Professional Documents
Culture Documents
Anatomical Features of Bougainvillea
Uploaded by
Bella ReadCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Anatomical Features of Bougainvillea
Uploaded by
Bella ReadCopyright:
Available Formats
Anatomical features of Bougainvillea (Nyctaginaceae)
Sarah Chew*
*This manuscript was prepared under the guidance of Professor John S. Greenwood, Department of Molecular and Cellular Biology, College of Biological Science, University of Guelph, Guelph, Ontario, Canada.
Abstract
Two potted plants of Bougainvillea were obtained from a local nursery to explore their anatomical features using a variety of microscopical methods and stains. Various anatomical structures revealed were consistent with previous literature and these features were related to the natural habitat and established internal and external defences of the plant. Results confirmed that the Bougainvillea is, in fact, a dicotyledonous plant that has evolved defensive features that permit its survival in hot and dry environments. Results indicated that features such as woody spines, the crystal inclusions in cells and the anomalous growth pattern are relatively unique to this species and important for its survival against predators.
Introduction
Bougainvillea is a genus of bright flowering plants that belongs to the family, Nyctaginaceae [1]. This genus is native to Brazil and is named after Louis Antoine de Bougainville, the first man to discover and record its existence in 1786 [2]. Today, Bougainvillea is now a popular plant in Southern California, Florida, the Caribbean and other areas with tropical and warm climates [3]. It is best grown in direct sunlight with frequent fertilization and requires little water to grow [2]. Since the original discovery of this genus, many varieties have been identified and named. Of these varieties, Bougainvillea spectabilis wiild and Bougainvillea glabra appear to be more commonly studied and are cited extensively in the literature relative to others. The primary use for Bougainvillea is as an ornamental because of its beautiful displays of colourful bracts. The specific cultivar that will be studied in this paper, Barbara Karst is known for its bright red bracts, but Bougainvillea cultivars have white, purple, lavender, and yellow bracts [1]. Bougainvillea can conform to a variety of habits such that, if left alone, it will assume the position of a sprawling shrub. If needed, this plant can be trained to cascade over walls, can be used as an espalier or can be transplanted into individual containers [1]. Bougainvillea tolerates trimming and shaping well, making it an easy plant to modify and control. Although Bougainvillea species respond well to horticultural use, this plant also has other useful properties. Recent research by Adebayo et al. has shown that extracts from Bougainvillea glabra leaves can have an anti-diabetic effect and can improve individual blood lipid profiles in rats [3]. Past anatomical research has emphasized the occurrence of anomalous secondary growth in stems and roots of Bougainvillea. Esau and Cheadle were two of the first researchers to reveal and explain anomalous secondary growth [4]. Following their work, researchers such as Stevenson and Popham studied the ontogeny of the primary thickening meristem in seedlings [5]. Subsequently, Zamaski described vascular continuity in the primary and secondary stem tissues [6] and more recently, Carlquist reviewed the work of the authors listed above in revisiting secondary growth in plants such as Bougainvillea in addition to others [7]. In this paper, emphasis will be placed on exploring the anatomical features of Bougainvillea while considering previous literature and applying that knowledge to the cultivar, Barbara Karst. In
addition, information obtained about the environment in which Bougainvillea thrives will be used to try to understand why certain anatomical features exist in this genus and not others.
Materials and Methods
Two potted plants of Bougainvillea were obtained from a local nursery to explore their anatomical features using a variety of stains and materials and methods as outlined in Plant Anatomy Laboratory exercises by Peterson et al.[8]. Fresh samples of shoots, petioles, roots, leaves, bracts, spines, and flowers were sectioned transversely and longitudinally with a two-sided razor blade and stained using TBO to reveal various cell wall components [8]. Shoots and roots were sectioned transversely and stained using phloroglucinol to reveal lignified secondary walls, sudan III/IV to reveal structures containing lipid bodies, acid fuschin to reveal protein, and berberine to reveal suberin, lignin and callose. Berberine was particularly useful in identifying Casparian bands in the endodermis and exodermis of roots. Sections of roots stained with berberine were viewed under UV and blue light, using an epifluorescence microscope. I2KI was used to stain statoliths, distinctive structures found in the root cap. Other techniques used that were particular to the shoot included aniline blue to reveal callose, a cell wall component of phloem. In addition to these techniques, macerates of shoots and petioles were obtained following the method outlined in Peterson et al., 2008 [8] and stained using TBO to reveal tracheids, fibres and vessel elements. Longitudinal sections of the spine and the floral apical meristem were embedded in paraffin wax and sectioned using a microtome according to the techniques described in Peterson et al. [8]. Sections were mounted on a slide, stained with TBO and viewed using a compound microscope to reveal crystal idioblasts, leaf primordia and trichomes [8].
