Professional Documents
Culture Documents
Refrigeration Cycles
Uploaded by
aravoof84Original Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Refrigeration Cycles
Uploaded by
aravoof84Copyright:
Available Formats
Chapter 10
Lyes KADEM [Thermodynamics II] 2007
Refrigeration Cycles
The objective of refrigeration cycles is to transfer the heat from a low temperature region to a high temperature region. - if the objective of the cycle is to decrease the lowest temperature, we call it: a Refrigeration cycle. - if the objective of the cycle is to increase the highest temperature, we call it: a heat pump. Carnot cycle As the Carnot cycle is the ideal cycle, let us first try to convert it to a refrigeration cycle, and then we will discuss practical limitations. Figure (10.1) shows a reversed Carnot cycle, the efficiency of this cycle will be:
=1
TL TH
However, if we try to apply the Carnot cycle, we will face some technical difficulties: 1-2: It is not advisable to compress a mixture of vapor and gas, since the liquid droplets would cause excessive wear. shift point 1 to the right (saturated vapor). 3-4: It would be quite expensive to construct a device to be used in the expansion process that would be nearly isentropic (no losses allowed). It is much simpler to reduce the pressure irreversibly by using an expansion valve (isenthalpic process).
Refrigeration cycles
44
Chapter 10
Lyes KADEM [Thermodynamics II] 2007
Figure.10.1. Reversed Carnot cycle. The Ideal vapor-compression refrigeration cycle 1-2: isentropic compression in a compressor. 2-3: isobaric heat rejection in a condenser. 3-4: throttling in an expansion device. 4-1: isobaric heat absorption in an evaporator.
Figure.10.2. Ideal vapor-refrigeration cycle.
Refrigeration cycles
45
Chapter 10
Lyes KADEM [Thermodynamics II] 2007
The coefficient of performance (COP) of a refrigeration cycle is:
COPR =
benefit Q = in Cost W in
The coefficient of performance (COP) of a heat pump is:
COPHP =
& benefit Q = out & Cost W in
COP can attain 4 for refrigerators and perhaps 5 for heat pumps. Actual vapor-compression refrigeration cycle The actual cycle differs from the ideal one due to irreversibilities, such as: Pressure drops due to friction in connecting pipes Heat transfer occurs from or to the refrigerant through the pipes connecting the components. Pressure drops occur through the condenser and evaporator tubes. Heat transfer occurs from the compressor.
Figure.10.3. Selecting a refrigerant The evaporation temperature of the refrigerant must be quite low, in the neighborhood of -25 C (so usually forget water ) The most common refrigerants used are: ChloroFluoroCarbons (CFC), ammonia , hydrocarbons (propane, ethane, ethylene, ), Carbon Dioxide, air,
Refrigeration cycles
46
Chapter 10
Lyes KADEM [Thermodynamics II] 2007
Usually R-11, R-12, R-22, R134a and R-502 where used (the trade name is Freon) before 1987, now isobutane (although it is a very dangerous gas ). For industry, they usually use ammonia since it is cheaper, doesnt damage the Ozone layer, has a high COP and greater delectability is case of a leak. The major drawback is the toxicity, which makes it unsuitable for domestic use. Important points for the design of a refrigeration cycle the temperature of the refrigerant within the condenser must be 5C to 15C higher than the exterior temperature, to allow efficient heat transfer. the temperature of the refrigerant within the evaporator must be 5C to 15C lower than the cooled space temperature, to allow efficient heat transfer. The equipments working at low pressure (evaporator) must be sufficiently large to account for a higher specific volume. The equipments working at high pressure (Condenser) must be designed to support the high pressure.
Multistage vapor refrigeration cycle The simple vapor-compression refrigeration cycle is the most widely refrigeration cycle used. However, if we are looking for a further decrease in the temperature, we have to use a cascade refrigeration cycle.
