Professional Documents
Culture Documents
Watten 1985 Aquacultural-Engineering
Uploaded by
Jorge RodriguezOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Watten 1985 Aquacultural-Engineering
Uploaded by
Jorge RodriguezCopyright:
Available Formats
Aquacultural Engineering 4 (1985) 271-297
Modeling Gas Transfer in a U-Tube Oxygen Absorption System: Effects of Off-Gas Recycling Barnaby J. Watten and L. Todd Beck
Pennsylvania Power and Light Company, Department of Technology and Energy Assessment, Brunner Island Aquaculture Project, York Haven, Pennsylvania 17370, USA
ABSTRA CT A computer model characterizing the performance o f a u-tube oxygen absorption system was developed based on finite difference-mass transfer calculations. Performance was assessed in terms o f oxygen utilization, transfer efficiency and economy. The system evaluated was unique in that oxygen not absorbed initially (off-gas) was captured and recycled. Mass transfer coefficients derived from pilot-scale test data were used to calibrate the computer model. A separate series o f tests served to verify the effects o f off-gas recycling as predicted by the program. Simulation data indicate off-gas recycling can result in substantial savings in variable and total costs o f oxygen transfer with only a minor increase in capital expenditures, both initially and when amortized. The benefits achieved will increase with lower oxygen flow rates, deeper shaft depths, and low influent dissolved oxygen concentrations. Performance algorithms developed should be useful to those establishing design conditions for pure oxygen u-tube systems.
NOMENCLATURE A Area o f gas-liquid interphase (m 2) Oxygen absorption efficiency (%) B Bunsen's coefficient at a given temperature and salinity (litre litre-atm -1) C Existing concentration o f a gas in solution (mg litre -1) C* Saturation concentration o f a gas in solution (mg litre -~) 271 Aquacultural Engineering 0144-8609/85/$03.30- Elsevier Applied Science Publishers Ltd, England, 1985. Printed in Great Britain
AE
272
B. J. Watten, L. Todd Beck
CF
D d dC DO DOt DOo ADO DN dt
e
F g
c/L HL
AHL i K k KL KLa
N n
e 2o
ei eo
PT Pw
Qg Qm
Qox Qw
R %R %S T
Multiplier used to correct for the effects of off-gas recycling, dimensionless U-tube shaft depth (m) Diameter of a gas molecule (A) Change in concentration of a gas in solution (mg litre -1) Dissolved oxygen concentration (mg litre -1) Influent DO concentration (mg litre -1) Effluent DO concentration (mg/litre -~) Change in dissolved oxygen concentration between u-tube inlet and outlet (mg litre -~) Dissolved nitrogen concentration (mg litre -~) Time elapsed (h) Combined efficiency of compressor or pump and motor Pipe friction loss factor (Pa m -1) Acceleration of gravity (m s-2) Oxygen-liquid ratio in u-tube influent (%) Head loss across u-tube (cm) Difference in hydraulic head loss across u-tube due to oxygen injection (cm) Gas species identifier Isentropic index for gas mixture, dimensionless Ratio of molecular weight to molecular volume for a gas (mg ml -~) Coefficient of diffusion for a gas (m h -a) Overall gas transfer coefficient (h -1) Mass of the gas in the gas phase (mg) ( K - 1)/K, dimensionless Finite difference step identifier Vapor pressure of water (mm Hg) Absolute compressor inlet pressure (kPa) Absolute compressor outlet pressure (kPa) Total pressure (mm Hg) Power (kW) Volumetric flow rate of off-gas (m 3 h -1) Mass flow of gas mixture (kg s-1) Volumetric flow rate of oxygen (m 3 h-~) Volumetric flow rate of water (m a h -l) Gas constant Off-gas recycle rate (%) Saturation level of dissolved oxygen in the influent (%) Temperature (C)
Modeling gas transfer in a u-tube oxygen absorption system
273
TE
TI
VL AZ
Ot
X
Pox
Transfer efficiency (Kg 02 k W h -~) Absolute temperature of gas at compressor inlet (K) Volume of gas at a specific temperature (litre) Volume of liquid (m 3) Change in depth within the u-tube shaft (-+ m) KLa of wastewater/KLa of pure water, dimensionless C* of wastewater/C* of pure water, dimensionless Unit weight of water, kN m -3 Mole fraction of the gas in the gas phase, dimensionless Mass density of oxygen, kg m -3
INTRODUCTION A limiting supply of dissolved oxygen (DO) is a condition that often restricts the production capacity of intensive aquatic culture systems (Westers and Pratt, 1977). Although numerous methods for supplementing DO have been described (Chesness et al., 1972; Colt and Tchobanoglous, 1981) pure oxygen absorption systems appear particularly attractive. These systems have the unique capability of providing saturated or supersaturated DO concentrations economically while avoiding temperature change, noise, and nitrogen supersaturation problems associated with diffused air and/or surface aeration equipment. Speece (1981) describes five types of pure oxygen contact systems, each of which provides a high oxygen utilization efficiency with reasonable capital costs and energy consumption ratios. The five systems are: (1) enclosed packed column, (2) u-tube, (3) downflow bubble contact aeration, (4) recycled diffused oxygenation and (5) rotating packed column. Of the above, the u-tube was selected for evaluation at the Brunner Island waste heat aquaculture project, York Haven, Pennsylvania. A preliminary design analysis indicated the pressure drop across the u-tube would be within the range of the hydraulic head that is available at the proposed site. This characteristic not only provides a saving in energy costs but more importantly reduces the risk of system failure through the elimination of electrically operated mechanical equipment. Furthermore, the u-tube requires little space, is simple to construct and control. As originally described by Bruijn and Hendrik (1958), a u-tube system incorporates two basic components, (1) a gas diffuser and (2) a
274
B. J. Watten, L. Todd Beck
vertical u-shaped conduit which provides a contact loop of above atmospheric pressure. In use, air or oxygen is dispersed at a fixed rate in water entering the system. The gas-liquid mixture is then directed down one side of the u-shaped conduit and up through the other. Water velocity in the clown-leg portion is maintained above the buoyant velocity of the entrained gas bubbles. As the gas-liquid mixture moves through the contact loop a temporary increase in hydrostatic pressure serves to increase the dissolved oxygen deficit which in turn accelerates the rate of oxygen transfer. Previous research (Speece e t al., 1969; Speece and Orasco, 1970; Mitchell, 1973; Speece e t al., 1980) has shown that oxygen transfer in a u-tube system is influenced primarily by shaft depth, inlet gas flow and composition, water velocity, diffuser depth, and inlet DO concentration. In this report, a mass transfer model is developed for purposes of characterizing the performance of a u-tube system in which commercial, or pure, oxygen is dispersed. The system studied is unique in that oxygen not absorbed in the first pass is collected and immediately recycled. Speece e t al. (1983) suggested the use of the recycle step as a means of improving oxygen utilization and thereby reducing transfer costs. The algorithms we develop should be of value to those establishing design conditions for pure oxygen u-tube systems.
BACKGROUND
Gas transfer rate
The primary resistance to oxygen and nitrogen transfer in a gas-liquid mixture is usually provided by a stagnant liquid film present at the interphase between the gas and the liquid (Lewis and Whitman, 1924). The rate at which transfer occurs is then proportional to the difference between the existing and saturation concentration of the gas in solution. In differential form the relationship is expressed as dC
dt =
KL A
(C*-C)
Because of the difficulty in measuring the area of the gas-liquid interphase (A), the diffusion coefficient (KL) is often combined with
Modeling gas transfer in a u-tube oxygen absorption system
275
the ratio A / V L to establish an overall transfer coefficient KLa, i.e. dC - - = K L a ( C * -- C) dt (2)
The overall transfer coefficient will reflect the conditions present in a specific gas-liquid contact system. Conditions of importance include turbulence, waste characteristics of the liquid, the extent of the gasliquid interphase and temperature. Values of KLa can be corrected for the effects of temperature using the following expression (APHA, 1975):
(KLa)T = (KLa)2o (1-024) T-2
(3)
Although each gas species in a contact system will have a unique value of KLa , it has been established that relative values for a specific gas pair are inversely proportional to their molecular diameters (Tsivoglou et al., 1965), i.e.
