Professional Documents
Culture Documents
Reacting System Examples
Uploaded by
saliljain2001Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Reacting System Examples
Uploaded by
saliljain2001Copyright:
Available Formats
Engineering Sciences 22 Systems
Summer, 2003
Reacting System Examples
Here are six examples of the chemical systems. The goal of this handout is to show you how to formulate a model of a chemical system, but some of the examples include solutions using methods you will learn later this week or later in the term, so that you get to see what happens in the system and build your intuition about system behavior. The first three examples are typical examples based on the methods you are expected to learn for the course. They include solutions, although the solutions are sometimes based on methods you will learn later in the course. Example 1: Sequential reactions. An example based on the chemical process industry. Example 2: Respiration in a submersible vessel. Example 3: Waste treatment pond. p. 1 p. 6 p. 7
The next two examples are included to demonstrate the wide variety of applications of these methods, although they are not typical examples for the material in 22. Models are developed by not solved. Example 4: Blood alcohol. A model for blood alcohol in the human body after consumption of alcoholic beverages. Example 5: Oxygen sag curve. Oxygen level in a stream or river downstream of a pollution source. Example 1. Sequential reactions. Background: In the chemical process industry, it is often the case that one or more reactants are converted to one or more desired chemicals under conditions such that the desired chemicals can also react further to form undesired byproducts. One example is in the cracking of crude oil, where high molecular weight nonvolatile liquid reactants are converted to intermediate molecular weight volatile liquids suitable for gasoline, but these intermediate molecular weight liquids also react to form low molecular weight gases. Such gases, although sold, are worth less than the intermediate molecular weight volatile liquids. So the goal is the form as much as possible of the volatile liquids, while avoiding allowing the liquids to react further and produce gasses. The simplest representation of a system with the general features described above involves two reactions:
1 B A 2C B k k
p. 8 p. 11
B is the desired product (e.g. volatile liquids), whereas C is not desired.
Reacting Systems
Page 1
Engineering Sciences 22 Systems
Summer, 2003
Problem Statement: Given the reaction described above, you wish to find the following quantities: Maximum value of B that can be realized in well-mixed reactors operated in either batch or steady-state continuous mode. The time or "residence time" at which this occurs, in both batch and continuous mode. The reactor volume required to produce 100 moles B/hr, again in batch and continuous mode.
The following information is given: CA,o = 1 mole/L;
1
CB,o = CC,o = 0
1
k1 = 3 hr , k2 = 0.7 hr
Reaction time for the continuous reactor = mean liquid residence time = V/Q = , where V = system volume, Q = volumetric flow rate. Assume that the reactors are well-mixed and have constant volume, that the reactions occur in the liquid phase, and (for the continuous reactor) that the input and output liquid flow rates are the same. Formulation of the system model.
a. Write the rate laws. Based on the indicated mechanism, we can write the rate laws for each chemical species:
rA = k1CA rB = k1CA k2CB rC = k2CB
b. Write appropriate material balance equations.
Batch case Material balance equations for each of the three reacting species:
dCA = rA = k1CA dt dCB = rB = k1CA k2 CB dt dCC = rC = k 2 CB dt
Reacting Systems
Page 2
Engineering Sciences 22 Systems
Summer, 2003
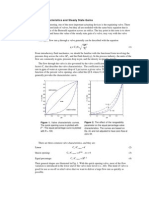
This is the system model for the batch case: three simultaneous first-order linear ordinary differential equations. Well come back to model formulation for the CSTR case after looking at a solution to the above equations. We can solve them by various means, which we will study later in the classone good choice, and one that you will learn soon, would be a numerical simulation using MATLAB. It is also possible to use the Laplace transform (as detailed in the appendix, although you arent expected to learn this until later in the term). The results of the solution are shown below.