Refrigeration cycles
47
Chapter 10
Lyes KADEM [Thermodynamics II] 2007
Figure.10.4. Two-stage refrigeration cycle. Indeed, this cycle will leads to a decrease in the temperature of the point 3, therefore increasing the refrigeration. The configuration will minimize the compressor work. Note that we need for that, 2 compressors, a heat exchanger and 2 expansion valves. So the additional costs for this must be justified by improved performance. For extremely low refrigeration temperatures, several stages may be used. The optimal intermediate pressure is:
Refrigeration cycles
48
Chapter 10
Lyes KADEM [Thermodynamics II] 2007
Pi =
PH PL
PH PL
the high pressure the low pressure
With this cycle, it is not an obligation to use the same refrigerant. It the refrigerants are different, the appropriate T-s diagram must be used. Example R134a is used in an ideal vapor refrigeration cycle operating between saturation temperatures of -20C in the evaporator and 39.39 C at the condenser outlet. The mass flow rate is 0.6 kg/s. Calculate the rate of refrigeration and the coefficient of performance. Now consider a two-stage cycle replacing the simple cycle for the same conditions. Determine the rate of refrigeration and the coefficient of performance.
Absorption refrigeration cycle
In conventional refrigeration cycles, the power input needed to operate the compressor is relatively large, since the fluid compressed is in the vapor state and has a very large specific volume ( w = v dP , with v high) .
1
The objective of an absorption refrigeration cylce is to reduce the power needed for the compression by converting the vapor into a liquid which will be pumped by a pump (liquid lower v lower w for the same pressure difference). To do so, several equipments are added to the conventional cycle. These equipments are: the absorber; the pump; the heat exchanger; the generator and a regulating valve. The most widly used absorption cycle is the ammonia-water system (Fig.10.5). It was patented by Ferdinand Carre (France) in 1859.
Refrigeration cycles
49
Chapter 10
Lyes KADEM [Thermodynamics II] 2007
Figure.10.5. Ammonia-water system. On the left side of the figure, it is exactly the same processes as for a conventional refrigeration cycle: condensation, throttling and evaporation. But on the right side, the compressor is replaced by the following process: The pure ammonia NH3 in the vapor state enters the absorber where it dissolves and reacts with water. The absorber contains therefore a mixture of NH3+H2O. As the amount of NH3 that can be dissolved in H2O is inversely proportional to the temperature. The absorber is cooled to maintain its temperature as low as possible. The liquid solution NH3+H2O, which is rich in NH3, is then pumped to the generator. Sometimes a heat exchanger is used between the pump and the generator to pre-vaporize the mixture NH 3+H2O. In the generator, an external source vaporize the mixture. The liquid is tranferred to the absorber through an expansion valve to reduce its pressure. The pure NH 3 vapor leaving the generator enters the condenser and follows the rest of the refrigeration cycle processes. Note: to analyze the absorption cycle, we must know the amount of refrigerant contained in a mixture (NH3+H2O here), both in liquid form and vapor form. This can be found with the aid of an equilibrium diagram.
The gas refrigeration cycle
If the flow of gas is reverted in the Brayton cycle, the gas undergoes an isentropic expansion process as it flows through the turbine, resulting in a substantional reduction in temperature.
Refrigeration cycles
50
Chapter 10
Lyes KADEM [Thermodynamics II] 2007
Figure.10.6. Reversed Brayton cycle. An open cycle is used in aircraft ; air is extracted from the atmosphere at state 1 and inserted into the passenger compartment at state 4, this provides both fresh air and cooling. Like for the regenerative Brayton cycle, the reversed Brayton cycle can be used with a regenerator to lower the temperature of the turbine inlet.
Figure.10.6. Reversed regenerative Brayton cycle.
Refrigeration cycles
51
Chapter 10
Lyes KADEM [Thermodynamics II] 2007
Heat pump
A heat pump utilizes the vapor refrigeration cycle, but to increase the temperature of the external medium, like a house (Figure). Although using a heat pump for heating is more expensive than other conventional systems, it saves money in the long run. The major problem with heat pumps occurs in humid regions where the temperature falls below 2 to 5C. This may cause frosting. The accumulation of frost is highly undesirable, since it seriously disturpt heat transfer. This problem can be solved by runing the system as an air conditionner.