( K L a ) J ( K L a ) 2 = (d)2/(d~)
(4)
The relationship above, based on Einstein's law of diffusion, provides a convenient means of establishing multicomponent gas transfer models (Speece and Orasco, 1970; Mitchel, 1973).
Gas solubility
The saturation concentration of a gas in solution (C*) will influence the direction as well as the rate of gas transfer (eqn (2)). The C* of a gas is a function of its partial pressure in the gas phase, liquid temperature, and liquid composition as related by Henry's law. In equation form (Colt, 1984),
( x ( P ~ -- Pr~o) ~ C* = B k 1 0 0 0 \ 760--0 /
(5)
The C* of oxygen in a u-tube system is increased temporarily by hydrostatic pressure. Those systems in which pure oxygen is dispersed further increase C* by increasing the mole fraction of oxygen in the gas phase. The increase in C* serves to (1) accelerate the rate of gas transfer (eqn (2)), and (2) provide the capability of achieving an effluent dissolved gas level in excess of the air saturation concentration at local barometric pressure.
276
B. J. Watten, L. Todd Beck
MODEL DEVELOPMENT A computer model of the gas transfer process was developed based on eqns (2)--(5). The program simulates the performance of an experimental u-tube system in which pure oxygen is dispersed and off-gas is recycled. Assuming carbon dioxide concentrations are negligible, two primary gas transfer operations will occur in such systems. These are (1) oxygen transfer from the gas phase to the liquid phase and (2) nitrogen transfer from the liquid phase to the gas phase (Speece, 1981). The program developed here accounts for these changes as it performs finite difference calculations. The logic and major components of the computer program are presented in Fig. 1.
READ INPUT /
I I
CALCULATE BACKGROUND PARAMETERS ESTIMATE INITIAL VALUES OFKL.aoxyg, nandKL'aoitrogen
I
I /
t
MAKE FINITE DIFFERENCE CALCULATIONS THROUGH BOTH LEGS OFTHEU.TUBE ISOFF.GAS RECYCLED?
Y E S
ISCexitSUFFICIENTLY CLOSE TOPREVIOUS Cexit? >
NO
BASED ONOFF.GAS CHARACTERISTICS AND OFF.GAS RECYCLE RATE CALCULATE NEWINLET GAS COMPOSITION ANDINLET GAS.LIQUID RATIO CALCULATE EXITCONDITIONS ANDSUMMARIZE PERFORMANCE
PRINT OUTPUT /
Fig. 1.
Computation sequence used in the computer model of the experimental u-tube system.
Modeling gas transfer in a u-tube oxygen absorption system
277
Finite difference calculations Finite difference calculations are similar to those employed by Speece and Orasco (1970). The following variables are recalculated at each distance step in the program: (1)mass of oxygen and nitrogen in the gas phase; (2) total pressure; (3) overall transfer coefficients; (4) dissolved gas deficits; and (5) changes in dissolved gas concentrations. At the start of each calculation series, the Ideal Gas law is used to relate volume, temperature, pressure and molar composition of the gas phase. Pressure at each step, n, neglecting pressure drop due to two-phase flow, is defined as follows:
(PT)n+I = (eT)n - - (AZPg)n -- (I A Z I)n ff
(6)
The change in hydrostatic pressure that occurs in a u-tube system affects the number, not the size, of the gas bubbles present (Speece and Orasco, 1970; Mitchell, 1973). Therefore, given the volume of gas at any two program steps n, a relative interfacial area to volume ratio can be determined, i.e.
(A/VL)nl/(A/VL)n2
=
(Vg)nl/(Vg)n2
(7)
Since KLa is the product of A/VL and the constant KL (eqn (1)), relative values of KLa may be established in a similar manner (Speece and Orasco, 1970): (KL at)nJ (KL ai),,: = ( Vg),n/( Vg)n: (8)
The expression above is used to calculate relative values of KLa at each step n with respect to u-tube inlet conditions. Based on eqn (4), values of KLa for nitrogen are assumed to be 0.9 times that estimated for oxygen. Gas saturation concentrations required to establish dissolved gas deficits are calculated using Henry's law (eqn (5)). Bunsen coefficients (Bi) and vapor pressures (PH20) used in this determination are obtained from equations presented by Weiss (1970) and ASHRAE (1972), respectively. Given the gas deficits and KLa values, the changes in dissolved gas concentrations are established using rate expression, eqn (2), i.e.
(Ci)n+l = (Ci)n + ( K L a i ( C ~ - - C i ) ) n
dt
(9)
The change in gas phase mass is determined in a similar manner by performing a mass balance on the volume segment represented by
278 step n.
B. J. Watten, L. Todd Beck
(~'li)n+l = (~t)n -- ( K L a i ( C * -- Ci)) VL dt
(10)
Upon completing finite difference calculations, exit conditions are identified and performance indicators calculated.
Performance indicators
U-tube performance is evaluated in terms of transfer costs, transfer efficiency and oxygen absorption efficiency. The latter represents the ratio of mass oxygen absorbed to mass oxygen applied, as defined in the following expression: A E = (Qw (D~-~0ox~DO01-3) 1 0 0 _ (11)
Transfer efficiency represents the mass of oxygen absorbed per unit of energy input. In the experimental u-tube, total energy input is the sum of the energy used in pumping water (Pw, pump) and recycling off-gas (Pw, compressor), e.g. TE = Qw (DOo -- DO010-, Pw, pump + Pw, compressor (12)
Energy used in pumping water is calculated as follows: Pw, pump = ((Qw/3600) (HE single phase + AHL) q,)/e (13)
An estimate of the energy required to recycle off-gas is obtained from the adiabatic compression formula (Yunt, 1979): Pw, compressor QmRTI[(Po~N__I] N ~ L\-~-i / (14)
MODEL CALIBRATION Prior to model use, two series of tests were undertaken to establish a predictive equation for head loss due to two-phase flow, and for KLaoxygen at the u-tube entrance (step n = 1). KLaoxygen values at other points are calculated using eqn (8). In all tests water flow was held constant at 197 litre min -~.
Modeling gas transfer in a u-tube oxygen absorption system
279
OFF.GAS RECYCLE [~'1" METERED 02FLOW--* OFF.GAS COLLECTOR DIS~,~,__. "--~
HIGH PRESSURE Jl] ~ WATER SUPPLY ~,S- WATER JETEXHAUSTER I..1 WATER FROM CONSTANT " HEAD RESERVOIR " ~ WATER SAMPLE PORT /
~,.~
--~ 6.35cm PIPE ,S 10.16cm CASING
DISCHARGE
50 LITER VOLUME ~
~' ~ WATER SAMPLE PORT
~
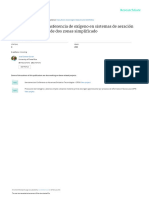
Fig. 2. Test apparatus
Diagram of the pilot scale u-tube oxygen absorption system incorporating off-gas recycling.
The experimental u-tube is illustrated in Fig. 2. As shown, the system consists of four major components. These are (1) a concentric pipe u-tube, (2) an oxygen sparger, (3) an off-gas collector, and (4) a water jet exhauster. The u-tube was fabricated with PVC pipe and standard threaded fittings. U-tube t y p e flow was achieved by positioning a 6.35 cm outside diameter pipe section (down-leg shaft) in the center and slightly off the b o t t o m o f a capped section of 10.16 cm outside diameter pipe. The cross sectional area o f the down-leg and annular space in the up-leg shafts were 30.3 cm 2 and 39-1 cm 2, respectively. Pure oxygen was dispersed through a 6 mm diameter orifice positioned near the discharge of the water jet exhauster (Fig. 2). A pressure regulator, throttle valve and rotameter (Brooks Model 1110) were used to meter the oxygen flow. A capped section of pipe 20.2 cm in diameter and 133 cm in length served as the off-gas collector. The effective volume o f the chamber was 50 liters which, when used, provided sufficient detection time for separation o f undissolved gases. The discharge end of the collector was elevated slightly (8-8% slope) to concentrate gas near the vacuum line inlet (Fig. 2). The water jet exhauster pro-
280
B. 3". Watten, L. Todd Beck
vided the vacuum necessary to recycle collected off-gas. The exhauster was operated at a water pressure of 241 kPa resulting in a water consumption rate of about 15 liter min -1. Water at this pressure was supplied by a jet pump coupled with a pressure tank and regulator. An elevated head tank was used to maintain gravity flow through the u-tube. The tank received unaltered water from the Susquehanna River pumped from a point near the Brunner Island Aquaculture Project. The head tank also provided water for the jet pump system. Water flow was measured with a stopwatch and barrel of known capacity. U-tube influent and effluent samples were obtained from sample ports located upstream of the exhauster and just below the discharge (Fig. 2). DO concentrations in samples were determined using the azide modification of the Winkler method (APHA, 1975). During head loss tests, manometer tubes connected to the water sample ports described above provided influent and effluent static pressure readings. Barometric pressure, water and gas temperature were measured with a standard mercury barometer and thermometer.
