Professional Documents
Culture Documents
Hypersenstivity Reaction
Uploaded by
Vikram KapurCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Hypersenstivity Reaction
Uploaded by
Vikram KapurCopyright:
Available Formats
Hypersensitivity reactions
Abdul Ghaffar, Ph.D.; e-mail: ghaffar@med.sc.edu)
Lectures 19-20 TEACHING OBJECTIVES: 1. 2. 3. 4. 5. Understand the classification of hypersensitivity reactions Know the diseases associated with hypersensitivity reactions Understand the mechanisms of damage in hypersensitivity reactions Know the methods for diagnosing conditions due to hypersensitivity Know the modes of treating disease due to hypersensitivity and their rationale
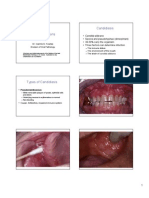
READING: Male, Brostoff, Roth and Roitt: Immunology, 7th Ed., Chapters 23-26. Hypersensitivity refers to undesirable (damaging, discomfort producing and sometimes fatal) reactions produced by the normal immune system. Hypersensitivity reactions require a presensitized (immune) state of the host. Hypersensitivity reactions can be divided into four types: type I, type II, type III and type IV, based on the mechanisms involved and time taken for the reaction. Frequently, a particular clinical condition (disease) may involve more than one type of reaction. Type I Hypersensitivity It is also known as immediate or anaphylactic hypersensitivity. The reaction may involve skin (urticaria and eczema), eyes (conjunctivitis), nasopharynx (rhinorrhea, rhinitis), bronchopulmonary tissues (asthma) and gastrointestinal tract (gastroenteritis). The reaction may cause from minor inconvenience to death. The reaction takes 15-30 minutes from the time of exposure to the antigen. Sometimes the reaction may have a delayed onset (10-12 hours).
B cell
IL13
TH2 Histamine, tryptase, kininegenase, ECFA
Leukotriene-B4, C4, D4, Newly prostaglandin D, PAF synthesized mediators
Figure 1: Induction and effector mechanisms in type I hypersensitivity
Immediate hypersensitivity is mediated by IgE. The primary cellular component in this hypersensitivity is mast cell or basophil. The reaction is amplified and/or modified by platelets, neutrophils and eosinophils. A biopsy of the reaction site demonstrates mainly mast cells and eosinophils. The mechanism of reaction involves preferential production of IgE, in response to certain antigens, allergens (Figure 1). Individuals prone to type-I hypersensitivity preferentially produce IL-4 and IL-13 that favor IgE class switch. IgE has very high affinity for its receptor (Fc; CD23) on mast cells and basophils. A subsequent exposure to the same allergen cross links the cell-bound IgE and triggers the release of various pharmacologically active substances (Figure 1). Cross-linking of IgE Fc-receptor is important in mast cell triggering. Mast cell degranulation is
1
preceded by increased Ca++ influx, which is a crucial process; ionophores which increase cytoplasmic Ca++ also promote degranulation, whereas, agents which deplete cytoplasmic Ca++ suppress degranulation. The agents released from mast cells and their effects are listed in Table 1. Mast cells may be triggered by other stimuli such as exercise, emotional stress, chemicals (e.g., photographic developing medium, calcium ionophores, codeine, etc.), anaphylotoxins (e.g., C4a, C3a, C5a, etc.). These reactions mediated by agents without IgE-allergen interaction are not hypersensitivity reactions, although they produce the same symptoms. Table 1. Pharmacologic Mediators of Immediate Hypersensitivity mediator Physiological effect preformed mediators in granules histamine tryptase kininogenase ECF-A (tetrapeptides) bronchoconstriction, mucus secretion, vasodialatation, vascular permeability proteolysis kinins and vasodialatation, vascular permeability, edema attract eosinophil and neutrophils newly formed mediators leukotriene B4 leukotriene C4, D4 prostaglandins D2 PAF basophil attractant same as histamine but 1000x more potent Eosinophil and basophil chemotactic, histamine-like but more potent edema and pain platelet aggregation and heparin release: microthrombi
The reaction is amplified by PAF (platelet activation factor) that causes platelet aggregation and release of histamine, heparin and vasoactive amines. Eosinophil chemotactic factor of anaphylaxis (ECF-A) and neutrophil chemotactic factors attract eosinophils and neutrophils, respectively, which release various hydrolytic enzymes that cause necrosis. Eosinophil may also control the local reaction by releasing arylsulphatase, histaminase, phospholipase-D and prostaglandin-E, although this role of eosinophils is now in question. Cyclic nucleotides appear to play a significant role in the modulation of immediate hypersensitivity reaction, although their exact function is ill understood. Substances which alter cAMP and cGMP levels significantly alter the allergic symptoms. Thus, substances that increase
2
