Professional Documents
Culture Documents
Optimization of The Acetone Plant Using CHEMCAD
Uploaded by
shahed IasirOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Optimization of The Acetone Plant Using CHEMCAD
Uploaded by
shahed IasirCopyright:
Available Formats
Chung,
Hodgson
GradesforProject2OptimizationofanExistingChemicalProcessFacility
Hodgson,
Roundy
ReportSection
Points
Possible
Points
earned
LetterofTransmittal 2 2
TitlePage 1 1
ExecutiveSummary 5 2
TableofContents 2 2
Introduction 10 10
Methods 15 15
ProcessDesign 70 70
Results 15 15
Discussion 15 15
Conclusion 5 5 Conclusion 5 5
References 5 5
AppendixCalculations 5 5
GrossTotal 150 147
AdjustmentsforReportAppearance,Spelling,Grammar,etc. 0
NetTotal 150 147
Created with novaPDF Printer (www.novaPDF.com). Please register to remove this message.
i
Optimization of the
Acetone Production Facility
Texas Tech University
Ch E 4555
February 26, 2007
Yongchul Chung
Cade Hodgson
Sam Roundy
Created with novaPDF Printer (www.novaPDF.com). Please register to remove this message.
ii
Executive Summary
The objective of this project is to design an improvement to an existing chemical
processing facility which produces acetone by the dehydrogenation of isopropyl alcohol.
It is recommended that a series of three compressors and two heat exchangers be used to
purify a hydrogen product stream to industrial grade and recover more acetone. This
design was optimized using a CHEMCAD simulation to determine the conditions
necessary to provide adequate separation.
The incremental revenue from the extra acetone and improved hydrogen is considered
against the capital cost of equipment and operating costs for that equipment. Capital
costs were obtained from CHEMCAD and CAPCOST while utility costs and revenues
were calculated manually. The resulting cash flows and rates of return are presented in
the table below.
The proposed expansion is extremely profitable, and the project team strongly
recommends that this proposal be implemented as soon as practical.
Table 1: Economic summary
Capital Investment Required $1,460,100
Incremental Annual Revenue $8,814,200
Incremental Annual Operating
Cost (COM
d
)
$2,805,500
Average Annual Net Profit $3,053,800
Before Tax Rate of Return 159%
After Tax Rate of Return 123%
After Tax Rate of Return on
Net Profit
117%
Created with novaPDF Printer (www.novaPDF.com). Please register to remove this message.
iii
Table of Contents
Introduction .... 1
Methods .. 2
Process Design ... 4
Results 8
Discussion 10
Conclusion ... 12
References 13
Appendix ...14
List of Tables
Table 1 ... ii
Table 2 ... 5
Table 3 ... 6
Table 4 ... 6
Table 5 ... 8
List of Figures
Figure 1 .. 4
Figure 2 .. 5
Figure 3 .. 8
Figure 4 .. 9
Created with novaPDF Printer (www.novaPDF.com). Please register to remove this message.
1
Introduction
The purpose of the project is to improve the profitability of the acetone production facility given
in Turton et al.
[6]
. This facility makes acetone via the dehydrogenation of isopropyl alcohol
(IPA). A complete description and economic analysis of the existing facility is available in a
previous report
[3]
. This optimization is performed as if the current facility is already built and
operating, so none of the economics of the base condition are relevant.
The four main costs of the facility are raw materials, utilities, waste treatment, and operating
labor. Unless significant equipment changes are made, little or no savings can be made from
operating labor. Waste treatment accounts for an insignificant amount of the total cost, so it was
also discarded as an optimization area. Utilities are a larger cost than waste treatment or
operating labor, but utilities can only be optimized to a certain extent, saving only a small
percentage. Raw material costs are by far the largest operating cost. The current process
achieves a 99.9% overall conversion of IPA to acetone, which leaves no room for significant
improvement. However, some of the acetone product is lost in the hydrogen byproduct.
The dehydrogenation process produces hydrogen which is separated out and sold as fuel gas
because it contains significant amounts of acetone. By improving the separation, the process
could recover additional acetone and thereby increase the revenue from the main product. At the
same time the hydrogen could be purified to industrial grade, which would greatly increase its
value. CHEMCAD was used to simulate the compressors, exchangers, and flash vessel for the
proposed expansion, and an optimum design was identified.
Created with novaPDF Printer (www.novaPDF.com). Please register to remove this message.