Methods Test
Series I
The effect of influent oxygen-liquid ratio and shaft depth on head loss due to two-phase flow was determined. Oxygen-liquid ratio here is defined as the volumetric flow rate of oxygen under standard conditions (21 .IC and 760 mm Hg pressure) divided by the volumetric flow rate of water. Oxygen was metered into u-tubes 6.10, 12.19 and 18.29 m deep. During tests, oxygen-liquid ratios were varied from 0 to 28%. Water flow was regulated by adjusting a valve positioned upstream of the influent sample port. Following data collection, head loss due to two-phase flow (AHL) was computed using recorded pressure drops across the system (HL) under single-phase (water) and two-phase (oxygen and water) flow conditions, i.e. AHL = HE two phase -- HL singlephase
Methods - T e s t S e r i e s II
(15)
In this second test series, the effect of influent oxygen-liquid ratio and shaft depth on oxygen transfer was evaluated. Oxygen was metered into u-tubes 6.10, 12.19 and 18.29m deep. Oxygen-liquid ratios were
Modeling gas transfer in a u-tube oxygen absorption system
281
varied from 2.3 to 11.7%. Water flow was regulated by adjusting the u-tube discharge valve. Because off-gas was not recycled, the water jet exhauster was operated with its vacuum line closed. At each established combination of depth and oxygen-liquid ratio, samples were taken for DO analysis once steady-state conditions had been maintained for a minimum of 10 min. Reagents used in the DO analyses were added immediately after sample collection:
Results - Test Series I
Data illustrating the effect of u-tube depth and influent oxygen-liquid ratio on head loss (AHL, eqn (15)) are presented in Fig. 3. Note that as the depth and/or oxygen-liquid ratios increased so did AHL. As a consequence, and due also to a limiting available hydraulic head, the range of oxygen-liquid ratios evaluated at a shaft depth of 6.10 m
1.78. U-TUBEDEPTH D 6.10m ~, 12.19m :, 18.29rn / J / ~
1.52.
1.27.
,~
1.02.
0.76_
0.51_
0.25_
0.00
;2
lh
~4
3~
INFLUENTOXYGEN.LIQUIDRATIO,PERCENT
Fig. 3. Effects of u-tube depth and influent oxygen-liquid ratio on head loss due to two-phase flow (ZM-/L, eqn (15)). Water temperature and influent DO during tests were 23.4-23-9C and 7-4-7-8 mg liter-z, respectively.
282
B. J. Watten, L. Todd Beck
exceeds the range evaluated at greater depths (12.19 and 18-29 m). Multiple linear regression was used to establish a model of the interaction observed between AHL, oxygen-liquid ratio, and depth: In AHL = 0.888 + 0-0758(D) + 1.021 (In G/L) R 2 = 0-98
R e s u R s - T e s t S e r i e s II
(16)
The effect of u-tube depth and influent oxygen-liquid ratio on oxygen transfer is illustrated in Fig. 4. As shown, increasing the oxygen flow rate increased effluent DO concentrations at a diminishing rate while reducing absorption efficiency. It can also be seen that both exit DO and absorption efficiency increased with greater shaft depths. The gas transfer program was used to establish values of KLaoxygen for each set of observations by determining the KLa value at the u-tube entrance that resulted in the observed effluent DO concentration. During this iteration procedure, influent dissolved nitrogen was assumed to be at the saturation concentration estimated using eqn (5). Established KLaoxygen values were adjusted to 20C using eqn (3). Multiple linear regression was then used to define KLaoxygen in terms of influent oxygen-liquid ratio and shaft depth:
(KLaoxygen)2oOc = 62-1161 --4-3331(D) + 67.7577(lnG/L)
R 2 = 0.90
(17)
The above expression, and that defining AHL established in Test Series I, were incorporated into the computer model of the u-tube system.
MODEL VERIFICATION Using the apparatus evaluated in Test Series I and II, the effect of off-gas recycling on oxygen transfer was determined for purposes of (1) demonstrating the feasibility of the recycle step and (2) verifying the effects of off-gas recycling as predicted by the calibrated computer model.
Modeling gas transfer in a u-tube oxygen absorption system
60-
283
48-
36+
24.
===
12
3510
~
E
28
14.
//
8.10
OXYGEN. LIQUIDRATIO,PERCENT
Fig. 4. Effects of u-tube depth and influent oxygen-liquid ratio on oxygen transfer. Original data had variations in influent DO (8.6-9.4 mg liter-l), temperature (26.7-27-2C) and barometric pressure (757-760 mm Hg). Data presented here have been adjusted to a standard set of conditions (temperature, 27C; influent DO, 7.95 mg liter-l; barometric pressure, 760 mm Hg) using the computer program and KLa values established for each observation.
Methods - Test Series III Off-gas recycle rates of 25, 50 and 60% were established at a shaft depth o f 12.19 m. Initial influent oxygen--liquid ratios were varied from 2.3 to 6.2%. As in Test Series II, water flow was regulated by adjusting discharge valves. When recycling off-gas at a specific rate (e.g. 50%),
284
B. J. Watten, L. Todd Beck
t h a t same f r a c t i o n o f u-tube flow was diverted t h r o u g h the off-gas collector. Undissolved gas entering this device was collected and i m m e d i a t e l y r e c y c l e d . Water flow in the recycle line (clear tubing) was k e p t to a m i n i m u m b y adjusting a v a c u u m r e g u l a t o r valve (Fig. 2). C o l l e c t o r e f f l u e n t was d i r e c t e d t h r o u g h a clear c a r b o y o f 19-liter capacity to visually test for c o m p l e t e s e p a r a t i o n and use o f available off-gas. Samples were o b t a i n e d for DO analysis a f t e r steady-state c o n d i t i o n s had b e e n m a i n t a i n e d for a m i n i m u m o f 10 min.
R e s u l t s - T e s t S e r i e s III
Due to a limiting h y d r a u l i c h e a d , observations at the 60% off-gas recycle rate were r e s t r i c t e d to an i n f l u e n t o x y g e n - l i q u i d ratio o f 2.3%. Table 1 presents a c o m p a r i s o n b e t w e e n Test Series III data and gas transfer m o d e l predictions. As s h o w n , m o d e l p r e d i c t i o n s are in very close a g r e e m e n t with the pilot s t u d y data. T h e relative e r r o r o f the estimates average just 2-9% with a range o f 0-9 t o 6.3%. Data in Table 1
TABLE 1
Comparison Between Observed (Test Series III) and Model Predicted Effluent DO Concentrations at Several Rates of Off-Gas Recycling. U-tube Shaft Depth, 12-19m, Temperature, 25-6-27.8C; Barometric Pressure, 757-762 mm Hg
Oxygenliquid ratio (%)
2.3 2.3 2.3 2.3 4.1 4-1 4.1 6-2 6.2 6.2
Off-gas recycle rate (%)
0 25 50 60 0 25 50 0 25 50
Influent DO (mg liter -1)
Mean AE (%)
Mean Predicted Residual effluent effluent as DO DO percent (mgliter -1) (mgliter -1)
2 3 3 3 21.8 23.3 24.4 27-3 27.9 30.5 31-1 31.4 34.4 35.6 22.3 23-7 25.1 26-7 28.9 30.2 32-2 33.4 34.7 36-9 + + + -2.3 1.7 2.9 2.2
9.4 9.0 8.3 9.4 9.4 8.8 8-3 9-4 8-5 8-3
41-7, 48-0, 54.3, 60.5,
N N N N
= = = =
34.6, N = 2 40-7, N = 3 43-1,N = 2 26.9, N = 2 31.6, N = 2 33.3, N = 2
+ 3.6 -- 1.0 +3.5 + 6.3 + 0-9 + 3.7
Modeling gas transfer in a u-tube oxygen absorption system
285
also demonstrate the substantial increase in oxygen utilization that can be achieved by off-gas recycling; e.g. with an influent oxygen-liquid ratio of 2.3%, recycling 60% of the available off-gas resulted in a 45% increase in absorption efficiency.