Results
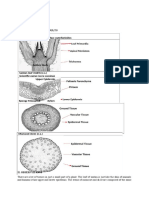
Stem Transverse and longitudinal sections were made of the young stem and stained to help reveal the characteristics of the Bougainvillea anatomy. Starting at the periphery of the stem and progressing inward, numerous multicellular trichomes arise from a single layer of epidermal cells. Just inside the epidermis, there are successive layers of newly arising cork cambium, parenchyma cells, phloem, tracheary elements of the xylem, and a thick band of fibres, all surrounding the central pith. Embedded in the pith are vascular bundles which are themselves composed of xylem and phloem (Fig. 1A). A closer examination of individual vascular bundles reveals fibres, both removed from and immediately surrounding the vasculature, the latter being composed of both phloem and tracheary elements of the xylem, presumably vessel elements. Interestingly, parenchyma lying near the fibres has thickened, sclerified cell walls (Fig.1B). In very young stems (Fig.1C), angular collenchyma, having primary cell walls thickened in the corners, lies immediately below the epidermis. Longitudinal sections of stem are shown in Figure 1 D, E, F. Fibres and sclerified parenchyma are illustrated in figure 1D. Figure 1E reveals the helical and scaliform secondary cell wall thickenings of the trachaery elements of the xylem. Peripheral to the xylem, phloem sieve tube members are identified by the presence of sieve plates (Fig. 1F). Phloroglucinol staining of a transverse section of a young stem reveals the highly lignified nature of the fibres associated with the xylem (pink staining Fig. 1G). The anamolous secondary growth seen in Bougainvillea is illustrated in a transverse section of a more mature stem (Fig. 1H), where individual layers of vasculature have been deposited. A new vascular cambium has been initiated in the parenchyma layer toward the outside of the stem, the successive vascular cambia defining anomalous growth.
Figure 1. Anatomy of Bougainvillea stem. 1A. Transverse section of young stem stained with TBO, and revealing the surface trichomes (Tr), single cell layer thick epidermis (E), and underlying forming cork layer (Co) also known as the periderm, phloem (PH), tracheary elements of the xylem (TE), a ring of fibres (FB) and the pith (PI) containing small vascular bundles (oval). 1B. Magnified view of the vascular bundles revealing a thin layer of fibres (FB towards the periphery of the stem, with a thicker layer capping the phloem (PH). Xylem tracheary elements (TE) are also surrounded by fibres and sclerified parenchyma (SP) 1C. Transverse section of a very young stem stained with TBO. A layer of angular collenechyma (AC) having irregularly thickened primary cell walls lies immediately under the epidermis (E) . FB, fibres; PH, phoem; TE, tracheary elements of the xylem 1D. Longitudinal section of young stem stained with TBO illustrating fibres (FB) and sclerified parenchyma (SP). 1E and F. Longitudinal section of stem stained with acid fuschin revealing the helical and scalariform nature of the cell wall thickenings of xylem tracheary elements (TE in 1 E) and sieve plates in the sieve tube members of the phloem (SP in 1F) 1G. Transverse section of a young stem stained with phloroglucinol HCl. Pink staining reveals lignified cell walls of the xylem tracheary elements and fibres. 1H. Transverse section of older stem revealing anomalous secondary growth. Successive vascular regions are added as the stem expands laterally. Two distinct regions of mature vasculature are shown with a tracheary element (TE) of the xylem indicated in each. A new layer of vascular cambium (VC), which will give rise to new xylem and phloem, has been initiated in the parenchyma more proximal to the outside of the stem . Macerates of the stem stained with TBO similarly revealed the helical secondary wall thickenings in some of the vessel elements (Fig. 2A). Fibres, vessel elements with simple perforation plates, and tracheids with bordered pits (Fig. 2B) and individual raphide crystals (Fig.2C) were also seen.
Figure 2. Stem macerates allow identification of elements of the vasculature and raphide crystal. All images stained with TBO 2A. A vessel element (VE) with simple perforation plate (pp) 2B. Elongated vessel element (VE) with simple perforation plate (pp) lies over a tracheid (T) having bordered pits. FB, fibre 2C. Numerous spear-shaped raphide crystals ( C ) were found in all macerates Roots Young roots in primary growth sectioned transversely and stained with TBO, revealed the formation of a diarch vaxcular arrangement with xylem having two protoxlyem poles with metaxylem between. The developing phloem formed two arches surrounding the xylem. Young roots also showed an extensive number of root hairs (Fig. 3A). As secondary growth began in the older roots (Fig. 1B), secondary tracheary elements were added outwards from the metaxylem and phloem began to surround the secondary xylem. The formation of a periderm was initiated at this point. Roots that were stained with berberine and viewed under UV light confirmed the same results as the TBO, showing the diarch arrangement, cortical parenchyma, hypodermis and epidermis (Fig. 3C). The berberine stain viewed under blue light revealed the diarch arrangement with Casparian bands formed in the surrounding endodermis (Inset Fig. 3C). It should be noted that a triarch arrangement was also identified in older regions of the root and when stained with berberine and viewed under blue light, tissue having a blue fluorescence, owing to the presence of suberin, was revealed (Fig. 3D). Longitudinal sections of the root tip stained with TBO revealed root cap cells, protoderm, procambium and ground meristem (Fig. 3E). A closer view of these root cap cells showed that statoliths are present (Inset Fig. 3E). Longitudinal sections stained with I2KI revealed an open organization of the root system and confirmed that statoliths contain starch (purple/black staining in root cap) (Fig. 3F).
