-20190525 PDFyantiNo ratings yet
- 2) Megaloblastic AnemiaDocument17 pages2) Megaloblastic AnemiaAndrea Aprilia100% (1)
- Anatomic Therapy English PDFDocument339 pagesAnatomic Therapy English PDFBabou Parassouraman100% (3)
- Gastric and Duodenal Disorders - Test 4Document21 pagesGastric and Duodenal Disorders - Test 4Vickie BuckerNo ratings yet
- Afifah FixedDocument25 pagesAfifah FixedAfifah Nur KartikasariNo ratings yet
- Infographic ProteinDocument1 pageInfographic ProteinMichele MarengoNo ratings yet
- SOCA Blok 12Document21 pagesSOCA Blok 12safiraNo ratings yet
- Expectations of Rehab After Leg AmputationDocument8 pagesExpectations of Rehab After Leg AmputationCristina Ortiz GuillénNo ratings yet
- Aspirin: The widely used pain reliever and fever reducerDocument4 pagesAspirin: The widely used pain reliever and fever reducerEithel EithelNo ratings yet
- Risk Management in Medical LaboratoriesDocument32 pagesRisk Management in Medical Laboratoriesapanisile14142No ratings yet