1 0.9 0.8 C [moles/L] 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 0 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8
CA CB CC
0.9
Time [hours]
At 0.63 hours, the concentration of B reaches a maximum value of 0.64 moles/liter. This volumetric productivity of this reactor is
0.64
moles L 0.63 hr
= 1.015
moles L hr
If we want to produce 100 moles/hr, we need a reactor of volume
moles hr V = moles 1.015 L hr 100
= 99 L
Reacting Systems
Page 3
Engineering Sciences 22 Systems
Summer, 2003
Continuous case For a continuous reactor, the material balance equation is of the form (for A)
Q dCA = (C A ,in CA , out ) + rA dt V
We are interested only in the steady state response, so set the derivative to zero.
CA ,in CA + rA = 0
where = V/Q, and Cout = C for a well-mixed reactor. We write an equation for each species, and substitute the rate laws to obtain three algebraic equations: CA ,in CA k 1CA = 0 CB,in CB + (k1 CA k2 CB ) = 0 CC ,in CC + k2 CB = 0 Only A is put into the reactor, at a concentration CA,in = CA,o, and CB,in=CC,in = 0. Solving the equations,
CA = CB = CC = CA , o 1 + k 1
k1CA ,o k1 CA = 1 + k 2 (1 + k2 )(1 + k1 ) 2 k 2 k1 CA ,o (1 + k 2 )(1 + k1 )
Again, CA + CB + CC = CA,o, as they should. In this problem, the independent variable is the residence time, = V/Q. We can use Matlab to plot the steady-state concentrations as a function of . This is not using Matlab to solve differential equations of anything of that sortjust as a plotting utility now that we know the solution.
% reacting systems example 2 % ENGS 22 % Charlie Sullivan CA0 = 1; k1 = 3; k2 = 0.7; % Moles/L % 1/hr % 1/hr
theta = linspace(0,4); CA = CA0 ./(1 + k1*theta); CB = theta.*k1.*CA./( 1 + theta*k2); CC = theta.*k2.*CB; plot(theta,CA, theta,CB,':', theta,CC,'--'), grid xlabel('Residence time, \theta [hours]'), ylabel('C [moles/L]') legend('C_A','C_B','C_C')
Reacting Systems
Page 4
Engineering Sciences 22 Systems
Summer, 2003
The maximum concentration of B, about 0.455 moles/liter, occurs with a residence time of 0.69 hr. The volumetric productivity is 0.45 moles L 0.68 hr moles L hr
= 0.67
To produce 100 moles/hr with this process, we would need a reactor volume of about 150 L. For this particular example, the batch reactor is superior to the continuous reactor.
1 0.9 0.8 0.7 C [moles/L] 0.6 0.5 0.4 0.3 0.2 0.1 0 0 0.5 1 1.5 2 2.5 Residence time, [hours] 3 3.5 4 CA CB CC
Intuitively, the superiority of the batch reactor makes sense. During the batch reaction cycle, CA is higher than its final value (increasing the rate of conversion of A to B) and CB is lower than its final value (decreasing the rate of conversion of B to C). By contrast, in the continuous reactor, CA and CB are at their effluent values for all time once the system is at steady state. Equivalent performance to the batch reactor may be realized in a continuous plug flow reactorthus providing the advantage of constant operation, with the better performance of the batch reactor.
Reacting Systems
Page 5
Engineering Sciences 22 Systems
Summer, 2003
Example 2: Respiration in a submersible vessel Problem statement Consider a submersible vessel designed for underwater exploration. How long can two persons be underwater, supported by the air initially present in the vessel? Heres what we know: Volume of the vessel = 20 m
3
Stoichiometry of respiration: C6H12O6 + 6 O2 6 CO2 + 6 H2O Properties of air at atmospheric pressure, 3 CO2 = 7 10 moles/liter CCO2 = 1 105 moles/liter Rate of metabolism, resting = 1 mole O2/hr per person Toxicity threshold, CCO2 1.8 103 moles/liter is toxic. Minimum oxygen to support human life, CO2 4 103 moles/liter required to support human life. Solution Rate law The rate of O2 consumption is fixed at 2 mole/hr (for 2 people), independent of concentration. The rate law is simply
rO 2 = 2 moles O 2 /hr 3 20 m = 0.1 moles O 3 m hr
2
= 0.0001
moles O L hr
The rate of CO2 production is the same as the rate of O2 consumption, since 1 mole of CO2 is produced for each mole of O2 consumed. Material balance The vessel may be modeled as a constant volume, well-mixed batch reactor with one reaction. The material balance equations are
Reacting Systems
Page 6
Engineering Sciences 22 Systems
Summer, 2003
dCO 2 = rO 2 dt dCCO 2 = rO 2 dt
This completes the formulation of this problem. At this point in the course, we generally cannot solve systems, except by using numerical methods. However, in this case we can, so it is useful to go ahead and solve it. Because these just have constants on the right hand side, we don't need any high-powered solution methods, and can just integrate them to get: CO 2 ,final CO 2 ,initial = rO 2 t CCO 2 , final CCO 2 ,initial = rO 2 t We sbstitute the toxicity thresholds for Cfinal and the nominal atmospheric concentrations for Cinitial, and solve for t.