Figure 3. Anatomy of Bougainvillea root. 3A. Transverse section of a very young root stained with TBO, revealing the diarch nature of the vasculature. The xylem consists of two protxylem (PX) poles with metaxylem (MX) between. Archs of phloem (PH) lie outside the xylem. Cortex (CT) surrounds the vasculature, which in turn is surrounded by an epidermal layer having numerous root hairs (RH) 3B. Transverse section of older root stained with TBO . Secondary growth has been initiated as indicated by the addition of secondary xylem adjacent to the metaxylem, phloem (PH) is more developed, and the periderm (PD) is starting to form with the lateral expansion of the root. 3C. Transverse section of young root stained with berberine hemisulfate and viewed under UV light shows the epidermis (E), hypodermis (H) , xylem (X) and the region of the endodermis (EN). Viewing under blue light confirms the region of the endodermis by reveling the Casprian band s associated with the cells of this layer (inset, arrows). 3D. Transverse section of old root stained with berberine hemisulfate and viewed under blue light illustrates the expansion of the secondary xylem (SX) around the region of meatxylem (MX). The blue tinge reveals the initiation of a periderm (PD), similar to that seen in 3 B, with suberin in these cells being responsible for the blue fluorescence. 3F. Longitudinal section of root tip stained with TBO and illustrating the locations of the procambium (PC), ground meristem (GM) and protoderm (PD). The apical meristem of the root is protected by a root cap (RC) which has statoliths (inset, ST). 3H. Longitudinal section of root stained with I2KI revealing an open organization of the root apex and demonstrating that the statoliths are most likely starch (purple-black deposits in root cap). Spines Spines of the Bougainvillea plant are very solid structures, which appear green when young (Fig. 4A) and woody (Fig. 4B) when mature. Spines sectioned transversely and stained with TBO revealed a very thick periderm, followed by a ring of fibres, overlaying a ring of sclerified parenchyma. Within this ring, a parenchymatous pith contains some vascular bundles (Fig. 4C), albeit very small (Fig. 4D). Longitudinal sections reflect what is seen in the transverse section, showing fibres and extensive sclerified parenchyma. Note that the sclerified parenchyma have very thickened cell walls (Fig. 4E). A closer view of this longitudinal section reveal some tracheary elements composed of vessel elements and tracheids seen among the fibres, albeit small and difficult to find (Fig.4F).
Figure 4. Anatomy of Bougainvillea protective spines. 4A and B. Spines arise as modified leaves (arrow, 4 A) and become wood y with age (4 B).
4C and D. Transverse section of spine stained with TBO revealing a well developed periderm (PD) surrounding a thick sclerenchymatous layer composed of fibres (FB) and sclerified parenchyma (SP). Small vascular bundles (VB) are present in a central pith-like region (4 C). The vascular bundles contain relatively few cells (oval, 4 D). 4E. Longitudinal section of spine stained with TBO and focusing on the sclerenchymatous layer reveals long fibres (FB) beside a region of short rectangular sclerified parenchyma cells (SC) having thickened cell walls. 4F. Longitudinal section of spine stained with TBO and focusing on the small vascular bundles. Xylem tracheary elements individually have bordered pits (presumably tracheids), and helical wall thickenings (tentatively vessel elements). Leaves Leaves of the Bougainvillea plant display an alternate, simple arrangement along the stem with an undulate leaf margin and ovate shape (Fig. 5A). Leaves and bracts that were sectioned transversely and stained with TBO revealed a variety of structures including the midvein (xylem and phloem), midrib parenchyma, palisade and spongy mesophyll, covering trichomes and raphide crystal bundles (Fig. 5B). A closer view of the transverse leaf viewed using brightfield and cross polarizing microscopy confirmed that these structures contained crystals (Inset Fig. 5B). Intact leaves viewed under the dissecting microscope revealed the venation pattern of the leaf, showing the midvein, lateral veins, areoles and trichomes (Fig. 5C). A closer view of a cleared leaf stained with TBO revealed numerous multicellular trichomes on the adaxial surface, although trichomes have been found on both surfaces (Fig.5D). Epidermal peels revealed that stomata complexes are generally found on the abaxial side of the leaf (Fig. 5E) while nail polish impressions further demonstrated this finding (Fig. 5F). Nail polish impressions also revealed that stomata were absent on the adaxial surface and that there were differences in the shapes of the pavement cells found on the abaxial versus the adaxial side of the leaf (Fig. 5G).
Figure 5. Morphology and anatomy of Bougainvillea leaves.