Figure.10.8. Heat pump.
Additional information
BTU: the BTU is a common unit used in refrigeration industry. A BTU (British Thermal Unit ) is the amount of heat energy needed to rise the temperature of one pound of pure water by one degree F. Gaz liquefaction One of the hottest application of refrigeration cycles is gas liquefaction. In fact, several engineering processes require very low temperatures. To liquefy a gas such as helium, hydrogen or nitrogen, the temperature must be lower than their critical temperature: -268C, -240C and -147C respectively.
Refrigeration cycles
52
Chapter 10
Lyes KADEM [Thermodynamics II] 2007
Figure.10.9. Transport of natural gas.
To reach so low temperatures several cycles may be used, usually using multistage compressors. The man who contributed the most significantly to gas liquefaction is Carl Von Linde [if you remember well, he was Diesels professor at Munich Polytechnics].
Although discovering oxygen and investigating its role in chemical reactions proved to be of crucial importance in changing the science of chemistry, initially oxygen could be produced only in the laboratory and in limited quantities, by chemical or electrolytic means: it had little importance outside the laboratory. It was the achievement of Carl von Linde (18421934) in 1902 to take oxygen from the air itselfand he was soon extracting it in quantities approaching one thousand cubic feet per hour. Oxygen became a common commodity that was supplied to hospitals and industries and was later used in rocket fuel, but this was not the German engineer's first important contribution. Linde, the son of a Lutheran minister, was educated in science and engineering at the Federal Polytechnic in Zurich, Switzerland. After working for locomotive manufacturers in Berlin and Munich, he became a faculty member at the Polytechnic in Munich. His research there on heat theory, from 1873 to 1877, led to his invention of the first reliable and efficient compressed-ammonia refrigerator. The company he established to promote this invention was an international success: refrigeration rapidly displaced ice in food handling and was introduced into many industrial processes. After a decade Linde withdrew from managerial activities to refocus on research, and in 1895 he succeeded in liquefying air by first compressing it and then letting it expand rapidly, thereby cooling it. He then obtained oxygen and nitrogen from the liquid air by slow warming. In the early days of oxygen production the biggest use by far for the gas was the oxyacetylene torch, invented in France in 1904, which revolutionized metal cutting and welding in the construction of ships, skyscrapers, and other iron and steel structures. One company formed to use Linde's later patents was the Linde Air Products Company, founded in Cleveland in 1907. In 1917 Linde Air Products joined with four other companies that produced acetylene, among other products, to form Union Carbide and Carbon Corporation. Recently, Linde Air again became an independent companyPraxair.
Refrigeration cycles
53
You might also like
- Chemistry NotesDocument12 pagesChemistry Notesnyu835nyuNo ratings yet
- Mini Solar Water HeaterDocument23 pagesMini Solar Water HeaterROCKY DUBEYNo ratings yet
- Experiment To Determine The Refractive Index of A Glass BlockDocument1 pageExperiment To Determine The Refractive Index of A Glass Blockmundu mustafaNo ratings yet