intracellular cAMP seem to relieve allergic symptoms, particularly broncho-pulmonary ones, and are used therapeutically (Table 2). Conversely, agents that decrease cAMP or stimulate cGMP aggravate these allergic conditions. Table 2: Relationship between allergic symptoms and cyclic-nucleotides
stimulation of -adrenergic receptor (nor-epinephrin, phenyl-epinephrin) or blocking of -adrenergic receptor (propanolol)
stimulation of -adrenergic receptor (epinephrine, isoproterenol) blocking of -adrenergic receptor (phenoxybenzamine) inhibition of phosphodiesterase (theophylline)
stimulation of -cholinergic receptor (acetyl choline, carbacol) WORSENING OF SYMPTOMS
binding of histamine-2 or PGE to their receptors IMPROVEMENT OF SYMPTOMS
Diagnostic tests for immediate hypersensitivity include skin (prick and intradermal) tests resulting in wheal and flare reaction, measurement of total IgE and specific IgE antibodies against the suspected allergens. Total IgE and specific IgE antibodies are measured by a modification of enzyme immunoassay (ELISA). Increased IgE levels are indicative of atopic condition, although IgE may be elevated in some non atopic diseases (e.g., myelomas, helminthic infection, etc.). There appears to be a genetic predisposition for atopic diseases and there is evidence for HLA (A2) association. Symptomatic treatment is achieved with antihistamines that block histamine receptors. Chromolyn sodium inhibits mast cell degranulation, probably, by inhibiting Ca++ influx. Late onset allergic symptoms, particularly bronchoconstriction which is mediated by leukotrienes are treated with leukotriene receptor blockers (Singulair, Accolate, Lukast) or inhibitors of cyclooxygenase pathway (Zileutoin). Symptomatic, although short-term, relief from bronchoconstriction is provided by bronchodilators (inhalants) such as isoproterenol derivatives (Terbutaline, Albuterol). Thophylline elevates cAMP by inhibiting cAMP-phosphodiesterase and inhibits intracellular Ca++ release is also used to relieve bronchopulmonary symptoms. IgG antibodies against the Fc portions of IgE that binds to mast cells has been approved for treatment of certain allergies, as it can block mast cell sensitization. Hyposensitization (immunotherapy or desensitization) is another treatment modality which is successful in a number of allergies, particularly to insect venoms and, to some extent, pollens. The mechanism is not clear, but there is a correlation between appearance of IgG (blocking) antibodies and relief from symptoms. Suppressor T cells that specifically inhibit IgE antibodies may play a role.
3
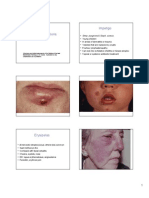
Type II Hypersensitivity It is also known as cytotoxic hypersensitivity and may affect a variety of organs and tissues. The antigens are normally endogenous, although exogenous chemicals (haptens) that can attach to cell membranes can also lead to type II hypersensitivity. Drug-induced hemolytic anemia, granulocytopenia and thrombocytopenia are such examples. The reaction time is minutes to hours. It is mediated, primarily, by antibodies of IgM or IgG class and complement (Figure 2). Phagocytes and K cells may also play a role (ADCC).
Figure 2: Type II hypersensitivity mechanisms
The lesion contains antibody, complement and neutrophils. Diagnostic tests include detection of circulating antibody against tissues involved and the presence of antibody and complement in the lesion (biopsy) by immunofluorescence. The staining pattern is normally smooth and linear, such as that seen in Goodpastures nephritis (renal and lung basement membrane) and pemphigus (skin intercellular protein, desmosome). Treatment involves anti-inflammatory and immunosuppressive agents.
Type III Hypersensitivity It is also known as immune complex hypersensitivity. The reaction may be general (e.g., serum sickness) or may involve individual organs including skin (e.g., systemic lupus erythematosus, Arthus reaction), kidneys (e.g., lupus nephritis), lungs (e.g., aspergillosis), blood vessels (e.g., polyarteritis), joints (e.g., rheumatoid arthritis) or other organs. This reaction may be the pathogenic mechanism of diseases caused by many microorganisms. The reaction may take 3-10 hours after exposure to the antigen (as in Arthus reaction). It is mediated by soluble immune complexes. They are mostly of IgG class, although IgM may also be involved. The antigen may be exogenous (chronic bacterial, viral or parasitic infections), or endogenous (non-organ specific autoimmunity: e.g., systemic lupus eythematosus-SLE). The antigen is soluble and not attached to the organ involved. Primary components are soluble immune complexes and complement (C3a, 4a and 5a). The damage is caused by platelets and neutrophils (Figure3). The lesion contains primarily neutrophils and deposits of immune complexes and complement. Macrophages infiltrating in later stages may be involved in the healing process.