5A. Leaves of Bougainvillea are arranged in a simple alternate arrangement on the stem. Leaves have ovate shape with undulate margin. 5B. Transverse section of leaf through the midvein and stained with TBO reveals a fairly typical arrangement of photosynthetic mesophyll, with a single layer of palisade mesophyll (PM) nearest the adaxial (upper) surface and spongy mesophyll (SM) below. The epidermis (E) is a single cell layer with multicellular trichomes (TR, difficult to distinguish in this section) The vascular bundle of the midvein has regions of xylem (X) and phloem (PH) surrounded by scattered regions of fibers (FB). There is an extensive layer of parenchyma tissue (PC) between the lower epidermis and the vascular bundle, which may aid in support. Bundles of raphide crystals (RB) are scattered throughout the mesophyll , the inset showing that the dense structures seen in brightfield microscopy (bf) contain crystal structures as observed under cross polarized light (pol). 5C. Intact leaf viewed under dissecting microscope shows net-venation pattern of the Bougainvillea leaf, with mid-vein (MV), lateral veins (LV) and areoles (A). Trichomes are numerous on the abaxial surface. 5D. Close up view of cleared leaf stained with TBO shows the very numerous multicellular trichomes of the leaf, the only structures seen in this image. 5E. Close up of epidermal peel of the abaxial surface of the leaf illustrating the guard cells (GC) of the stomtal complex and pavement cells (PC) of the epidermis. The stomatal complex lacked obvious subsidiary cells. 5F and G.. Nail polish impressions of the abaxial and adaxial leaf surfaces illustrate that stomatal complexes exist only on the abaxial surface (ovals, 5F) . Pavement cells of the abaxial surface have the shape of jigsaw puzzle pieces, whereas those on the adaxial surface (PC) are more regular in shape. Flower Longitudinal sections of the flower left unstained revealed the internal anatomy including the perianth, filaments, anthers and pollen grains (Fig. 6A). On the external surface of the perianth, pinkpurple rrichomes were evident. A macro photo shows the tubular shape of the flower, surrounded by the bright pink bract (Inset Fig 6A). Cross polarization of the flower also revealed these pink-purple trichomes as well as pollen grains and numerous crystal bundles seen throughout the entire organ (Fig. 6B).
Figure 6. Mophology and anatomy of Bougainvillea flowers. 6A and B. Bougainvillea flowers are perfect flowers (F) that reside in groups of three above colourful leaf-like bracts (Br, inset 6A). The fused perianth is slender and tubular in nature (inset 6A, PE 6A) and has numerous trichomes (TR) on the outer surface (6A). Stamens with anthers (AN) and filaments (Fi) were obvious within the sectioned flower. As with the leaves, bundles of raphide crystals are present in the perianth, presumably as a protective measure against herbivores. 6A, bright field unstained, 6B cross polarizing microscopy. Pollen grains (PG), having thick almost crystalline cell walls are revealed using polarized light (6B).
Discussion
Anomalous growth has been characterized by the presence of multiple cambia that are ontogenetically related yet forming independently of one another [4]. Each year, parenchyma cells in the stem will dedifferentiate and become meristematic again forming new phloem and xylem tissue where phloem is always to the periphery and xylem to the middle of the stem [4]. As growth continues, the vasculature appear to be randomly dispersed due to the activity of each independent cambium. The organization of the vascular bundles themselves appears to exhibit a collateral arrangement with fibres, sclerenchmya and possible angular collenchyma found surrounding these bundles. These cell types provide extra protection and add support to the growing stem. The Bougainvillea plant is also characterized by its woody stem as it matures and is accompanied by a periderm layer. Sections of the mature periderm were difficult to obtain. The periderm is normally present in older Bougainvillea stems and adds extra protection to the stem as it grows. The suberized phellem would be of great benefit to the Bougainvillea plant growing in the hot, dry climate as it helps to seal o ff water loss. Very young shoots did not have mature periderm but rather an epidermal layer characterized by primary cell walls. Just inside this epidermis, angular collenchyma (characteristic of stems) are found which aid in the support of the rapidly elongating stem and then
parenchyma containing chloroplasts, signifying the photosynthetic activity of the young stem. The thin layer of fibres found just outside the vascular bundles exists to protect the outer most layer of phloem. Moving further inwards, a second fibre band with sclerified parenchyma appears. This thick fibre band surrounds the innermost vascular bundles. As secondary growth proceeds, some of the parenchyma cells found within and outside of this band will become meristematic and contribute to the formation of new vascular cambia and a new layer of fibres, providing them with extra strength and flexibility as the stem continues to grow expand. The diarch and triarch arrangements found within the root are further evidence that Bougainvillea is a dicotyledonous plant. Esau & Cheadle [4] and Stevenson & Popham [5] also took note of these two arrangements and in particular, the diarch arrangement in young roots of Bougainvillea. The switch to a triarch arrangement occurred due to the widening diameter of the older root and the addition of secondary xylem and phloem. In the older sections of the root, much of the cortex had been crushed and sloughed off, including the endodermis and the pericycle and leaving the xylem and phloem surrounded by a newly forming layer of periderm, ultimately to protect this area of the root from losing water to the environment. According to Esau and Cheadle, much of the phloem associated with the normal secondary xylem increment is crushed during the expansion growth of the root, thus only the most recently formed phloem is active [4]. Sections stained with berberine and viewed under blue light did reveal Casparian bands. Due to the dry climate in which Bougainvillea thrives, it would have adapted these to help keep water locked in its roots [8]. Another adaptation that also would have helped with water retention is the cortex which appears to contain secondary wall deposits in its cell walls. TBO and I2KI revealed statoliths consisting of starch in the root cap cells; these aid in directing the downwards growth of the root, a process known as gravitropism [8]. The sharp structures located near leaf buds on the stem are referred to as spines and are modified leaves. These structures are another feature of this plants defence system. Young spines are green and involved in photosynthesis. As these structures age and become woodier, their photosynthetic abilities cease. The vascular bundles also become much smaller and eventually collapse. This allows for the plant to divert its energy sources to other structures. Eventually, a mature spine will be mostly composed of sclerified parenchyma, fibres, and a thick periderm which aid in creating a strong structure for protection and deterrence to pests. The leaves of the Bougainvillea plant have adapted to a hot, dry climate in several ways. Their flat surface makes it ideal for maximum light interception while the wide surface allows for a denser distribution of stomata complexes. The leaves also contain a thick epidermal layer, covered by a thin cuticle layer (cuticle layer not shown). The cuticle layer allows the leaf to prevent water loss in the hot environment [10]. The stomata complexes, all found on the abaxial side of the leaf, allows the leaf to carry out gas exchange during the hot day without the direct heat of the sun drying out the leaf. Other adaptations of the Bougainvillea leaves include a dense covering of epidermal hairs and the distribution of raphide crystals. The epidermal hairs, also known as covering trichomes, function to deter herbivory by insects, prevent organisms from inhabiting the surface, screen out some UV light and reduce water loss by forming a buffer against air flow [11]. The raphide crystals are a part of the plants passive defence system and act in deterring herbivory. These crystals have also been found throughout many other structures including the stem, root, flower and spine. The Bougainvillea flower is a true, perfect flower that is small and tubular in shape. The actual flower itself is white or yellow and is surrounded by showy, vibrant bracts [9]. The colourful bracts are, in fact, not petals but modified leaves, adapted to attract pollinators to the colourless and scentless flowers residing on the upper surface. The research of Xu et al. aided in determining structures found in the Bougainvillea flower such as the perianth, filaments, anthers and pollen grains [12]. These researchers also made note of the pink-purple trichomes protruding from the tubular perianth.