moles moles 4 103 7 103 CO 2 , final CO 2 ,initial L L tO 2 = = = 30 hr moles rO 2 0.0001 L hr moles moles 0.01 103 1.8 103 CCO 2 , final CCO 2 ,initial L L t CO 2 = = = 17.9 hr moles rO 2 0.0001 L hr The CO2 buildup is the ultimate constraint. The time limit is just under 18 hr. The divers will suffer hypercapnia (too much CO2) before they become hypoxic (too little O2).
Example 3: Waste treatment pond Continuous feed, constant volume, well-mixed reactor, Cout = CA = C Material balance:
Q Q dC f = C + r + Cin V V dt
Reaction mechanism: A B with rate constant k rf = kC Combine material balance with rate law,
dC Q Q = C kC + Cin dt V V
Reacting Systems Page 7
Engineering Sciences 22 Systems
Summer, 2003
Steady state solution: Steady state implies derivatives are equal to zero. Setting the derivative equal to zero, we find: Css = Q V C = (Q V + k ) in
1 C (1 + kV Q) in
The concentration of pollutant leaving the pond can be reduced by increasing the volume or decreasing the flow rate (stuff stays around longer before being washed out), or designing a better reaction (higher k). For unsteady (transient) situations, we can use the differential equations above in a computer simulation, or solve them analytically, as we will learn later in the course. (The result comes out as
Cin t t C(t ) = C(0)exp + 1 exp (1 + kV Q )
1 Q V + k
V Q (1 + kV Q )
V/Q is the time constant in the absence of the reaction, also known as the residence time, or average time that a molecule of A is in the pond. That time is shortened by the reaction, since the reaction provides an alternate pathway to simply being washed out by the flow.) Example 4. Blood alcohol. Background. Quantitative modeling of biological systems is of interest both to test our understanding and to predict behavior of such systems. Understanding the dynamic behavior of blood alcohol levels, the subject of this example, is of obvious interest in terms of informing behavior and related guidelines aimed at responsible use of alcohol.
The following simplified model for blood alcohol absorption and reaction is based on that presented by Fogler (Elements of Chemical Reaction Engineering, 3rd ed., Prentice Hall, 1999.). This two-compartment model (Fig. 1) features ingested alcohol entering the gastrointestinal (GI) tract, transported across the stomach wall from the GI to the body fluid (BF), and then reaction in the body fluid. A more elaborate model would probably consider transport from the body fluid into the urine and subsequent excretion but these features are not examined here.
