Professional Documents
Culture Documents
Ariad
Uploaded by
aditisinghaniaCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Ariad
Uploaded by
aditisinghaniaCopyright:
Available Formats
1 2
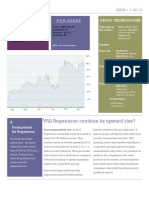
ARIAD
+ ARIA-Nasdaq
Market capitalization 3.83 B Company value 3.64 B Revenues 5.58 M Net income (-220.9M) Employees 150
PER SHARE
Value (MRQ) $0.99 Price/ Book (MRQ) 21.2x Earning -$1.36 Revenue $0.00 Dividend $0.00 Currently in the market
DRUGS
Iclusig- chronic myeloid leukemia resistant or intolerant to tyrosine kinase inhibitor
Pipeline Phase III
Iclusig- Additional indications and regions
Phase III/II
AP26113- inhibitor of cancer causing molecular pathways implicated in cancers Ridaforolimusmetastic soft- tissue and bone sarcoma
Phase I
AP1903- prostate cancer and hematologic malignancies
Will Ariads perseverance finally pay off?
Ariad has spent the last twenty years in research and designing drugs targeting specific mutations that cause cancer, without a commercial product. But finally, in December they received an accelerated approval in the US for their cancer drug, Iclusig. Clinical trials for Iclusig showed an impressive response in half the patients that previously showed resistance to various cancer drugs including Gleevec, Nilotinib and Dastanib. Iclusig treats Philadelphia chromosome positive (Ph+) chronic myeloid leukemia (CML) that is resistant to tyrosine kinase inhibitor (TKI) treatment. At the moment Gleevec commonly refered to as imatinib, is a TKI to treat CML. It targets the fusion protein BCR-ABL1. Iclusig is predicted to reach $800 M in annual sales and forecasted to reach blockbuster status with sales surpassing $1.5B if it gets approved for first line of treatment for CML. Currently, a comparative study between Iclusig and Gleevec is on the way.
Iclusig
Although Iclusig has been shown to be effective against chronic myeloid leukemia (CML) resistant to tyrosine kinase inhibitor (TKI) therapy, its initial sales did not meet the forecasted targets as some severe side effects were not disclosed.
1 2 3
Global sales of dastanib from Bristol-Myers Squibb (treats CML) was ~ 38M in the first year Global sales of nilotinib from Novartis (treats CML) was ~ 24M in the first year Estimates of Global sales of Iclusig, launched in the US -2012, only includes CML indication
Next in line first line therapy - taking on Novartis and Bristol- Myers Squibb.
Ariad launched a randomized phase III clinical study in July 2012 that is testing Iclusig and Gleevec head to head in people newly diagnosed with CML. The data from this study is expected in mid 2014. In addition, Ariad has initiated a study with Newcastle University on the behalf of the UK National Cancer Research Institute to investigate the impact of Iclusig as a first line of treatment in patients recently diagnosed with CML. If the results show Iclusig to be more effective as first line of treatment than Gleevec, it will broaden its use and assuredly turn Iclusig into a blockbuster by capturing a large portion of the $4.5B market, which is currently being dominated by Novartis and BristolMeyers Squib. Iclusig is also the only TKI that can target the T315I mutation present in a subset of CML patients. As personalized medicine is gathering momentum, patients that have T315I mutations could be preferentially prescribed Iclusig as first line of treatment. All the signs point towards Iclusig being a successful.
The market has seen many generations of TKIs, with Iclusig being a part of generation III. Novartis and Bristol-Myers Squibb are the current market leaders of the TKI market. Their annual sales in 2011 was nearly $3B in the US and ~$5B worldwide. The number of newly diagnosed patients was ~ 13000 in 2011 and is expected to grow to ~ 14,000 in the next 5 years.