According to their research, these hairs are referred to as a stylar brush, which might be responsible for inhibiting contact between the flowers pollen mass and stigma between the cluster of flowers found on each bract, reducing sterility in this plant. They have also proposed that the stylar brush contains betalains, (a compound responsible for the purple colour found in beets), which accumulate in the bracts and perianth as they grow. It has been strongly suggested that these betalains function to protect the plant from the stresses of high temperature and strong light which coincides with the hot and dry climate Bougainvillea thrives in [12]. As noted in the leaf, the tubule flower and bracts also contain raphide crystals which again serve as a passive defence mechanism for this plant. Most of the features of the anatomy of Bougainvillea are fairly typical of plants that exist in temperate to tropical environs. Features that are unusual in Bougainvillea include the spines, the crystal inclusions in cells and the anomalous secondary growth pattern. These adaptations may be more related to defence against herbivory. The spines provide the plant with an obvious defence against large herbivores. The spear-shaped raphide crystals, most likely composed of calcium oxalate, would act as both a physical and a toxic chemical deterrent against both small and large herbivores [4]. Finally, the anomalous secondary growth with the accompanying layers of fibres that are laid down result in scale-like pattern of fibrous protection around the vasculature. This would be difficult for any herbivore to penetrate. Thus, in attempting to relate plant anatomy to environment, we must consider both the abiotic and biotic factors in that environment.
References
1. Gilman, F., E. 1999. Fact sheet: Bougainvillea spp. Available from the Environmental Horticulture Department, Florida U.S. Publishing. 2. Hammad, I. 2009. Genetic variation among Bougainvillea glabra cultivars (Nyctaginaceae) detected by Rapd markers and Isozymes patterns. Journal of Agriculture and Biological sciences. 5 (1): 63-71. 3. Adebayo, I.G., Alabi T. O., Owoyele, V., B., and Soladoye, O., A. 2009. Anti-diabetic properties of the aqueous leaf extract of Bougainvillea glabra on alloxan-induced diabetic rats. Rec. Nat. Prod. 3 (4): 187-192. 4. Esau, K., and Cheadle, I,V. 1969. Secondary growth in Bougainvillea. Ann. Bot. 33: 807-819. 5. Stevenson,W.D., and Popham, A.R. 1973. Ontogeny of the primary thickening meristem in seedlings of Bougainvillea spectabilis. Am. J. Botany. 60 (1):1-9. 6. Zamski, E. 1979. Vascular continuity in the primary and secondary stem tissues of Bougainvillea (Nyctaginaceae). Ann. Bot. 45: 561-567 7. Carlquist, S. 2007. Sucessive cambia revisited: ontogeny, histology, diversity and functional significance. Torrey J. Bot. 134 (2): 301-322. 8. Peterson L., Greenwood J., Lee B., McClellan D. 2009. Plant Anatomy (Bot*3410) Laboratory Exercises. Department of molecular and cellular biology. University of Guelph 9. Kobayashi, D.K., McConnell, J., Griffis, J. 2007. Bougainvillea. Available from the Department of Tropical Plant and Soil Sciences, published by the College of Tropical Agriculture and Human Resources 10. Purves, K.W., Sadava, D. Orians, H.G., Heller, C.H. 2004. The plant body. In The science of biology. Sinauer Associates, Inc., Sunderland, MA. Pp.