- Advanced Fluid Mechanics Lab ManualDocument55 pagesAdvanced Fluid Mechanics Lab ManualchristianNo ratings yet
- Radiation View Factors and TransferDocument29 pagesRadiation View Factors and Transfernauman khanNo ratings yet
- UTech Jamaica Electronic Devices Rectification and Smoothing CircuitsDocument8 pagesUTech Jamaica Electronic Devices Rectification and Smoothing CircuitsMonique HepburnNo ratings yet
- Haber Process and AmmoniaDocument2 pagesHaber Process and AmmoniaAaron HongNo ratings yet
- A.zerrouki Et El The Natural Circulation Solar Water Heater ModelDocument11 pagesA.zerrouki Et El The Natural Circulation Solar Water Heater ModelJuan-Pierre HerbothNo ratings yet
- Aluminium PresentationDocument12 pagesAluminium PresentationDaniel McknightNo ratings yet
- Gas Power Cycles Study Guide in Powerpoint: To AccompanyDocument68 pagesGas Power Cycles Study Guide in Powerpoint: To AccompanyexceptionalhighdeeNo ratings yet
- School of Civil, Environmental & Chemical EngineeringDocument38 pagesSchool of Civil, Environmental & Chemical EngineeringJair BarruetaNo ratings yet
- Boiling and Condensation PDFDocument11 pagesBoiling and Condensation PDFYeditha Satyanarayana MurthyNo ratings yet
- 545 ChemistryDocument24 pages545 Chemistrykitderoger_391648570No ratings yet
- Lecture NoteDocument34 pagesLecture Notenelson100% (1)
- UNIT II: Fuels: SyllabusDocument26 pagesUNIT II: Fuels: SyllabusMAYUR BHOSALE100% (1)
- Homer Lab 04Document9 pagesHomer Lab 04DiegoFernandoRojasTorresNo ratings yet
- Stefan's Law of RadiationDocument7 pagesStefan's Law of RadiationHema AnilkumarNo ratings yet
- Lab 2 Report ThermodynamicsDocument9 pagesLab 2 Report ThermodynamicsOse Colix Jr.100% (1)
- The Heat of Solution LabDocument4 pagesThe Heat of Solution Labapi-310957734No ratings yet
- A Textbook of Machine Design by R.S.KHURMI AND J.K.GUPTA - 216Document1 pageA Textbook of Machine Design by R.S.KHURMI AND J.K.GUPTA - 216Vivek MishraNo ratings yet
- Chemical ThermodynamicsDocument33 pagesChemical ThermodynamicsAkash Ghosh0% (1)
- IC Engines GuideDocument47 pagesIC Engines GuidearulNo ratings yet
- PFR vs. CSTR: Size and Selectivity: V R V RDocument6 pagesPFR vs. CSTR: Size and Selectivity: V R V RSerkan KayacanNo ratings yet
- Combustion in Premixed & Diffusion FlamesDocument16 pagesCombustion in Premixed & Diffusion FlamesRahul Singh TomarNo ratings yet
- Unit 5 Electric Vehicle Maintenance and ServiceDocument15 pagesUnit 5 Electric Vehicle Maintenance and ServiceTamil selvan100% (1)
- The Haber and Contact ProcessesDocument3 pagesThe Haber and Contact Processesjavison_501100% (1)
- Thermodynamics h2Document209 pagesThermodynamics h2ezoramajnun100% (1)
- Kneader Mixer Lab Report by Group E (SECTION A)Document16 pagesKneader Mixer Lab Report by Group E (SECTION A)Maryam Fatima100% (1)
- Compression Test Lab ReportDocument11 pagesCompression Test Lab ReportRobert K OtienoNo ratings yet
- Chemistry Notes (Organic Chem)Document6 pagesChemistry Notes (Organic Chem)Teo Jia Ming NickolasNo ratings yet
- FIE Physics Lab Report 2Document8 pagesFIE Physics Lab Report 2Sharvind Kumar Sharvind KumarNo ratings yet
- Experiment 6: Fluid Friction Apparatus This Is A Sample Report, It Is The Perfect FormatDocument15 pagesExperiment 6: Fluid Friction Apparatus This Is A Sample Report, It Is The Perfect FormatBryan Pashian ManaluNo ratings yet
- Radiation Heat TransferDocument3 pagesRadiation Heat TransferAnonymous 2BJgxbxJNo ratings yet