Fig. 1. Schematic representation of blood alcohol model (modified from Fogler) Ingestion GI Tract Absorption Body Fluid Reaction
Reacting Systems
Page 8
Engineering Sciences 22 Systems
Summer, 2003
The rate of transport ( rtransport, g alcohol/(L GI volumehr) ) is a function of the alcohol concentrations in the GI and BF, CA,GI and CA,BF respectively: rtransport = ktrans(CA,GI CA,BF)
where
ktrans = transport rate constant 10 hr-1
The rate of reaction of alcohol is limited by the concentration of a required co-enzyme rather than by the concentration of alcohol. Thus the rate can be said to be zeroth order (independent of concentration) with respect to alcohol as long as the concentration of alchol in the body fluid is greater than 0. The rate of ethanol being depleted by reaction is 0.192 g alcohol/(hL body fluid). Thus we have rreaction = 0.192 the sign of r depends on whether we are considering the GI (negative) or the BF (positive). Additional information: For m = body mass (kg) Body fluid volume, VBF = (0.5 L/kg)m Gastrointestinal tract fluid volume, VGI = (0.02 L/kg)m Amount of ethanol in a tall martini: 40 g Legal limits on blood alcohol, in terms of CE,BF (g ethanol/L body fluid) United States: 1 g/L Sweden: 0.5 g/L Following initial ingestion of alcohol, the GI fluid and body fluid may be modeled as well-mixed batch reactors. Each of these can be assumed to have constant volume over time, although their volumes differ from each other. We would like to know what time period is required after consuming two tall martinis before the blood alcohol level will be below the legal limit for the following cases: U.S. limit, 50 kg and 100 kg person Swedish limit, 50 kg and 100 kg person Formulation of the system model a. Rate laws (given above).
b. Material balances (this time for the same component in two different locations).
Reacting Systems Page 9
Engineering Sciences 22 Systems
Summer, 2003
For the GI, assuming that we have calculated an initial value of CA,GI following ingestion of alcohol:
dCA,GI = rtrans = - ktrans (CA,GI CA,BF ) (units: g/(L GI fluidhr) ) dt
[1]
For the BF:
VBF dCA,BF = VGI ktrans CE,GI + VBFrreaction (units: g/hr) dt
[2]
Dividing by VBF we obtain
dCA,BF = 0.04 ktransCA,GI + rreaction (units: g/(L body fluidhr) ) dt
[3]
Equations [1] and [3] constitute the system model.
Reacting Systems
Page 10
Engineering Sciences 22 Systems
Summer, 2003
Example 5. Oxygen sag curve. Background. Analysis of reacting systems arises can be applied to the environment with respect to the dynamic behavior of both engineered systems to consume a pollutant and also the behavior of the environment itself. This example concerns the derivation of a classic equation arising in the latter context, the Streeter-Phelps equation, which can be used to predict the dissolved concentration downstream of a point of pollutant discharge. Typically, we want to find the minimum oxygen concentration that will result, and where this will occur. This minimum can be compared to threshold values to determine if a proposed discharge is acceptable. Model formulation. The concentration of dissolved oxygen CO2 is a critical determinant of water quality. When wastewater (either municipal or industrial) containing organic matter is discharged into a stream, CO2 is determined by the balance between two competing processes: 1) oxygen depletion (deaeration) due to consumption of organic matter by aerobic organisms (primary microorganisms), 2) oxygen addition (reaeration) due to mass transfer from the air above the river. Consumption of organic matter commonly modeled as first order with respect to the concentration of organic matter, COM. Thus rOM = - k1COM It is standard to express COM in units of mg O2 necessary for organic matter oxidation per liter. With this convention, the consumption of organic matter is also equal to the rate of deaeration. Reaeration is commonly modeled as being proportional to the difference between the concentration of dissolved oxygen in equilibrium with air and the actual concentration of dissolved oxygen in the river. The concentration of oxygen in equilibrium with air is a physical property independent of the system state corresponding to the solubility of oxygen, denoted CO2.sat. Thus rreaeration = k2(CO2,sat CO2) Formulate a system model for the concentration of dissolved oxygen in the stream, modeling the stream as a plug flow reactor. Problem formulation. As in the previous example, the problem statement includes the rate laws, so we proceed directly to the material balance. Using a frame of reference moving at the velocity of the river, we can write material balance equations for both organic matter and oxygen: Organic matter: Oxygen:
dCO 2 dt
dCOM = rOM = - k 1COM dt
= rOM + rreaeration = - k1 COM + k 2 (CO 2 , sat - CO 2 )
Reacting Systems
Page 11
Engineering Sciences 22 Systems
Summer, 2003
Appendix: Solution to the first example problem using Laplace transforms, for reference. Laplace transformers
will be introduced later in the course. You arent expected to be able to follow this now, but if, after you learn Laplace transforms, you want to see how they can be applied to a chemical example, here you are. (It is rare that they can be applied, since usually chemical systems are nonlinear, but these equations are linear.)