11. Peterson, R. L., Peterson, A.C, Melville, H.L. 2008. Teaching Plant Anatomy. NRC Press, Ottawa, Ontario. 12. 12. Xu, S., Huang, Q., Shu, Q., Chen, C., Vick, A.B. 2009. Reproductive organography of Bougainvillea spectabilis Willd. Scientia Horticulturae. 120 (3): 399-405
You might also like
- John PFTDocument231 pagesJohn PFTAlexander Santiago ParelNo ratings yet
- Outstanding 12m Bus DrivelineDocument2 pagesOutstanding 12m Bus DrivelineArshad ShaikhNo ratings yet
- Diversity (CHAPTER 30) Development (CHAPTER 35) Biology 8 Plant Evolution and Biology (CHAPTER 5)Document5 pagesDiversity (CHAPTER 30) Development (CHAPTER 35) Biology 8 Plant Evolution and Biology (CHAPTER 5)Suzette Bantiles AseoNo ratings yet
- Carbapenamses in Antibiotic ResistanceDocument53 pagesCarbapenamses in Antibiotic Resistancetummalapalli venkateswara raoNo ratings yet
- BIOLAB FROG Organ HistologyDocument7 pagesBIOLAB FROG Organ HistologyCloie Anne Rabinetas100% (1)
- American Woodworker No 171 April-May 2014Document76 pagesAmerican Woodworker No 171 April-May 2014Darius White75% (4)
- Miedy Corazon Tabang Sta. Monica High School PhilippinesDocument68 pagesMiedy Corazon Tabang Sta. Monica High School PhilippinesArman Austria100% (1)
- Introduction To Plant SystematicsDocument10 pagesIntroduction To Plant SystematicsIzba SanaNo ratings yet
- Cat Anatomy and Physiology: 4-H Cat Project - Unit 3 - Em4289EDocument44 pagesCat Anatomy and Physiology: 4-H Cat Project - Unit 3 - Em4289EmasindNo ratings yet
- Class: 8 Science Chapter - 8 Cell - Structure and FunctionsDocument10 pagesClass: 8 Science Chapter - 8 Cell - Structure and FunctionsAmiteshwar SinghNo ratings yet
- Plant HistologyDocument2 pagesPlant HistologyNurul Rayhana100% (3)
- Eating and HealingDocument19 pagesEating and HealingMariana CoriaNo ratings yet
- BWT Septron Line 31-61 Rev01!08!05-18 Opm enDocument56 pagesBWT Septron Line 31-61 Rev01!08!05-18 Opm enDavide Grioni100% (1)
- A. What Is Balanced/objective Review or Criticism?Document11 pagesA. What Is Balanced/objective Review or Criticism?Risha Ann CortesNo ratings yet
- Module III - Unit 3 - THE NATURE AND COMPOSITION OF PLANTSDocument10 pagesModule III - Unit 3 - THE NATURE AND COMPOSITION OF PLANTSRechel SamontinaNo ratings yet
- Answers To Worksheets: Worksheet 8.1 The Pathway of Water Movement Through A PlantDocument1 pageAnswers To Worksheets: Worksheet 8.1 The Pathway of Water Movement Through A Plantapi-306720794No ratings yet
- Siegfried Kracauer - Photography (1927)Document17 pagesSiegfried Kracauer - Photography (1927)Paul NadeauNo ratings yet
- Fisiologi TumbuhanDocument63 pagesFisiologi TumbuhanMasnawati WatiNo ratings yet
- Report SheetDocument11 pagesReport SheetMikhail LandichoNo ratings yet
- 00 Key For The Identification of Different BambooDocument14 pages00 Key For The Identification of Different BambooShanaia BualNo ratings yet
- Amex Case StudyDocument12 pagesAmex Case StudyNitesh JainNo ratings yet
- AP Bio Plant Anatomy (KFogler)Document26 pagesAP Bio Plant Anatomy (KFogler)julie rainesNo ratings yet
- The Primary and Secondary Growth of PlantDocument31 pagesThe Primary and Secondary Growth of PlantNOFAIZAH RAMLINo ratings yet
- Network Structure in Root, Stem, and LeafDocument5 pagesNetwork Structure in Root, Stem, and LeafMarwana SuaibNo ratings yet
- The Anatomy of Zea MaysDocument2 pagesThe Anatomy of Zea MaysCharm Dizon BasilioNo ratings yet
- Tissues: Chapter - 6Document17 pagesTissues: Chapter - 6Aditya Jha100% (1)
- Dicot-Monocot Stem AnatomyDocument6 pagesDicot-Monocot Stem AnatomyAdlet100% (1)
- Chapter 5 Morphology of Flowering PlantsDocument16 pagesChapter 5 Morphology of Flowering PlantsYashiNo ratings yet
- Secondary GrowthDocument33 pagesSecondary Growthgsr_sasiNo ratings yet
- MimosaceaeDocument15 pagesMimosaceaeZaryabTarhilyNo ratings yet
- PsilotumDocument9 pagesPsilotumDilshadNo ratings yet
- Palaeobotany: Types Nomenclature of FossilsDocument21 pagesPalaeobotany: Types Nomenclature of FossilsPawan KatiyarNo ratings yet
- Root Anatomy: RootsDocument6 pagesRoot Anatomy: RootsKim John NateNo ratings yet