MODEL APPLICATION Effects of off-gas recycling
Absorption efficiency Following Test Series III, the computer program was used to simulate the response of u-tube effluent DO to changes in design and operating conditions. Results of a simulation run used to establish the effects of the off-gas recycle rate are illustrated in Fig. 5. Note that as the recycle rate approached 100%, effluent DO rose steadily from 15.0 to 28.5 mg liter -1. The improvement in oxygen utilization occurs in response to the increase in the effective gas-liquid ratio despite the concurrent reduction in the oxygen purity level of the influent gas mixture (Fig. 5). Additional simulation runs indicate the improvement in oxygen utilization, resulting from a fixed rate of off-gas recycling, will increase with (1) greater shaft depths (Fig. 6), (2) lower pure oxygen flow rates (Fig. 6), and (3) lower levels of DO in the influent (Fig. 7). Temperature has a relatively minor effect at a given saturation level of DO in the influent (Fig. 7). The rise that occurs in the gas-liquid ratio with off-gas recycling will be more dramatic than indicated in Fig. 5 at higher pure oxygen flow rates. This effect may limit the off-gas recycle rate, i.e. the two-phase flow that occurs in a u-tube is, under normal conditions, represented by the bubble flow regime, a continuous liquid phase in which a gas is homogenously dispersed as bubbles (Mitchell, 1973). If the gas-liquid ratio becomes excessive, bubbles coalesce forming large bullet-shaped slugs of gas. The transition to slug flow should be avoided as it results in discharge surging and a rapid increase in A H L. The maximum acceptable gas-liquid ratio is influenced primarily by velocity (Speece et al., 1969), although shaft depth and diameter may also have an effect (Mitchell, 1973). Speece et al. (1969) suggest a 20% air-liquid ratio is about the limit at a velocity of 1.22 m s-1 while at velocities of 1.832.44 m S -1 a 25% air-liquid ratio was satisfactory. In Test Series I,
286
B. J. Watten, L. Todd Beck
COMPUTERSIMULATION
8-
~_d
6-
=~
o
o
42-
--~ ~ z
....
OXYGEN " "
60
0 958065-
, ' ,--""
I S O 3.0
2.5
2.0 ~
"
t5 ~
/
35
, , ,
ABSORPTIOEFFICIENC' N ~
, 1.0
13.0
25E
"
20-
-11.25~9. ~z5 -0 E
15-
. . . . DISSOLVED NITROGEN 7.75 DISSOLVED OXYGEN
20
40 60 80 RECYCLE RATE, PERCENT
100
6.00
Fig. 5. Effect of off-gas recycling on effluent DO and DN, oxygen absorption efficiency, transfer efficiency, effective gas-liquid ratio and off-gas composition. Temperature, 22.5C; influent DO, 4.32 mg liter-1, influent DN, 14-23 mg liter-Z; shaft depth, 18.29 m; velocity in down-leg 1-08 m s-l; velocity in up-leg, 0.84m s-z.
Modeling gas transfer in a u-tube oxygen absorption system COMPUTERSIMULATION
287
1.40 1
1'321 1.24-
oo
1.16-
......................
~--~
2.0%GIL
1.08-
..... 3.0%GIL - - - 5.0% G/L
1.O0 4.0
810
12'0
li0
20.0
SHAFTDEPTH,METERS
Fig. 6. Effect of initial oxygen-liquid ratio (G/L) and u-tube shaft depth on the increase in DO resulting from an off-gas recycle rate of 60%. Temperature, 30C; influent DO, 7.54 mg liter-l; velocity in down-leg, 1-08 m s-l; velocity in up-leg, 0-84 m s-1.
1.7
COMPUTER SIMULATION
1.8
==_
o. =E
1.51.4
15.0C 1.3- - - 22.5C ..... 30.0C
12
2'5
sb
;5
100
-I
INFLUENTDO(PERCENT OFSATURATION)
Fig. 7. Effect of influent DO (% of saturation) and water temperature on the increase in DO resulting from an off-gas recycle rate of 60%. U-tube shaft depth, 18.29 m;initial oxygen-liquid ratio, 2%.
288
B. J. Watten, L. Todd Beck
pressure oscillations were observed when the oxygen-liquid ratio exceeded 28% (shaft depth, 6-10 m; system flow rate, 197 liter min-1).
Transfer efficiency Simulation runs indicate transfer efficiency will vary widely with operating conditions. At low influent oxygen-liquid ratios, there is an off-gas recycle rate that will maximize transfer efficiency (Fig. 5). At higher oxygen flow rates, off-gas recycling will result in reduced transfer efficiency. When off-gas is not recycled, there is an oxygen flow rate that will provide maximum transfer efficiency at a given u-tube depth. It is also clear that u-tubes of shallow construction will favor transfer efficiency, e.g. with an influent oxygen-liquid ratio of 5%, transfer efficiency at shaft depths of 18.29 and 6.10 m would be 4.02 and 13.87 kg 02 kW h -1, respectively (temperature, 30C, percent saturation of DO in the influent, 100%). Transfer economy Amortized capital and bulk oxygen are the expenditures of primary importance in the operating budget of a pure oxygen u-tube system. Unlike conventional aeration systems (e.g. surface aerators) energy costs are minimal. Figure 8 provides a comparison of oxygen transfer costs established for a u-tube of 18.29 m depth with and without off-gas recycling. In the analysis, unit oxygen and electricity costs were assumed to be $0-146kg-IO2 and $0-055 kWh -1, respectively. The initial capital costs used are summarized in Table 2. From this comparison the following observations were made:
1. Incorporation of the recycle step results in a minimal (8.4%) increase in the total annualized equipment cost (Table 2). 2. Off-gas recycling reduced variable costs by 49-55%. Total transfer costs were reduced by 25-41%. The savings achieved increase with greater effluent concentrations of DO (Fig. 8). 3. When off-gas is not recycled, there is an effluent DO at which total transfer costs are minimized (Fig. 8). 4. Variable costs for both systems evaluated increase with greater concentrations of effluent DO (Fig. 8).
Performance algorithms
U-tube simulation data were modeled to establish performance algorithms for those who do not have access to computer equipment.
Modeling gas transfer in a u-tube oxygen absorption system
289
0.660-~ 0.5940.5280.462~ 0.3960.330 0.550.44--
COMPUTERSIMULATION
t'a
~ 0.33o 0.22-
~ 0.11
0,00
.....
NO OFF.GASRECYCLING WITH 90% OFF.GASRECYCLE RATE
20
2'3
2'6
3'2
EFFLUENT DO,mglLITER
Fig. 8. Effect of off-gas recycling on transfer economy. Temperature, 22-5C; influent DO, 4-32 mg liter-Z; shaft depth, 18-29 m; velocity in down-leg, 1.08 m s-l; velocity in up-leg, 0-84 m s-z.
The algorithmic functions established describe data generated in a series of 432 computer runs in which at each o f three u-tube depths (6-10, 12-19, and 18.29 m) all combinations of the following independent variables were tested: influent oxygen-liquid ratio, 2, 5, 8 and 12%; water temperature, 15, 22-5 and 30C; percent saturation of DO in the influent, 0, 50 and 100%; and off-gas recycle rate, 0, 30, 60 and 90%. The configuration of the system evaluated is the same as that used in Test Series I-III. Barometric pressure and liquid flow were held constant at 760 m m Hg and 197 liter min -~, respectively.