After the Laplace transform is applied to the system model, it becomes:
k 1CA (s ) +
(s + k1 )CA (s) (s + k 2 )CB (s)
= CA , o = CB , o
k 2 CB (s ) + sC C (s ) = CC ,o We can just solve these algebraic equations top to bottom: CA (s) = CA , o s + k1 CB,o CB (s) = + s + k2 CC (s) =
k 1CA ,o = (s + k1 )(s + k 2 )
k1C A ,o (s + k 1 )(s + k2 )
CC ,o k 2 CB ,o k 1k 2 CA ,o k1 k2 CA ,o + + = s s(s + k2 ) s (s + k1 )(s + k2 ) s (s + k1 )(s + k 2 )
where we have used the fact that the initial concentrations of B and C are zero. Inverting the transforms by the usual means leads to
CA (t ) = CA ,o exp ( k1 t ) CB (t ) = CC (t ) k1 k1 CA ,o exp ( k1 t ) C A ,o exp ( k 2 t ) k2 k1 k2 k1 k2 k1 = CA , o CA ,o exp ( k1 t ) + CA , o exp ( k2 t ) k2 k1 k2 k1
Note that CA + CB + CC = CA,o, indicating that the total number of moles of the reacting species in the batch reactor must be the same for all time. The following Matlab code will plot the results % reacting systems example 1 % ENGS 22, Charlie Sullivan CA0 = 1; k1 = 3; k2 = 0.7; % Moles/L % 1/hr % 1/hr
t = linspace(0,5); CA = CA0 * exp(-k1*t); CB = k1*CA0/(k2-k1) * (exp(-k1*t) - exp(-k2*t)); CC = CA0 - CA - CB; plot( t, CA, t,CB,':', t,CC,'--') xlabel('Time [hours]'), ylabel('C [moles/L]') legend('C_A','C_B','C_C')
Reacting Systems
Page 12
You might also like
- Reaction Engineering Notes IDocument15 pagesReaction Engineering Notes Isaliljain2001No ratings yet
- CRE Book ErrataDocument3 pagesCRE Book Erratasaliljain2001No ratings yet
- KPA - Presentation - ABP 2012-13 & TARGET 13 - 14 PDFDocument30 pagesKPA - Presentation - ABP 2012-13 & TARGET 13 - 14 PDFsaliljain2001No ratings yet
- Chapter 29 Capital Budgeting PDFDocument28 pagesChapter 29 Capital Budgeting PDFlalita pargaiNo ratings yet
- Advanced CalculusDocument749 pagesAdvanced Calculussaliljain2001100% (10)
- The Time Value of MoneyDocument24 pagesThe Time Value of Moneysaliljain2001No ratings yet
- Math Prelims SolnDocument97 pagesMath Prelims SolnChristopher ThongNo ratings yet
- PCI For BF and Future of IronmakingDocument11 pagesPCI For BF and Future of Ironmakingsaliljain2001No ratings yet
- Coke Reactivity & Nut Coke EffectDocument8 pagesCoke Reactivity & Nut Coke Effectsaliljain2001No ratings yet
- Boilers and ThermicFluidHeatersDocument42 pagesBoilers and ThermicFluidHeatersvallamreddyNo ratings yet
- Quiz 1 SolutionDocument3 pagesQuiz 1 Solutionsaliljain2001No ratings yet
- Intro To Chem Engg (Exam)Document7 pagesIntro To Chem Engg (Exam)saliljain2001No ratings yet
- HW01 SolutionDocument8 pagesHW01 Solutionsaliljain2001No ratings yet
- Valve CharacteristicsDocument2 pagesValve Characteristicssaliljain2001No ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (120)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Melde's MethodDocument2 pagesMelde's MethodDR.P.V.Kanaka Rao0% (1)
- TSI 271210 GHH Two-Stage Compressor Repair InstructionsDocument18 pagesTSI 271210 GHH Two-Stage Compressor Repair InstructionsMax HudsonNo ratings yet