4
Figure 3: Mechanism of damage in type-III hypersensitivity
The affinity of antibody and size of immune complexes are important in production of disease and determining the tissue involved. Diagnosis involves examination of tissue biopsies for deposits of Ig and complement by immunofluorescence. The immunofluorescent staining in type III hypersensitivity is granular (as opposed to linear in type II: Goodpasture). Presence of immune complexes in serum and depletion in complement level are also diagnostic. Polyethylene glycol mediated turbidity (nephelometry), binding of C1q and Raji cell test are utilized to detect immune complexes. Treatment includes anti-inflammatory agents.
Type IV Hypersensitivity It is also known as cell mediated or delayed type hypersensitivity. The classical example of this hypersensitivity is tuberculin (Montoux) reaction that peaks 48 hours after the injection of antigen (PPD or old tuberculin). The lesion is characterized by induration and erythema.
APC
IL2 TNF IFN NO2
TH1
IL2, TNF IFN
NK
LAK
Type IV hypersensitivity is involved in the pathogenesis of many autoimmune and infectious diseases (tuberculosis, leprosy, Figure 4. Mechanisms of damage in delayed blastomycosis, histoplasmosis, toxoplasmosis, hypersensitivity leishmaniasis, etc.) and granulomas due to infections and foreign antigens. Another form of delayed hypersensitivity is contact dermatitis (poison ivy, chemicals, heavy metals, etc.) in which the lesions are more papular. Type IV
FGF
hypersensitivity can be classified into three categories depending on the time of onset and clinical and histological presentation (Table 3). Mechanisms of damage in delayed hypersensitivity include T lymphocytes and monocytes and/or macrophages. Cytotoxic T cells (Tc) cause direct damage whereas helper T (TH1) cells secrete cytokines that activate cytotoxic T cells and recruit and activate monocytes and macrophages, which cause the bulk of the damage (Figure 3). The delayed hypersensitivity lesions mainly contain monocytes and a few T cells. Major lymphokines involved in delayed hypersensitivity reaction include monocyte chemotactic factor, interleukin-2, interferon-, TNF , etc. Table 3. Delayed hypersensitivity reactions type contact tuberculin granuloma reaction time 48-72 hr 48-72 hr 21-28 days clinical appearance eczema local induration hardening histology antigen and site
lymphocytes, followed by epidermal ( organic macrophages; edema of chemicals, poison ivy, epidermis heavy metals, etc.) lymphocytes, monocytes, macrophages macrophages, epitheloid and giant cells, fibrosis intradermal (tuberculin, lepromin, etc.) persistent antigen or foreign body presence (tuberculosis, leprosy, etc.)
Diagnostic tests in vivo include delayed cutaneous reaction (e.g. Montoux test) and patch test (for contact dermatitis). In vitro tests for delayed hypersensitivity include mitogenic response, lympho-cytotoxicity and IL-2 production. Corticosteroids and other immunosuppressive agents are used in treatment. A comparative summary of all four types of hypersensitivity reactions have been presented in Table 4 below.
Table 4. Comparison of Different Types of hypersensitivity type-I (anaphylactic) IgE exogenous 15-30 minutes wheal & flare basophils and eosinophil antibody allergic asthma, hay fever type-II (cytotoxic) IgG, IgM cell surface minutes-hours lysis and necrosis antibody and complement antibody type-III (immune complex IgG, IgM soluble 3-8 hours erythema and edema, necrosis type-IV (delayed type) None tissues & organs 48-72 hours erythema and induration
characteristics antibody antigen response time appearance histology transferred with examples
complement and monocytes and lymphocytes neutrophils antibody T-cells tuberculin test, poison ivy, granuloma
SLE, farmers erythroblastosis fetalis, Goodpastures lung disease nephritis
You have learned: Distinctions between different types of hypersensitivity. Mechanisms of immune-mediated damages. Examples of different types of hypersensitivity and overlap among them. Diagnostic test for hypersensitivity diseases and treatments.