290
B. J. Watten, L. Todd Beck TABLE 2
Summary of Costsa for a Concentric Pipe U-tube 18.29 m Deep. Inner Pipe Diameter, 0-203 m ;Outer Casing Diameter, 0.305 m. It is Assumed That Adequate Hydraulic Head is Available for U-tube Operation
Parameter US dollar, 1983
U-tube (PVC) Shaft drilling @ $171 m -1 Casings installed @ $122-6 m-1 Flow control valve Miscellaneous fittings Liquid flowmeter Assembly Recycle equipment Off-gas collector Off-gas blower Assembly Oxygen delivery system Liquid oxygen tank (rental) Oxygen metering system Oxygen sparger Assembly Annualized equipment cost b Without off-gas recycling With off-gas recycling
3 129 2 242 245 463 976 240 2 100 473 120 3 300 year-1 1 600 320 270 4 693 5 088
a Equipment cost estimates are based on bids received for construction at Brunner Island. b Capital recovery factor used is based on a 12% interest rate and a 15-year amortization period.
Following data collection, multiple linear regression was used in conj u n c t i o n w i t h c o m p u t e d correlation o f coefficient values to establish the m o s t appropriate m o d e l form for a given data set. A regression e q u a t i o n predicting e f f l u e n t DO as a f u n c t i o n o f the i n d e p e n d e n t variables tested, excluding %R, is listed below:
Modeling gas transfer in a u-tube oxygen absorption system DOo = -- 3.77 + 12-198(1n G/L) + 0-9069(D) -- 0.1405 (T) + 0-0575 (%S) R 2 = 0.92; SE = 2.97
291
(18)
To correct the above for the effects of off-gas recycling, a dissolved gas multiplier o f the following form was established: D O C F = (DOo)recycling/(DOo)no recycling The regression equation defining the DO o multiplier is In DOCF = 0-16347 -- 0-0922(ln G/L) + 0 . 0 0 2 7 9 ( D ) -- 0-00143 (T) -- 0-00078 (%S) + 0.00393 (%R) R 2 = 0.86; SE = 0.049 A multiplier such as that defined in eqn (19) was established to similarly correct the influent gas-liquid ratio for the effect o f off-gas recycling. The multiplier was postulated to be of the following quadratic form: G / L C F = (1/2X 2) + 1 (21) (20) (19)
The term X is defined below as a function of the independent variables included in eqn (20): l n X = -- 1.0216 + 0.1363(ln G / L ) ~ 0.01(D) + 0 . 0 0 0 9 9 ( T ) + 0.00043(%S) + 0 . 0 2 0 5 6 ( % R ) R 2 = 0-99; SE = 0.055 The algorithms presented here, when used in conjunction with models described in previous sections, provide a relatively simple means of predicting u-tube performance within the range o f operating conditions evaluated. For example, given shaft depth, water temperature, influent DO saturation and gas-liquid ratio, the response o f effluent DO to various rates of off-gas recycling can be characterized (eqns (18)-(20)). Oxygen absorption efficiency can be obtained from eqn (11). To establish transfer efficiency, the power required to recycle off-gas (Pw, compressor) and p u m p water (Pw, pump) must be known (eqn (12)). Pw, pump can be determined by adding the estimated head loss resulting from pipe friction, velocity and elevation changes to the head loss due to two-phase flow (eqn (13)). The latter, AHL, is obtained (22)
292
B. J. Watten, L. Todd Beck
from eqn (16) using a corrected influent gas-liquid ratio (eqns (21) and (22)). Pw, compressor is calculated using the expression for adiabatic compression (eqn (14)). The absolute pressure differential required, neglecting gas conduit friction losses, is taken as the total pressure drop across the u-tube. The volumetric flow of off-gas being compressed can be derived by subtracting the pure oxygen flow rate from the effective gas--liquid ratio, i.e. ag = (((G/LCF" G/L) -- G/L)/100) Qw (23)
To obtain the mass flow rate from the above, an estimate of the molar composition of the off-gas is required. An expression describing the off-gas mixture (primarily O~ + N2) has not been provided. However, if one assumes the gas is pure oxygen or air, a reasonable approximation of the mass flow and hence Pw, compressor, can be obtained. Disperser depth, system flow rate, and water quality are design variables that have not been incorporated in the computation sequence just described. Their potential effects are addressed briefly in the following paragraphs. Disperser depth Increasing disperser depth will result in reduced oxygen transfer and AHL (Speece et al., 1969). Implicit in the performance algorithms presented above is that commercial oxygen and recycled off-gas is introduced in water at the top of the down-leg shaft. The dispersion of gas at this point will maximize oxygen utilization while also minimizing compressor power requirements. System flow rate The algorithmic functions derived assume a velocity of 1.08 m s-1 in the down-leg and 0.84 m s-1 in the up-leg shafts. Higher velocities may result in a moderate reduction of both effluent DO and AHL (Speece et al., 1969; Mitchell, 1973; Speece et al., 1980). Figure 9 gives a comparison of oxygen transfer data obtained at two flow rates in the experimental u-tube system (Fig. 2). Note that oxygen transfer at 274 liter min -x is nearly the same as that obtained at 197 liter min -~ despite a 39% reduction in residence time. A comparison of corresponding KLa values derived as in Test Series II, and plotted below the transfer data, indicate the reduction in residence time was off-set in this case by increases in the overall transfer coefficient. The increases in KLa are
Modeling gas transfer in a u-tube oxygen absorption system
25.0-
293
20.0|& |
t5.0-
I
t:
FLOW:274 LITERIMIN. FLOW: 197 LITERIMIN.
=d
loo5.0-
0.0 360-
288-
210-
~ 14472"
oo
31o
61o
OXYGEN.LIQUIDRATIO,PERCENT
mlo
12'o
Fig. 9. Effect of system flow rate on oxygen transfer (ADO) and overall oxygen transfer coefficient (KLa). Shaft depth, 6-10 m. Data have been adjusted to a standard set of conditions (temperature, 27C; influent DO, 7.95 mg liter-l; barometric pressure, 760 mm Hg) using the computer program as in Fig. 4.
attributed to the greater levels o f turbulence associated with the higher flow rate (Mauvinic and Bewtra, 1976).
Water quality
The performance algorithms were established with the assumption that pure water is being treated. Wastewater characteristics such as BOD, COD and suspended solids will, if present, affect both the rate and extent o f gas transfer. The alpha factor (a) is used to represent the ratio o f KLa in wastewater to KLa in pure water under a c o m m o n set o f operating conditions (Gilbert, 1979), i.e. ot
=
(K La ) w a s t e w a t e r / ( g L a )pua-e water
(24 )
294
B. J. Watten, L. Todd Beck
Similarly, the beta factor (13) represents the ratio o f C* in wastewater to C* in pure water (Gilbert, 1979):
13 -'~ ( C * )wastewater/ ( C * )pure water
(25)
o~ and (3 values were incorporated into eqns (9) and (10) of the computer model. A series o f computer runs were then used to simulate the effects of various combinations of the waste correction factors. The effect o f two combinations on oxygen transfer are given in Table 3. Note that the percent reduction in the change in DO (ADO)remained relatively constant for a given combination o f ot and 13 despite changes in influent oxygen-liquid ratio. Additional runs indicated the percent reduction in ADO was also independent of shaft depth, water temperature and influent DO. Thus the effect o f a given combination of waste correction values can be approximated using a contingency table of ADO multipliers established for a single set o f operating conditions. Multipliers established for a u-tube o f 18.29 m depth are plotted in Fig. 10. It is apparent that the response o f ADO to changes in both ot and 13 is linear. Under the worst case condition represented (i.e.
TABLE 3 Effect of Wastewater Quality on Oxygen Transfer in a U-tube Oxygen Absorption System. Data are Effluent DO in mg liter-1 Followed by the Percent Reduction in ADOa due to a and /3 Values of < 1. Temperature, 22.5C; DO Saturation in Influent, 50%; U-tube Depth, 18.29 m; Velocity in Down-leg Shaft, 1-08 m s-1;Velocity in Up-leg Shaft, 0-84 m s-a
]nfluen t oxygen-liquid ratio (%) Wastewater quality ~ and /3 = 0. 9 a and /3 = 0.8
2 5 8 12
13-4 (15.0%) 27.5 (14.1%) 34-1 (14-4%) 39-2 (14.5%)
11.8 (30.0%) 23-7 (28.2%) 29-1 (28.8%) 33.4 (28.7%)
a ADO = (DO)emuent -- (DO)influent.