- Experiment No. 5 Emissivity 1. Objective: q Eσ (T T Wm KDocument6 pagesExperiment No. 5 Emissivity 1. Objective: q Eσ (T T Wm KJanine Abiegale PeraltaNo ratings yet
- Projeect PPT-1Document28 pagesProjeect PPT-1Shubham PawarNo ratings yet
- MS - 57 SolvedDocument8 pagesMS - 57 Solvedprajwolrajaryal4980No ratings yet
- Load Forecasting ClassDocument24 pagesLoad Forecasting ClassSumit Dhingra100% (1)
- Properties of Pure Substances: Noor Hafizah UyupDocument109 pagesProperties of Pure Substances: Noor Hafizah UyupDIey ChokiEyNo ratings yet
- A Solar Adsorption Refrigeration System Operating at Near Atmospheric PressureDocument167 pagesA Solar Adsorption Refrigeration System Operating at Near Atmospheric Pressureali105No ratings yet
- UO 4 Solid Handling UnitDocument17 pagesUO 4 Solid Handling UnitNoor FadzleenaNo ratings yet
- Seminar On Nano Fluid Based Solar Thermal SystemsDocument13 pagesSeminar On Nano Fluid Based Solar Thermal SystemsSARATH SASINo ratings yet
- Algebra QuestionsDocument29 pagesAlgebra QuestionsRandy ViolaNo ratings yet
- Free VibrationsDocument27 pagesFree Vibrationshelllooo00No ratings yet
- ME 8391 Engineering Thermodynamics Workbook - UNIT 1Document154 pagesME 8391 Engineering Thermodynamics Workbook - UNIT 1BIBIN CHIDAMBARANATHANNo ratings yet
- PHY203 - Oscillations and WavesDocument237 pagesPHY203 - Oscillations and WavesMitrabhanuNo ratings yet
- Cooling Tower ReportDocument11 pagesCooling Tower Reportbae zazNo ratings yet
- Chapter 3 - Free Damped Vibrations - Mechanical VibrationsDocument21 pagesChapter 3 - Free Damped Vibrations - Mechanical Vibrationsumangthechamp100% (1)
- I. Principles of Wind EnergyDocument3 pagesI. Principles of Wind EnergyJohn TauloNo ratings yet
- ECE 3040 Lecture 22: Numerical Solution of Differential EquationsDocument34 pagesECE 3040 Lecture 22: Numerical Solution of Differential EquationsAqe KitamNo ratings yet
- Eme Lab Manual PDFDocument45 pagesEme Lab Manual PDFpatidar montuNo ratings yet
- A Major Project ON Biodiesel Production From Waste Cooking Oil (Literature and Planning)Document20 pagesA Major Project ON Biodiesel Production From Waste Cooking Oil (Literature and Planning)Ankur SemleNo ratings yet
- Thermodynamic CyclesDocument25 pagesThermodynamic CyclesMoonsoul ChildNo ratings yet
- Mechanical Workshop ReportDocument4 pagesMechanical Workshop ReportHumaid Al-'AmrieNo ratings yet
- Cooking With Nature 2011Document8 pagesCooking With Nature 2011Linda MuhamadNo ratings yet
- Experiment No,-3 (A) : Mass Transfer Lab IIT KGPDocument3 pagesExperiment No,-3 (A) : Mass Transfer Lab IIT KGPSiddharth MohapatraNo ratings yet
- Solar Collectors: By: Dr. Amandeep Singh Oberoi Assistant Professor Mechanical Engineering DepartmentDocument24 pagesSolar Collectors: By: Dr. Amandeep Singh Oberoi Assistant Professor Mechanical Engineering DepartmentSachin MalhotraNo ratings yet
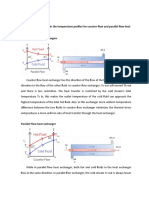
- Describe The Difference in The Temperature Profiles For Counter-Flow and Parallel Flow Heat Exchangers. Counter Flow Heat ExchangersDocument3 pagesDescribe The Difference in The Temperature Profiles For Counter-Flow and Parallel Flow Heat Exchangers. Counter Flow Heat Exchangersbyun baekNo ratings yet
- Chapter 10: Refrigeration CyclesDocument14 pagesChapter 10: Refrigeration CyclesGhulam AbbasNo ratings yet
- Refrigeration CycleDocument8 pagesRefrigeration CycleMohamed HassanainNo ratings yet