Ridaforolimus and APCapabilities and pipeline
Ariad has a very promising pipeline. The two drugs farthest along in their development are ridaforolimus and AP26113. Ridaforolimus are small molecules that inhibit the mammalian target of rapamycin or mTOR. If approved it will be used to treat patients suffering from metastatic soft tissue and bone sarcomas. It was first discovered internally by Ariad and was later licensed to Merck in 2010. Merck is responsible for all the development, manufacturing and commercialization costs of ridaforolimus. Trials continue to gather information about the efficacy and side effects of ridaforolimus. Under the license agreement Merck paid Ariad $50 M in initial fees and has agreed to pay around $154M upon successful development and commercialization of the drug. Unfortunately, in 2012 a federal advisory panel rejected ridaforolimus for not being effective and backed a drug developed by GlaxoSmithKline. This adversely affected Ariad as the drugs failure to get approved might lead to a termination of their license agreement. As Ariad is a small company it needs the funding from Merck to continue its future R&D operations. However, on a more positive note, ridaforolimus has been shown to treat cardiovascular indications. In pre-clinical studies, ridaforolimus inhibited processes that lead to narrowing and blockage of arteries. An analog of mTOR has been approved for use in drugdelivering stents and has proved successful in lowering re-blockage rates. As a result of the new findings Ariad has entered into non-exclusive license agreements with a leading innovator in stent technology, Medinol Ltd and an emerging company, ICON. They are working
together to develop stents and other The shining armor in Ariads medical devices to prevent reblockages of injured arteries. Under Ariad around $39.3M and will receive royalties when the product is sold. As a result of ridaforolimus additional indication all will be not be lost if it looses out on the cancer approval. Furthermore, the partnerships with medical device companies will results in a potent force and ensure the stent users dependence on ridaforolimus. Although, ridaforolimus is now downgraded in its status it has the potential to be a revenue source for Ariad. pipeline is largely believed to be AP26113. It is an inhibitor of ALK, were confirmed as targets in nonsmall cell lung cancer (NSCLC). It accounts for 90% of all lung cancers. According to the NIH lung cancer accounts for 14% of all cancers diagnosed and accounts for nearly 29% of all cancer deaths. Many different mutations lead to NSCLC, EGFR contributes to about 20% of mutations and ALK about 1%. Presently, Erlotinib, Gefitnib and Afatinib are prescribed for NSCLC. But in patients with stage IV EGFR
mutant lung cancer, first line EGFR TKIs have a median progression free survival of 12 months. Therefore, there is a need for drugs that can act on EGFR TKI resistant lung cancer. So far there has been no effective drug(s) that can overcome EGFR resistance. At present no drug is effective in treating NSCLC in the long term except for Pfizers Crizotinib that targets ALK, ROS1 and cMET mutations which accounts for about ~2% of the mutations. But after 1 year of use patients develop resistance against it. Presently, their two competitors, Novartis and Chugai are working on second generation drugs to target NSCLC. Their drugs are at the same stage of development as AP26113.When compared to the competition AP26113 can more broadly target different ALK mutations. It is also shown to have lesser side effects. In general it is more potent in patients that have developed ALK resistance. Ariad is poised to begin phase II trials in mid 2013. They estimate that currently there are 14,200 ALK+ and 35,400 EGFR+ patients with advanced and metastatic NSCLC in the US, Europe and Japan, which according to the healthcare providers will be eligible for treatment with AP26113. This drug has the potential to stabilize Ariads financial position. If AP2113 gets approved and only manages to capture the second line, it will enter into $2B lucrative EGFR market.
the license agreement, Medinol paid ROS1 and EGFR kinases, which
- sodales. Ariad has just launched their first drug Iclusig. Although it did not perform as well as market expectations, it is going strong and is forecasted to achieve blockbuster status. In addition an IMS study found that the lackluster performance could be due to the failure of specialized pharmacies to report sales. The company has a very robust pipeline with at least one potential blockbuster. Ariad together with Merck, may have run into problems with ridaforolimus, but it has broadened its indications and entered licensing agreements with two medical device companies. Furthermore, the company has developed and optimized computational and structural approaches to design small molecules that can overcome resistance to existing cancer medications. This technology will enable Ariad to specifically design drugs targeting many different types of cancer that become resistant to first line treatments. By 2015 Ariad is forecasted to become profitable and is poised bring in ~$1B in sales by the year 2017. Ariad is a company with robust scientific footing, which is looking to grow rather than being acquired by a big Pharma. Its future looks bright. BUY