Modeling gas transfer in a u-tube oxygen absorption system
295
1.00-
COMPUTER SIMULATION~
0.~-
0.82- ~
~=o.~
0,76-
0,70
0.80
o.~s
ALPHA
o.~o
o.~s
~.~o
Fig. 10. Effect of a and 13waste correction values on oxygen transfer. Temperature, 22-5C; influent DO, 4.32 mg liter-l; velocity in down-leg, 1.08 m s-l; velocity in up-leg, 0.84 m s-1. ADO multiplier = (mDO)w~ewater/(,~DO)pure water.
t~ = 0.8; (~ = 0-8), oxygen transfer is reduced to a level o f about 72% o f that expected with pure water. In addition to ~ and ~ factors, u-tube performance will also be affected b y influent dissolved nitrogen (DN) and carbon dioxide (DCO2) concentrations. The algorithms established assume DN and DCO2 are at normal saturation concentrations. Treatment of water with DN and DCOz above C* will reduce further the mole fraction of 02 in the gas phase within the u-tube which, in turn, will decrease oxygen transfer (eqns (2) and (5)). The extent o f the effect will increase with (1) the supersaturation level o f DN and DCO2, and (2) the off-gas recycle rate. In summary, data presented in this report indicate off-gas recycling can result in a substantial savings in both variable and total costs of oxygen transfer with only a minor increase in capital expenditures. The great flexibility in effluent DO concentration provided by u-tube oxygen absorption systems has also been demonstrated. This performance characteristic, combined with simple construction and low energy requirements, makes aquacultural applications of such systems highly attractive.
296
B. J. Watten, L. Todd Beck ACKNOWLEDGEMENTS
We thank T. Fridirici, B. Nguyen and M. Srnyser for assistance in data collection and J. Colt, V. Mudrak, R. Soderberg and R. Speece for reviewing this article.
REFERENCES APHA (1975). Standard Methods for the Examination of Water and tCastewater, 14th edn, American Water Works Association, and Water Pollution Control Federation, American Public Health Association, Washington, DC. ASHRAE Handbook of Fundamentals (1972). American Society of Heating, Refrigeration and Air Conditioning Engineers, New York. Bruijn, J. & Hendrick, H. (1958). The relationship between depth of u-tubes and the aeration process. J. Am. Water Works Assoc., 50, 879-83. Chesness, J. L., Stephens, J. L. & Hill, T. K. (1972). Gravity flow aerators for raceway fish culture systems. Coll. Agric. Exp. Sta., Univ. Georgia, Athens. Res. Rep. 137. Colt, J. (1984). Computation of dissolved gas concentrations in water as functions of temperature, salinity, and pressure. American Fisheries Society Special Publication 14, Bethesda, Maryland. Colt, J. E. & Tchobanoglous, G. (1981). Design of aeration systems for aquaculture. In: Proceeding of the Bio-engineering Symposium for Fish Culture, eds L. J. Allen and E. C. Kinney, Fish Culture Section of the American Fisheries Society and the Northeast Society of Conservation Engineers, Bethesda, Maryland, pp. 138-48. Gilbert, R. G. (1979). Measurement of alpha and beta factors. In: Proceedings: Workshop Toward an Oxygen Transfer Standard, EPA-600/9-78-021, ed. W. C. Boyle, US Environmental Protection Agency, Cincinnati, Ohio, pp. 147-62. Lewis, W. K. & Whitman, W. C. (1924). Principles of gas adsorption. J. Ind. Eng. Chem., 16, 1215-20. Mavinic, D. S. & Bewtra, J. K. (1976). Efficiency of diffused aeration systems in wastewater treatment. J. l~at. Poll. Contr. Fed., 48, 2273-83. Mitchell, R. C. (1973). U-tube aeration. EPA 670/2-73-031, US Environmental Protection Agency, Cincinnati, Ohio. Speece, R. E. (1981). Management of dissolved oxygen and nitrogen in fish hatchery waters. In: Proceedings of the Bio-engineering Symposium for Fish Culture, eds L. J. Allen and E. C. Kinney, Fish Culture Section of the American Fisheries Society and the Northeast Society of Conservation Engineers, Bethesda, Maryland, pp. 53-62.
Modeling gas transfer in a u-tube oxygen absorption system
297
Speece, R. E. & Orosco, R. (1970). Design of u-tube aeration systems. Am. Soc. Civil Eng., 96 (SA4), 715-25. Speece, R. E., Adams, J. L. & Wooldridge, C. B. (1969). U-tube aeration operating characteristics. Am. Soc. Civil Engr., 95 (SA3), 563-74. Speece, R. E., Gallagher, D., Krick, C. & Thomson, R. (1980). Pilot performance of deep u-tubes.Prog. Water Technol., 12,395-407. Speece, R. E., Eheart, J. W. & Givler, C. A. (1983). U-tube aeration sensitivity to design parameters. J. Wat. Poll. Contr. Fed., 55, 1065-9. Tsivoglou, E. C., O'Connell, R. L., Walter, C. M., Godsil, P. J. & Logsdon, G. S. (1965). Tracer measurements of atmospheric reaeration - 1. Laboratory studies. J. IVat. Poll. Contr. Fed., 37, 1343-62. Weiss, R. F. (1970). The solubility of nitrogen, oxygen, and argon in water and sea water. Deep-Sea Res., 17, 721-35. Westers, H. & Pratt, K. M. (1977). Rational design of hatcheries for intensive salmonid culture, based on metabolic characteristics.Prog. Fish-Cult., 39, 157-65. Yunt, F. W. (1979). Gas flows and power measurement. In: Proceedings: Workshop Toward an Oxygen Transfer Standard, EPA-600/9-78-021, ed. W. C. Boyle. US Environmental Protection Agency, Cincinnati, Ohio, pp. 105-27.