- Refrigeration and LiquefactionDocument51 pagesRefrigeration and LiquefactionAlbert ShesmanNo ratings yet
- Airconditioningforlargemultistorybuildings 110522204801 Phpapp02Document23 pagesAirconditioningforlargemultistorybuildings 110522204801 Phpapp02aravoof84No ratings yet
- Valve & Type of ValesDocument82 pagesValve & Type of Valesvipinct83% (6)
- Calculator Programmable ThermostatDocument5 pagesCalculator Programmable Thermostataravoof84No ratings yet
- Air Duct CalculatorDocument1 pageAir Duct Calculatoraravoof84No ratings yet
- How To Determine The Sitting and Facing of A Home, Apartment or BuildingDocument8 pagesHow To Determine The Sitting and Facing of A Home, Apartment or Buildingaravoof84No ratings yet
- Sustainable HVACDocument28 pagesSustainable HVACaravoof84No ratings yet
- Hvac Load CalculationsDocument68 pagesHvac Load CalculationsPrecisionetica100% (2)
- Hvacprogrammematerial 090703025103 Phpapp02Document95 pagesHvacprogrammematerial 090703025103 Phpapp02aravoof84No ratings yet
- Cooling Coil FormulasDocument2 pagesCooling Coil Formulasaravoof84No ratings yet
- Mechanical FormulasDocument5 pagesMechanical FormulasSenthil KumarNo ratings yet
- Fire Apparatus Pump Theory 1Document25 pagesFire Apparatus Pump Theory 1aravoof84No ratings yet
- How To Determine The Sitting and Facing of A Home, Apartment or BuildingDocument8 pagesHow To Determine The Sitting and Facing of A Home, Apartment or Buildingaravoof84No ratings yet
- Why Change The Chilled Water Temperature RangeDocument6 pagesWhy Change The Chilled Water Temperature RangeShabeer HamzaNo ratings yet
- Do Building Energy Codes Go Far EnoughDocument28 pagesDo Building Energy Codes Go Far Enoughvarshneyrk@rediffmail.comNo ratings yet
- Induction unit system design manualDocument35 pagesInduction unit system design manualNahiyan AbdullahNo ratings yet
- Flow FansDocument35 pagesFlow Fansaries26march100% (2)
- Calculation of Invert LevelsDocument8 pagesCalculation of Invert Levelsaravoof84100% (1)
- Almost Everthing For MEPDocument55 pagesAlmost Everthing For MEPdkpushp100% (4)
- Module 123Document7 pagesModule 123aravoof84No ratings yet
- Hong Kong Stormwater Drainage Manual: Planning, Design and ManagementDocument108 pagesHong Kong Stormwater Drainage Manual: Planning, Design and ManagementFree Rain Garden Manuals0% (1)
- Workshop Exercise - Fans and BlowersDocument3 pagesWorkshop Exercise - Fans and BlowersShukla SuyashNo ratings yet
- Pump Selection CriteriaDocument5 pagesPump Selection CriteriaAbbas RizviNo ratings yet
- Air SeperatorDocument20 pagesAir Seperatoraravoof84No ratings yet
- Calculations/Design Procedures Basic Fire Protection Requirements For Hydrocarbon Hazards Offshore PlatformsDocument3 pagesCalculations/Design Procedures Basic Fire Protection Requirements For Hydrocarbon Hazards Offshore Platformsaravoof84No ratings yet
- Tips For Filling Out The Performance Planning and Appraisal Form (PPAF)Document5 pagesTips For Filling Out The Performance Planning and Appraisal Form (PPAF)Sudhakar ChollangiNo ratings yet
- Description & Design of Sprinkler SystemsDocument20 pagesDescription & Design of Sprinkler SystemsIrfanshah2013100% (4)
- Mechanical FormulasDocument5 pagesMechanical FormulasSenthil KumarNo ratings yet
- Air-Water Systems For Air Conditioning Design ManualDocument60 pagesAir-Water Systems For Air Conditioning Design ManualSchreiber_Dieses100% (1)