- Review On Animal Tissues PDFDocument7 pagesReview On Animal Tissues PDFTitiNo ratings yet
- Class: Dicotyledonae: 1. Annona Squamosa-Custard Apple 2. A.reticulata 3. Annona MuricataDocument3 pagesClass: Dicotyledonae: 1. Annona Squamosa-Custard Apple 2. A.reticulata 3. Annona MuricataDani MathewNo ratings yet
- Insect BodywallDocument3 pagesInsect BodywallDillawar KhanNo ratings yet
- Biology and Cultivation of BrinjalDocument31 pagesBiology and Cultivation of BrinjalVaibhav DafaleNo ratings yet
- Cell WallDocument15 pagesCell WallAlex CarnajeNo ratings yet
- Anomalous secondary growth in monocots like yucca and dracaenaDocument12 pagesAnomalous secondary growth in monocots like yucca and dracaenaIRDINA ADLIN BINTI FIRDAUS MoeNo ratings yet
- History of Zoology: Evolution of Animal StudyDocument4 pagesHistory of Zoology: Evolution of Animal StudyBen Gabriel MaghuyopNo ratings yet
- Top 3 Shoot Apical Meristem TheoriesDocument11 pagesTop 3 Shoot Apical Meristem TheoriesAbhimanyu Pandey100% (2)
- Transport System in PlantDocument16 pagesTransport System in PlantAbu-Omar50% (4)
- Respiratory System: StructureDocument29 pagesRespiratory System: StructureDr. Abir Ishtiaq100% (1)
- ChordataDocument28 pagesChordatatinchu guptaNo ratings yet
- Slide For Presentation Leaf DevelopmentDocument13 pagesSlide For Presentation Leaf DevelopmentJalal AhmedNo ratings yet
- Podocarpus: The Largest Genus of ConifersDocument9 pagesPodocarpus: The Largest Genus of ConifersFouzia Youseph100% (1)
- Forests of KarnatakaDocument10 pagesForests of Karnatakasanjay kumarNo ratings yet
- Secondary and Anomalous Growth of Bougainvillea PDFDocument9 pagesSecondary and Anomalous Growth of Bougainvillea PDFIza SalvadorNo ratings yet
- Eamcet QR Botany SR Botany 05pterisDocument4 pagesEamcet QR Botany SR Botany 05pterisRajat Kumar SabharwalNo ratings yet
- BrassicaceaeDocument18 pagesBrassicaceaeTimothy JohnsonNo ratings yet
- Lab Report - Phloem & XylemDocument6 pagesLab Report - Phloem & XylemAriadna ApolonioNo ratings yet
- Pengertian, Sejarah Dan Aplikasi Kultur JaringanDocument20 pagesPengertian, Sejarah Dan Aplikasi Kultur JaringanUsman MaukNo ratings yet
- Jurnal Keanekaragaman Jenis Vegetasi Tepian Sungai Kaili Desa Labuan Kungguma Kecamatan LabuanDocument9 pagesJurnal Keanekaragaman Jenis Vegetasi Tepian Sungai Kaili Desa Labuan Kungguma Kecamatan Labuanrahmat hidayatNo ratings yet
- Plant TissuesDocument24 pagesPlant TissuesKen Torres Paycana100% (1)
- Plant Histology and AnatomyDocument39 pagesPlant Histology and AnatomyN Madhusudhan50% (2)
- Lap Pap 2 Daun Monokotil Dan Dikotil-WahDocument13 pagesLap Pap 2 Daun Monokotil Dan Dikotil-WahChaya Stia ClaluiNo ratings yet
- NostocDocument2 pagesNostocAshish DhakalNo ratings yet
- Effectiveness of Kenaf Fibre Composite Plate for Reinforcing Concrete BeamsDocument81 pagesEffectiveness of Kenaf Fibre Composite Plate for Reinforcing Concrete BeamsIfkar Azmi100% (1)
- Chapman System of ClassificationDocument6 pagesChapman System of Classificationvineetvishal73No ratings yet
- Anatomy Review Blood VesselDocument6 pagesAnatomy Review Blood VesselPaula ClayNo ratings yet
- Ant Internal Anatomy DiagramDocument4 pagesAnt Internal Anatomy DiagramHfNo ratings yet
- LYGINOPTERIS OLDHAMIA B.Sc. Part II Botany Hons. Prof. (DR.) Manorma Kumari, Botany, ANCDocument6 pagesLYGINOPTERIS OLDHAMIA B.Sc. Part II Botany Hons. Prof. (DR.) Manorma Kumari, Botany, ANCJannatul MalaNo ratings yet
- Morphology of Flowering PlantsDocument87 pagesMorphology of Flowering PlantsRenu80% (5)
- Nerve PhysiologyDocument11 pagesNerve PhysiologyKeth LaurelNo ratings yet
- Cycas Rumphii Vs Cycas CircinalisDocument1 pageCycas Rumphii Vs Cycas CircinalisjohncrusoeNo ratings yet
- AkarDocument74 pagesAkarAnonymous LCr6APfKaNo ratings yet
- Meristematic Tissues - Plant Cell Bab 2Document6 pagesMeristematic Tissues - Plant Cell Bab 2Siti NormaidahNo ratings yet
- Exercise 19 Serial Transverse Section of A 48 Hour Chick Embryo PDFDocument11 pagesExercise 19 Serial Transverse Section of A 48 Hour Chick Embryo PDFMichaela FayeNo ratings yet
- Fitohormon IntroDocument27 pagesFitohormon Introvitri_indrianiNo ratings yet