You might also like
- ORE Sample QuestionsDocument16 pagesORE Sample QuestionsVikram Kapur91% (23)
- Guide To Dental Treatment BandsDocument2 pagesGuide To Dental Treatment BandsVikram Kapur100% (1)
- Fungi HODocument9 pagesFungi HOVikram KapurNo ratings yet
- Bacterial Infections PDFDocument10 pagesBacterial Infections PDFVikram KapurNo ratings yet
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (399)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (120)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- Mossbauer SpectrosDocument7 pagesMossbauer SpectroscyrimathewNo ratings yet
- Introduction To Microelectronic Fabrication PDFDocument332 pagesIntroduction To Microelectronic Fabrication PDFChristy Moore92% (13)
- 3-A Y 3-B Brenda Franco DíazDocument4 pages3-A Y 3-B Brenda Franco DíazBRENDA FRANCO DIAZNo ratings yet
- ManualDocument24 pagesManualCristian ValenciaNo ratings yet
- 1id Abstracts Season 2 Episode 6Document406 pages1id Abstracts Season 2 Episode 6Jennifer BrownNo ratings yet
- Earth Science NAME - DATEDocument3 pagesEarth Science NAME - DATEArlene CalataNo ratings yet
- Full Download University Physics With Modern Physics 14th Edition Young Test Bank PDF Full ChapterDocument13 pagesFull Download University Physics With Modern Physics 14th Edition Young Test Bank PDF Full Chapterpoetrycloudyzjm12q100% (19)
- The Handmaid's TaleDocument40 pagesThe Handmaid's Taleleher shahNo ratings yet
- General Return Service Agreement (RSA) GuidelinesDocument2 pagesGeneral Return Service Agreement (RSA) GuidelinesJune Francis AngNo ratings yet
- Ferrero A.M. Et Al. (2015) - Experimental Tests For The Application of An Analytical Model For Flexible Debris Flow Barrier Design PDFDocument10 pagesFerrero A.M. Et Al. (2015) - Experimental Tests For The Application of An Analytical Model For Flexible Debris Flow Barrier Design PDFEnrico MassaNo ratings yet
- Present Perfect Simp ContDocument14 pagesPresent Perfect Simp ContLauGalindo100% (1)
- Construction Drawing: Legend Notes For Sanitary Piping Installation General Notes NotesDocument1 pageConstruction Drawing: Legend Notes For Sanitary Piping Installation General Notes NotesrajavelNo ratings yet
- An Introduction To Routine and Special StainingDocument13 pagesAn Introduction To Routine and Special StainingBadiu ElenaNo ratings yet
- Handout Waste Catch BasinDocument2 pagesHandout Waste Catch BasinJonniel De GuzmanNo ratings yet
- SANDWICH Elisa (Procedure) - Immunology Virtual Lab I - Biotechnology and Biomedical Engineering - Amrita Vishwa Vidyapeetham Virtual LabDocument2 pagesSANDWICH Elisa (Procedure) - Immunology Virtual Lab I - Biotechnology and Biomedical Engineering - Amrita Vishwa Vidyapeetham Virtual LabsantonuNo ratings yet
- Buddha Mind PDFDocument32 pagesBuddha Mind PDFVishal GadeNo ratings yet
- God Reproducing Himself in UsDocument6 pagesGod Reproducing Himself in UsLisa100% (1)
- UserProvisioningLabKit 200330 093526Document10 pagesUserProvisioningLabKit 200330 093526Vivian BiryomumaishoNo ratings yet
- (Schottel) Aspects of The Design Procedure For Propellers Providing Max Bollard PullDocument10 pages(Schottel) Aspects of The Design Procedure For Propellers Providing Max Bollard Pulldevu2chodankarNo ratings yet
- Hackerearth Online Judge: Prepared By: Mohamed AymanDocument21 pagesHackerearth Online Judge: Prepared By: Mohamed AymanPawan NaniNo ratings yet
- RS-All Digital PET 2022 FlyerDocument25 pagesRS-All Digital PET 2022 FlyerromanNo ratings yet
- Nascsa - Sponsor Solicitation List: January 06, 2021Document35 pagesNascsa - Sponsor Solicitation List: January 06, 2021Prasoon SimsonNo ratings yet
- Aircraft Flight Control SystemDocument25 pagesAircraft Flight Control Systemthilina jayasooriyaNo ratings yet
- Ortho TechnologyDocument196 pagesOrtho Technologyr3doc3No ratings yet
- All About TarlacDocument12 pagesAll About TarlacAnonymous uLb5vOjXNo ratings yet
- Ims DB DCDocument90 pagesIms DB DCpvnkraju100% (1)
- Setting and Plot: Old YellerDocument8 pagesSetting and Plot: Old YellerWalid AhmedNo ratings yet
- Linux and The Unix PhilosophyDocument182 pagesLinux and The Unix PhilosophyTran Nam100% (1)
- Oracle SOA Suite Oracle Containers For J2EE Feature Overview OC4JDocument10 pagesOracle SOA Suite Oracle Containers For J2EE Feature Overview OC4JLuis YañezNo ratings yet
- What Is Urban PlanningDocument33 pagesWhat Is Urban PlanningDivine Grace FernandoNo ratings yet