- Air Duct CalculatorDocument1 pageAir Duct Calculatoraravoof84No ratings yet
- WWDocument13 pagesWWonspsnonsNo ratings yet
- Chapter-6 IscaDocument1 pageChapter-6 IscakishorejiNo ratings yet
- Nichrome60 Wire Data SheetDocument2 pagesNichrome60 Wire Data SheetvvingtsabtaNo ratings yet
- Electrical Measurements LabDocument40 pagesElectrical Measurements Labmdayyub100% (4)
- Stress-Strain Behaviour of Steel-Fibre-Reinforced Recycled Aggregate Concrete Under Axial TensionDocument16 pagesStress-Strain Behaviour of Steel-Fibre-Reinforced Recycled Aggregate Concrete Under Axial TensionAndrucruz CruzNo ratings yet
- Establishing OPC UA Connectivity With Rockwell Automation® Integrated ArchitectureDocument3 pagesEstablishing OPC UA Connectivity With Rockwell Automation® Integrated ArchitecturehuiyitNo ratings yet
- 9853 1239 01 - COP 54 Service Poster - LOWDocument1 page9853 1239 01 - COP 54 Service Poster - LOWValourdos LukasNo ratings yet
- MI MetadataDocument310 pagesMI MetadataMatthew McCreadyNo ratings yet
- Testing Machines For TextilesDocument35 pagesTesting Machines For TextilesAmarech YigezuNo ratings yet
- ZXONE Quick Installation Guide - V1.0Document56 pagesZXONE Quick Installation Guide - V1.0kmad100% (2)
- List of Linkages2016Document74 pagesList of Linkages2016engrwho0% (1)
- Mixers Towable Concrete Essick EC42S Rev 8 Manual DataId 18822 Version 1Document84 pagesMixers Towable Concrete Essick EC42S Rev 8 Manual DataId 18822 Version 1Masayu MYusoffNo ratings yet
- XZX ZX ZXDocument4 pagesXZX ZX ZXWong VoonyeeNo ratings yet
- 6GK52160BA002AA3 Datasheet en PDFDocument6 pages6GK52160BA002AA3 Datasheet en PDFgrace lordiNo ratings yet
- Manual 800 KvaDocument87 pagesManual 800 Kvavicvarg100% (3)
- Department of Mechanical Engineering, Uet Lahore Refrigeration and Air Conditioning LaboratoryDocument7 pagesDepartment of Mechanical Engineering, Uet Lahore Refrigeration and Air Conditioning LaboratoryTauQeer ShahNo ratings yet
- Wireless Mouse m325 Quick Start GuideDocument2 pagesWireless Mouse m325 Quick Start GuideFabolos 9No ratings yet
- VGS 8.1.2 Rev.20 - UTDocument29 pagesVGS 8.1.2 Rev.20 - UTPaul-Petrus MogosNo ratings yet
- Partlist Smsport 110RDocument74 pagesPartlist Smsport 110RThai YunNo ratings yet
- JUS StandardsDocument28 pagesJUS StandardsgarejkaNo ratings yet
- Foxpro For O LevelDocument3 pagesFoxpro For O LevelShiv PathakNo ratings yet
- Account Manager Business Development in San Jose CA Resume Mark WestonDocument2 pagesAccount Manager Business Development in San Jose CA Resume Mark WestonMarkWeston2No ratings yet
- HalideDocument195 pagesHalidejadecolourNo ratings yet
- Bbraun Infusomat Service MaualDocument4 pagesBbraun Infusomat Service Maualalfie frankie diezNo ratings yet
- LV12 - Drive Shafts - Issue 1Document19 pagesLV12 - Drive Shafts - Issue 1Đức HòangNo ratings yet
- Inventory Management PreetDocument28 pagesInventory Management PreetKawalpreet Singh MakkarNo ratings yet
- As 4123.4-2008 Mobile Waste Containers Containers With Four Wheels With A Capacity From 750 L To 1700 L WithDocument7 pagesAs 4123.4-2008 Mobile Waste Containers Containers With Four Wheels With A Capacity From 750 L To 1700 L WithSAI Global - APACNo ratings yet
- VisiLogic Software Manual-LadderDocument158 pagesVisiLogic Software Manual-LadderEduardo Vasquez CastroNo ratings yet
- How To Install GmtsarDocument24 pagesHow To Install GmtsardedetmixNo ratings yet
- WATCHDocument9 pagesWATCHGANTORONo ratings yet