- Staghorn Corals of the World: A Revision of the Genus AcroporaFrom EverandStaghorn Corals of the World: A Revision of the Genus AcroporaNo ratings yet
- Drosophila Raw DataDocument6 pagesDrosophila Raw DataBella ReadNo ratings yet
- Cell Bio 1Document2 pagesCell Bio 1Bella ReadNo ratings yet
- Proposed Outline of Webinar PresentationDocument1 pageProposed Outline of Webinar PresentationBella ReadNo ratings yet
- Compilation of Field Exercises in Bio 160 - Feb 2014Document28 pagesCompilation of Field Exercises in Bio 160 - Feb 2014Bella ReadNo ratings yet
- Physics Problem Set For CircuitsDocument1 pagePhysics Problem Set For CircuitsBella ReadNo ratings yet
- CharacterizationDocument1 pageCharacterizationBella ReadNo ratings yet
- PAWERPOYNTDocument20 pagesPAWERPOYNTBella ReadNo ratings yet
- Review of Related LiteratureDocument5 pagesReview of Related LiteratureBella ReadNo ratings yet
- Anatomical Features of The Roots and LeavesDocument4 pagesAnatomical Features of The Roots and LeavesBella ReadNo ratings yet
- Bougainvillea InfoDocument3 pagesBougainvillea InfoBella ReadNo ratings yet
- Anatomical StudyDocument8 pagesAnatomical StudyBella ReadNo ratings yet
- Bougainvillea InfoDocument3 pagesBougainvillea InfoBella ReadNo ratings yet
- Integumentary System of VertebratesDocument4 pagesIntegumentary System of VertebratesBella ReadNo ratings yet
- Lks Bahasa Inggris Kelas Vii Semester 1 Dan 2Document6 pagesLks Bahasa Inggris Kelas Vii Semester 1 Dan 2ꓰꓡꓡꓰꓠ.ꓓꓰꓖꓰꓠꓰꓣꓰꓢꓢ.No ratings yet
- Team Dynamics and Behaviors for Global ExpansionDocument15 pagesTeam Dynamics and Behaviors for Global ExpansionNguyênNo ratings yet
- Design of Steel Structures Handout 2012-2013Document3 pagesDesign of Steel Structures Handout 2012-2013Tushar Gupta100% (1)
- Ford 30 V600Document7 pagesFord 30 V60008088338No ratings yet
- 10CV54 Unit 05 PDFDocument21 pages10CV54 Unit 05 PDFvinodh159No ratings yet
- Afrah Summer ProjectDocument11 pagesAfrah Summer Projectاشفاق احمدNo ratings yet
- Digestive System Song by MR ParrDocument2 pagesDigestive System Song by MR ParrRanulfo MayolNo ratings yet
- Women Safety AppDocument18 pagesWomen Safety AppVinod BawaneNo ratings yet
- Biotechnology Eligibility Test (BET) For DBT-JRF Award (2010-11)Document20 pagesBiotechnology Eligibility Test (BET) For DBT-JRF Award (2010-11)Nandakumar HaorongbamNo ratings yet
- After EffectsDocument56 pagesAfter EffectsRodrigo ArgentoNo ratings yet
- 26th April 2021 ES Submission - CloudKitchens - ProfessorSriramDocument16 pages26th April 2021 ES Submission - CloudKitchens - ProfessorSriramSamarth LahotiNo ratings yet
- Experimental Investigation On The Properties of Compressed Earth Blocks Stabilised With A Liquid ChemicalDocument7 pagesExperimental Investigation On The Properties of Compressed Earth Blocks Stabilised With A Liquid ChemicalDeb Dulal TripuraNo ratings yet
- Mehdi Semati - Media, Culture and Society in Iran - Living With Globalization and The Islamic State (Iranian Studies)Document294 pagesMehdi Semati - Media, Culture and Society in Iran - Living With Globalization and The Islamic State (Iranian Studies)Alexandra KoehlerNo ratings yet
- TSGE - TLGE - TTGE - Reduce Moment High Performance CouplingDocument6 pagesTSGE - TLGE - TTGE - Reduce Moment High Performance CouplingazayfathirNo ratings yet
- FRABA - Absolute - Encoder / PLC - 1 (CPU 314C-2 PN/DP) / Program BlocksDocument3 pagesFRABA - Absolute - Encoder / PLC - 1 (CPU 314C-2 PN/DP) / Program BlocksAhmed YacoubNo ratings yet
- Sci7 Q1 Wk-5 Module-5Document15 pagesSci7 Q1 Wk-5 Module-5Lester Noel RosalesNo ratings yet
- John Williams - WikipediaDocument2 pagesJohn Williams - Wikipedia三木和代No ratings yet
- Trabajo de Investigación FormativaDocument75 pagesTrabajo de Investigación Formativalucio RNo ratings yet
- Costos estándar clase viernesDocument9 pagesCostos estándar clase viernesSergio Yamil Cuevas CruzNo ratings yet
- Bosch Committed to Outsourcing to Boost CompetitivenessDocument4 pagesBosch Committed to Outsourcing to Boost CompetitivenessPriya DubeyNo ratings yet
- Issue 189Document38 pagesIssue 189Oncampus.net100% (1)