You might also like
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (119)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (265)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (587)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2219)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (890)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- M96SC05 Oleo StrutDocument6 pagesM96SC05 Oleo Strutchaumont12345No ratings yet
- Wall ChartDocument2 pagesWall ChartAhmed Fittoh Mosallam0% (1)
- Effects of Sucrose Concentration On Cell Respiration in YeastDocument7 pagesEffects of Sucrose Concentration On Cell Respiration in YeastRachel Utomo83% (23)
- Hybridization Review WorksheetDocument6 pagesHybridization Review WorksheetRejed VillanuevaNo ratings yet
- CASE Study PTBDocument53 pagesCASE Study PTBmeleanaquino94% (16)
- Personal Development: Quarter 1 - Module 5: Developmental Tasks and Challenges of AdolescenceDocument16 pagesPersonal Development: Quarter 1 - Module 5: Developmental Tasks and Challenges of AdolescenceMary Joy CejalboNo ratings yet
- Ageism PowerpointDocument11 pagesAgeism Powerpointapi-254132646No ratings yet
- Case Digest 16Document2 pagesCase Digest 16Mavic MoralesNo ratings yet
- Regen PDFDocument6 pagesRegen PDFaditisinghaniaNo ratings yet
- GHDXDocument3 pagesGHDXaditisinghaniaNo ratings yet
- GHDXDocument3 pagesGHDXaditisinghaniaNo ratings yet
- AriadDocument5 pagesAriadaditisinghaniaNo ratings yet
- Regen PDFDocument6 pagesRegen PDFaditisinghaniaNo ratings yet
- REGENDocument6 pagesREGENaditisinghaniaNo ratings yet
- DaloDocument2 pagesDalojosua tuisawauNo ratings yet
- 016-5032-002-C - SmarTrax - Case IH STX-Steiger-Quadtrac (AccuGuide-Ready) and New Holland TJ-T90X0-T9XXX (IntelliSteer-Ready) - Installation ManualDocument26 pages016-5032-002-C - SmarTrax - Case IH STX-Steiger-Quadtrac (AccuGuide-Ready) and New Holland TJ-T90X0-T9XXX (IntelliSteer-Ready) - Installation ManualAndreyNo ratings yet
- Statistics of Design Error in The Process IndustriesDocument13 pagesStatistics of Design Error in The Process IndustriesEmmanuel Osorno CaroNo ratings yet
- A Comparative Study Between Various Models of Eco-Brick and Hollow BlocksDocument9 pagesA Comparative Study Between Various Models of Eco-Brick and Hollow BlocksMykaila Ysa ValdezNo ratings yet
- 4front Projects: BbbeeDocument12 pages4front Projects: BbbeeBrand Media OfficeNo ratings yet
- Knorr FinalDocument25 pagesKnorr Finalimbree100% (3)
- Food Regulations MalaysiaDocument4 pagesFood Regulations MalaysiaSyafi'ie Syukri100% (1)
- Sodium Chloride MSDSDocument5 pagesSodium Chloride MSDSIbaharmovic LpuNo ratings yet
- Chapter - 10 NanoshellsDocument13 pagesChapter - 10 NanoshellskarthikNo ratings yet
- Akron Grad School Statement of Purpose PDFDocument2 pagesAkron Grad School Statement of Purpose PDFapi-406039291No ratings yet
- Corn Genetics and Chi Square AnalysisDocument2 pagesCorn Genetics and Chi Square AnalysisBonifacius Budi NugrohoNo ratings yet
- HRLM - Catalogue # Ex Apparatus - AC-Z Series Explosion Proof Plug and ReceptaclesDocument2 pagesHRLM - Catalogue # Ex Apparatus - AC-Z Series Explosion Proof Plug and Receptaclesa wsNo ratings yet
- Lit Crit TextDocument8 pagesLit Crit TextFhe CidroNo ratings yet
- Sem-V Principle of Taxation Law PDFDocument3 pagesSem-V Principle of Taxation Law PDFAnantHimanshuEkkaNo ratings yet
- Meditation ProjectDocument2 pagesMeditation Projectapi-411448305No ratings yet
- Will BrinkDocument10 pagesWill BrinkJoao TorresNo ratings yet
- Lesson 1:: Introduction To Science, Technology and SocietyDocument17 pagesLesson 1:: Introduction To Science, Technology and SocietyAlexis A. AguilarNo ratings yet
- Exudate Detection For Diabetic Retinopathy With Circular HoughDocument7 pagesExudate Detection For Diabetic Retinopathy With Circular HoughAshif MahbubNo ratings yet
- PSP TablesDocument32 pagesPSP TablesLucas Cariño LlaconaNo ratings yet
- NCM 117 Rle NAME: Guerzo, Danniel Dave Y. DATE/TIME: March 9, 2023 Section/Year Level: BSN 3B Group No:10 CIDocument2 pagesNCM 117 Rle NAME: Guerzo, Danniel Dave Y. DATE/TIME: March 9, 2023 Section/Year Level: BSN 3B Group No:10 CISherlyn Miranda GarcesNo ratings yet
- Rorschach y SuicidioDocument17 pagesRorschach y SuicidioLaura SierraNo ratings yet
- Soa Group Health TrackDocument2 pagesSoa Group Health TrackwasabiwafflesNo ratings yet