- GFM Lab ReportDocument102 pagesGFM Lab ReportÁlvaroDeLaGarza100% (1)
- Project Management Dashboard TemplateDocument10 pagesProject Management Dashboard Templateindra prasetyaNo ratings yet
- Ce 506 Prestressed Concrete Design: M R M M MDocument2 pagesCe 506 Prestressed Concrete Design: M R M M Mbadr amNo ratings yet
- Non Vital BleachingDocument19 pagesNon Vital BleachingPriyabrat Pattanaik100% (1)
- ERZG Blanking Plug Technical DatasheetDocument3 pagesERZG Blanking Plug Technical DatasheetEvolution Oil ToolsNo ratings yet
- Design CriteriaDocument6 pagesDesign CriteriaAllyssa Jane ErcillaNo ratings yet
- Parts Catalog: MF9280Cdn/9220Cdn/9170/ 9150/9130/8450 SeriesDocument94 pagesParts Catalog: MF9280Cdn/9220Cdn/9170/ 9150/9130/8450 SeriesAdorjan Sandor ZoltanNo ratings yet
- ASTMD2444 FixedImpactDocument8 pagesASTMD2444 FixedImpactGerardo Lopez GochiNo ratings yet
- GSM 06 InterfacesDocument17 pagesGSM 06 Interfacesamitcool26No ratings yet
- J Fluids Engineering 2009 Vol 131 N4Document120 pagesJ Fluids Engineering 2009 Vol 131 N4Нильва АлександрNo ratings yet
- SA35AC E01 MergedDocument87 pagesSA35AC E01 MergedClassyNo ratings yet
- MC600 ManDocument156 pagesMC600 ManEsteban PauloNo ratings yet
- Kata LogDocument48 pagesKata LogsofiakeramikNo ratings yet
- Total Chloride in Alumina Supported Catalysts by Wavelength Dispersive X-Ray FluorescenceDocument5 pagesTotal Chloride in Alumina Supported Catalysts by Wavelength Dispersive X-Ray FluorescenceJesus Gonzalez GracidaNo ratings yet
- CPAR Summary - WK 144Document6 pagesCPAR Summary - WK 144NagarajNo ratings yet
- Peri Up Flex Core ComponentsDocument50 pagesPeri Up Flex Core ComponentsDavid BourcetNo ratings yet
- Air Compressor Anatomy 101Document20 pagesAir Compressor Anatomy 101Rahul ChandrawarNo ratings yet
- Under Balanced OperationsDocument104 pagesUnder Balanced OperationsJavier Ignacio Meriles100% (1)
- The Centaur Upper Stage Vehicle HistoryDocument12 pagesThe Centaur Upper Stage Vehicle HistoryjuniormirandaNo ratings yet
- Pvu-L0880er GaDocument1 pagePvu-L0880er GaarunghandwalNo ratings yet
- Interactive CatalogDocument76 pagesInteractive CatalogahmedelhajNo ratings yet
- Microgel Particles and Their Effect On The Textural Properties of FoodsDocument9 pagesMicrogel Particles and Their Effect On The Textural Properties of Foodsmsa_imegNo ratings yet
- Poster Flender Com1 PDFDocument1 pagePoster Flender Com1 PDFvijaykumarnNo ratings yet
- Red MarsDocument16 pagesRed Marsfz.fathima50% (2)
- Wire Line Operation and EquipmentDocument122 pagesWire Line Operation and Equipmentmissaoui100% (3)
- Condensation Reactions ADocument28 pagesCondensation Reactions ANino FelicesNo ratings yet
- PC Smart Ptsgob8wDocument11 pagesPC Smart Ptsgob8wJose LopezNo ratings yet
- Chapter One Wind Load Analysis and Design 1.1 Wind ForcesDocument13 pagesChapter One Wind Load Analysis and Design 1.1 Wind ForcesYalem MesfinNo ratings yet