You might also like
- Case New Holland Kobelco Iveco Komatsu F4CE F4DE F4GE F4HE Service Repair Manual For Engine Overhaul (Mechanical Injection and Electronic Common Rail) PDFDocument277 pagesCase New Holland Kobelco Iveco Komatsu F4CE F4DE F4GE F4HE Service Repair Manual For Engine Overhaul (Mechanical Injection and Electronic Common Rail) PDFpckey100% (46)
- GAS ABSORPTION ExperimentDocument24 pagesGAS ABSORPTION ExperimentJoanne YapNo ratings yet
- Gas Transfer and AerationDocument33 pagesGas Transfer and Aerationherutok100% (1)
- Aeration Calculation PDFDocument20 pagesAeration Calculation PDFBrian Thomas100% (1)
- Oxygen Transfer Mechanism in Wastewater: First DraftDocument6 pagesOxygen Transfer Mechanism in Wastewater: First DraftSirajuddin AhmedNo ratings yet
- Hackney 1982 Aquacultural-Engineering PDFDocument21 pagesHackney 1982 Aquacultural-Engineering PDFJorge RodriguezNo ratings yet
- Predicting Oxygen Transfer of Fine Bubble Diffused AerationDocument9 pagesPredicting Oxygen Transfer of Fine Bubble Diffused AerationpiticmicNo ratings yet
- Gas TransferDocument10 pagesGas TransferSy-Dar LiouNo ratings yet
- 1 s2.0 S0043135414001389 MainDocument9 pages1 s2.0 S0043135414001389 MainLuciaMarinaR.OrizaNo ratings yet
- Oxygen Transfer Measurements at Surface Aerators in Waste Water As Basis For Energy Saving in AerationDocument6 pagesOxygen Transfer Measurements at Surface Aerators in Waste Water As Basis For Energy Saving in AerationIrving VazquezNo ratings yet
- AerationDocument7 pagesAerationEddiemtongaNo ratings yet
- Oxygen Transfer Model Development Based On Activated Sludge and Clean WaterDocument9 pagesOxygen Transfer Model Development Based On Activated Sludge and Clean WaterSol AngelNo ratings yet
- Written Report PDEDocument6 pagesWritten Report PDECesar Augusto GarechNo ratings yet
- Effects of Impurities On Oxygen Transfer Rates in Diffused Aeration SystemsDocument8 pagesEffects of Impurities On Oxygen Transfer Rates in Diffused Aeration SystemsJenniferNo ratings yet
- Tokumura 2007 Dynamic Modeling andDocument7 pagesTokumura 2007 Dynamic Modeling andAle EcoNo ratings yet
- Adsorption With Water: (CO2) (CO2)Document3 pagesAdsorption With Water: (CO2) (CO2)cat5117No ratings yet
- American Journal of Engineering Research (AJER)Document8 pagesAmerican Journal of Engineering Research (AJER)Ali Abdul-RahmanNo ratings yet
- Absorber Version 1Document4 pagesAbsorber Version 1Jose Eduardo MoralesNo ratings yet
- Modelación Transferencia de OxigenoDocument21 pagesModelación Transferencia de OxigenoOscar Humberto Sandoval FajardoNo ratings yet
- Aeration Lab 1Document3 pagesAeration Lab 1ZafirahAhmadFauziNo ratings yet
- Null DikonversiDocument17 pagesNull DikonversiBayu PratamaNo ratings yet
- Carbon Dioxide Capture and Hydrogen Purification From Synthesis Gas by Pressure Swing AdsorptionDocument6 pagesCarbon Dioxide Capture and Hydrogen Purification From Synthesis Gas by Pressure Swing Adsorptionpedrorios26No ratings yet
- Assessment of Aeration Capacity of Stepped Cascade System For Selected GeometryDocument9 pagesAssessment of Aeration Capacity of Stepped Cascade System For Selected GeometrySong Nguyen NguyenNo ratings yet
- Lab ReportDocument11 pagesLab Report777jas83No ratings yet
- Oxgen Transfer in ReactorsDocument8 pagesOxgen Transfer in Reactorsabarriga78No ratings yet
- Comparison of Several Packings For CO2 Chemical Absorption in A Packed ColumnDocument7 pagesComparison of Several Packings For CO2 Chemical Absorption in A Packed ColumnianphilanderNo ratings yet
- Zhang Xu-A Simple Airlift PBR For Microalgal Mass Culture-2002-PublDocument6 pagesZhang Xu-A Simple Airlift PBR For Microalgal Mass Culture-2002-PublmoneymakerhalilNo ratings yet
- InTech Air Change MeasurementsDocument43 pagesInTech Air Change MeasurementsjonathanuptonNo ratings yet
- Chinese Journal of Chemical Engineering: Wei Yang, Xiaodan Yu, Jianguo Mi, Wanfu Wang, Jian ChenDocument8 pagesChinese Journal of Chemical Engineering: Wei Yang, Xiaodan Yu, Jianguo Mi, Wanfu Wang, Jian ChenDiana Marcela UribeNo ratings yet
- Gas-Liquid Mass Transfer in Taylor Flow Through A CapillaryDocument5 pagesGas-Liquid Mass Transfer in Taylor Flow Through A CapillaryArunNo ratings yet
- Gas-Liquid Mass Transfer in Taylor Flow Through A CapillaryDocument5 pagesGas-Liquid Mass Transfer in Taylor Flow Through A CapillaryArunNo ratings yet
- Rectangular Surface AeratorsDocument12 pagesRectangular Surface AeratorsJesus PerezNo ratings yet
- Section - 11377 - Air Diffuser Equipment - Fine BubbleDocument18 pagesSection - 11377 - Air Diffuser Equipment - Fine BubbleamrezzatNo ratings yet
- Gas Transfer: CEE 453: Laboratory Research in Environmental Engineering Spring 2002Document8 pagesGas Transfer: CEE 453: Laboratory Research in Environmental Engineering Spring 2002ChiruVardhanBandreddiNo ratings yet
- Full 4102Document8 pagesFull 4102piticmicNo ratings yet
- A Simple Model For Falling Film Absorption On (Bagus)Document9 pagesA Simple Model For Falling Film Absorption On (Bagus)Fadli Ryan ArikundoNo ratings yet
- Evaluating The Use of Airlift Pumps For Bioreactor ApplicationsDocument8 pagesEvaluating The Use of Airlift Pumps For Bioreactor ApplicationsDobri CundevNo ratings yet
- Contribution To The Study of Hydrodynamics Gourichh Et AlDocument7 pagesContribution To The Study of Hydrodynamics Gourichh Et AlAlpha ValerioNo ratings yet
- Wetted Wall ColumnDocument4 pagesWetted Wall Columnendang dian lestariNo ratings yet
- Membrane SeparationDocument12 pagesMembrane Separationchemicaly12No ratings yet
- Mass Transfer Study Using An Electrochemical MethodDocument7 pagesMass Transfer Study Using An Electrochemical MethodinstrutechNo ratings yet
- Ijest Vol1 No1 pp.1 15Document15 pagesIjest Vol1 No1 pp.1 15prabhjot singh1No ratings yet
- Received 15 February 1985 in Revised Form 22 August 1985Document11 pagesReceived 15 February 1985 in Revised Form 22 August 1985Francisco OppsNo ratings yet
- A Technical and Economic Assessment of Ammonia-Based Post-Combustion CO2 Capture at Coal-Fired Power PlantsDocument10 pagesA Technical and Economic Assessment of Ammonia-Based Post-Combustion CO2 Capture at Coal-Fired Power PlantsBánh Cuốn Tôm ThịtNo ratings yet
- OTRDocument51 pagesOTRNithi AnandNo ratings yet
- An Investigation of Effect of Stepped Chutes WithDocument14 pagesAn Investigation of Effect of Stepped Chutes Withsudah sudahiNo ratings yet
- Mass Transfer Work PlanDocument3 pagesMass Transfer Work PlanGorgi PavlovNo ratings yet
- Equation-Based Rigorous Modelling of The NO Absorption Process: Model Development and Process OptimizationDocument6 pagesEquation-Based Rigorous Modelling of The NO Absorption Process: Model Development and Process OptimizationAlberto TousNo ratings yet
- Sparger and Surface Gas Transfer For Cell Culture BioreactorsDocument12 pagesSparger and Surface Gas Transfer For Cell Culture BioreactorsdanNo ratings yet
- Gas Holdup in A Gasliquid Up Flow Bubble Column in The Presence of Double Cone Promoter PDFDocument8 pagesGas Holdup in A Gasliquid Up Flow Bubble Column in The Presence of Double Cone Promoter PDFCastañeda ValeriaNo ratings yet
- Wet Wall AbsorptionDocument11 pagesWet Wall Absorptionhagt813No ratings yet
- G. Tiwari 2006Document11 pagesG. Tiwari 2006oswaldoNo ratings yet
- Romain - Lemoine - Final - Hydrodynamics, Mass Transfer and Modeling of The Toluene Oxidation ProcessDocument399 pagesRomain - Lemoine - Final - Hydrodynamics, Mass Transfer and Modeling of The Toluene Oxidation Processandrei12320003181No ratings yet
- Calculating Accidental Release Flow RatesDocument5 pagesCalculating Accidental Release Flow RatesakashawalkerNo ratings yet
- Activated Sludge - Types of Processes and Modifications: 1 ConventionalDocument33 pagesActivated Sludge - Types of Processes and Modifications: 1 ConventionalJon Bisu DebnathNo ratings yet
- Air StrippingDocument9 pagesAir StrippingCésarNo ratings yet
- Dynamic Numerical Simulation of Gas-Liquid Two-Phase Flows Euler/Euler Versus Euler/LagrangeDocument16 pagesDynamic Numerical Simulation of Gas-Liquid Two-Phase Flows Euler/Euler Versus Euler/Lagrangeamin_zargaranNo ratings yet
- Wickins 1987 Aquacultural-EngineeringDocument12 pagesWickins 1987 Aquacultural-EngineeringJorge RodriguezNo ratings yet
- Shepherd 1987 Aquacultural-EngineeringDocument15 pagesShepherd 1987 Aquacultural-EngineeringJorge RodriguezNo ratings yet
- Provenzano 1987 Aquacultural-EngineeringDocument12 pagesProvenzano 1987 Aquacultural-EngineeringJorge RodriguezNo ratings yet
- Ross 1987 Aquacultural-EngineeringDocument3 pagesRoss 1987 Aquacultural-EngineeringJorge RodriguezNo ratings yet
- Robertson 1987 Aquacultural-EngineeringDocument16 pagesRobertson 1987 Aquacultural-EngineeringJorge RodriguezNo ratings yet
- Watten 1987 Aquacultural-EngineeringDocument14 pagesWatten 1987 Aquacultural-EngineeringJorge RodriguezNo ratings yet
- Coconut-In Support of Good Health in The 21st CenturyDocument27 pagesCoconut-In Support of Good Health in The 21st CenturyDan RoneyNo ratings yet
- Poxton 1987 Aquacultural-Engineering 1Document3 pagesPoxton 1987 Aquacultural-Engineering 1Jorge RodriguezNo ratings yet
- Pellegrini 1987 Aquacultural-EngineeringDocument7 pagesPellegrini 1987 Aquacultural-EngineeringJorge RodriguezNo ratings yet
- Manthe 1987 Aquacultural-EngineeringDocument10 pagesManthe 1987 Aquacultural-EngineeringJorge RodriguezNo ratings yet
- Poxton 1987 Aquacultural-EngineeringDocument22 pagesPoxton 1987 Aquacultural-EngineeringJorge RodriguezNo ratings yet
- Airlift Pump Article 1987Document14 pagesAirlift Pump Article 1987lychekNo ratings yet
- Plaia 1987 Aquacultural-EngineeringDocument11 pagesPlaia 1987 Aquacultural-EngineeringJorge RodriguezNo ratings yet
- Madenjian 1987 Aquacultural-EngineeringDocument18 pagesMadenjian 1987 Aquacultural-EngineeringJorge RodriguezNo ratings yet
- Dryden 1987 Aquacultural-Engineering 1Document12 pagesDryden 1987 Aquacultural-Engineering 1Jorge RodriguezNo ratings yet
- Madenjian 1987 Aquacultural-Engineering 1Document17 pagesMadenjian 1987 Aquacultural-Engineering 1Jorge RodriguezNo ratings yet
- Kreiberg 1987 Aquacultural-EngineeringDocument11 pagesKreiberg 1987 Aquacultural-EngineeringJorge RodriguezNo ratings yet
- Huggins 1987 Aquacultural-EngineeringDocument17 pagesHuggins 1987 Aquacultural-EngineeringJorge RodriguezNo ratings yet
- Costa Pierce 1987 Aquacultural EngineeringDocument12 pagesCosta Pierce 1987 Aquacultural EngineeringJorge RodriguezNo ratings yet
- Kruner 1987 Aquacultural-EngineeringDocument18 pagesKruner 1987 Aquacultural-EngineeringJorge RodriguezNo ratings yet
- Holm 1987 Aquacultural-EngineeringDocument14 pagesHolm 1987 Aquacultural-EngineeringJorge RodriguezNo ratings yet
- Dryden 1987 Aquacultural-EngineeringDocument18 pagesDryden 1987 Aquacultural-EngineeringJorge RodriguezNo ratings yet
- Fabregas 1987 Aquacultural-EngineeringDocument10 pagesFabregas 1987 Aquacultural-EngineeringJorge RodriguezNo ratings yet
- Don 1987 Aquacultural-EngineeringDocument6 pagesDon 1987 Aquacultural-EngineeringJorge RodriguezNo ratings yet
- (First Author) 1987 Aquacultural-EngineeringDocument2 pages(First Author) 1987 Aquacultural-EngineeringJorge RodriguezNo ratings yet
- Twu 1986 Aquacultural-EngineeringDocument16 pagesTwu 1986 Aquacultural-EngineeringJorge RodriguezNo ratings yet
- (First Author) 1987 Aquacultural-Engineering 1Document1 page(First Author) 1987 Aquacultural-Engineering 1Jorge RodriguezNo ratings yet
- Cathcart 1987 Aquacultural-EngineeringDocument21 pagesCathcart 1987 Aquacultural-EngineeringJorge RodriguezNo ratings yet
- Wang 1986 Aquacultural-EngineeringDocument2 pagesWang 1986 Aquacultural-EngineeringJorge RodriguezNo ratings yet
- Shleser 1986 Aquacultural-EngineeringDocument15 pagesShleser 1986 Aquacultural-EngineeringJorge RodriguezNo ratings yet
- ValvesDocument75 pagesValvesAmaury M Costa JuniorNo ratings yet
- 2628 Laja Project Coordination MTG #94Document31 pages2628 Laja Project Coordination MTG #94pollito190303No ratings yet
- Elster Jeavons-J125 1.5Document9 pagesElster Jeavons-J125 1.5hataefendiNo ratings yet
- Scuba Diving - November - December 2016Document76 pagesScuba Diving - November - December 2016IgorNo ratings yet
- Eska Valve ERG-S Series Double Stage Pressure RegulatorDocument4 pagesEska Valve ERG-S Series Double Stage Pressure RegulatorRONAL QUISPE MAMANINo ratings yet
- 4VOL R01ipDocument9 pages4VOL R01ipzhyhhNo ratings yet
- Parker COMOSO 0600P E Complete 3-28-2013 Valvair II SeriesDocument40 pagesParker COMOSO 0600P E Complete 3-28-2013 Valvair II SeriesmohammedNo ratings yet
- 122 6006Document14 pages122 6006Paulo Jurandir Santos PereiraNo ratings yet
- Manifold Systems Catalog PDFDocument28 pagesManifold Systems Catalog PDFRam BabuNo ratings yet
- Sport Diver Magazine UK - June 2011Document139 pagesSport Diver Magazine UK - June 2011dragos.dd100% (1)
- Vent Free FireplaceDocument43 pagesVent Free FireplaceAngel MorrisNo ratings yet
- Eclipse Tube Firing Burners: Models TFB030, TFB075, TFB200Document22 pagesEclipse Tube Firing Burners: Models TFB030, TFB075, TFB200RiyazNo ratings yet
- 18 PartsDocument2 pages18 PartsHarraKiriNo ratings yet
- G12 - Pe 4 - Module 2Document21 pagesG12 - Pe 4 - Module 2John Lois VanNo ratings yet
- Breathing ApparatusDocument34 pagesBreathing ApparatusSwapnil BhargavNo ratings yet
- GN8 Oxy-Fuel Gas Daily Checklist1a1Document1 pageGN8 Oxy-Fuel Gas Daily Checklist1a1sanketpavi21No ratings yet
- f32 Am1a Briz Motors Technical and Repair ManualDocument131 pagesf32 Am1a Briz Motors Technical and Repair ManualAndres Sorin100% (2)
- Fresenius 2008 Hemodialysis System - Troubleshooting GuideDocument0 pagesFresenius 2008 Hemodialysis System - Troubleshooting GuideEricka Lj Robles DimaculanganNo ratings yet
- QuatroDocument73 pagesQuatroAriel Oscar HuertaNo ratings yet
- DragerDocument2 pagesDragerOkan TekinNo ratings yet
- SCBA Inspection SIMON PMDocument9 pagesSCBA Inspection SIMON PMCesar CoronelNo ratings yet
- DL650 S5Document34 pagesDL650 S5Ferdi EsmerNo ratings yet
- WeishauptDocument24 pagesWeishauptpatitay036817No ratings yet
- Manual PDS55S - 5B2 - E1 - (2) (39600 - 78420)Document22 pagesManual PDS55S - 5B2 - E1 - (2) (39600 - 78420)singoNo ratings yet
- Double Regulating Valves (2601V)Document7 pagesDouble Regulating Valves (2601V)Sujit RajanNo ratings yet
- BHMY 887 Flexflo IOM 31683C 0920 EnglishDocument16 pagesBHMY 887 Flexflo IOM 31683C 0920 EnglishSajjad MehdiNo ratings yet
- Safety Induction: An ISO 9001-2008 and OHSAS 18001 - 2007 CompanyDocument19 pagesSafety Induction: An ISO 9001-2008 and OHSAS 18001 - 2007 CompanyRudra RoyNo ratings yet
- Cumminssec7 PDFDocument28 pagesCumminssec7 PDFjosecarlosvjNo ratings yet
- Топливная Система Siemens Sid 201Document105 pagesТопливная Система Siemens Sid 201Vytautas Mackonis67% (3)