2
Methods
In this project, it quickly became obvious that at the current pressure of the hydrogen stream
(stream 7 in Figure 1), cooling alone would not separate out the acetone. For this reason,
compressors were added to raise the pressure before cooling. Simulating the current process in
CHEMCAD suggested that for a temperature of -15 C, the stream would need to be compressed
to somewhere between 50 and 100 bar. Using this information, the plant was designed more
rigorously in the CHEMCAD simulation according to the following procedures, assumptions,
and guidelines.
Compressor Design
Compressors are applied in series to achieve the desired final pressure. The compression ratio
for each compressor is limited to 4:1 based on heuristics for single stage compression
[6]
.
Theoretical power for each compressor is calculated by CHEMCAD based on the outlet
pressures specified. The efficiency for all compressors was assumed to be 80%
[6]
. CHEMCAD
provided the installed cost for each compressor based on compressor size and type. All
compressors are reciprocating compressors because CHEMCAD does not allow the user to select
a rotary compressor which is probably more reasonable for the compressor sizes considered. All
other cost factors in CHEMCAD were left at default settings.
Heat Exchanger Design
Heat exchangers cool the compressed gas stream. Cooling water is available at 30
o
C and low
temperature refrigerant is available at -20
o
C. Because the plant already uses refrigerated water,
it was assumed that low temperature refrigerant would be available. Very low temperature
refrigerant (-50
o
C) was not considered because the existing plant utilities probably cannot
supply it. The inlet temperature to the first heat exchanger is calculated by CHEMCAD based on
adiabatic compressor operation. The lowest temperature achievable with cooling water is 41
o
C
if an 11
o
C approach is maintained with counter-current flow. The refrigerant can cool down to -
15
o
C while maintaining a 5
o
C approach. These approach values were specified based on heat
exchanger heuristics
[6]
.
CHEMCAD calculates duty from the specified outlet temperature for each heat exchanger. The
heat transfer area of each exchanger is generated manually from the log mean temperature
difference, CHEMCADs calculated duty, and a heat transfer coefficient of 150 Btu/hr ft
2
o
F
based on heuristics for condensers
[6]
. CHEMCAD generated installed costs for the heat
exchangers based on the heat transfer area. For cost estimating purposes, the heat exchanger
using cooling water was designated a shell and tube heat exchanger with U tubes, and the heat
exchanger using refrigerant was designated a refrigeration exchanger. Costs were compared for
installing two heat exchangers versus one heat exchanger at each pressure.
Costs
Two types of costs are associated with this project: utility and capital costs. The utility for the
compressors is electricity, and the power consumption is obtained directly from CHEMCAD.
The price of electricity is obtained from the Energy Information Administration website
[1]
. The
utilities for the heat exchangers are cooling water and refrigerant. The costs for heat exchanger
utilities were obtained from Turton
[6]
then indexed with the electricity price from the Energy
Information Administration website
[1]
because most costs for cooling water are based on
Created with novaPDF Printer (www.novaPDF.com). Please register to remove this message.
3
electricity (compression and pumping). For all utilities, price is multiplied by hourly usage to
calculate hourly cost. The annual cost is calculated from the hourly cost based on a stream factor
of 0.9. As discussed above, the capital costs of each heat exchanger and compressor is obtained
from CHEMCAD based on current values of the CEPCI. Capital cost of the vessel is obtained
from CAPCOST using the same dimensions as vessel V-402 in the existing process
[3]
.
Revenues
The revenue of this project comes from two sources: recovering more acetone into the saleable
product and purifying the hydrogen stream to increase its value. It is assumed that currently, the
hydrogen can only be used as fuel gas. A price of $0.208/100 scf was applied to this hydrogen
based on the value of the fuel gas
[1]
with a heating value equivalent to the hydrogen stream
[1],[4]
.
The purified hydrogen is worth $3.50/100 scf
[2]
. The value of acetone is $1.46/kg
[3]
.
CHEMCAD was used to determine the flow rates and purities resulting from the proposed
process improvements. Flow rates were multiplied by unit prices to calculate hourly revenue.
Annual revenue is calculated from hourly revenue with a stream factor of 0.9.
Optimization Method
The proposed addition was simulated in CHEMCAD in order to find the optimal design. The
goal of the optimization performed was to minimize the objective function. The objective
function was defined as the present cost of the capital expenditure and annual utility costs. It
was assumed that the revenue produced would be constant as would the cost of the single vessel.
Present values of the utility costs are based on a 10 years life at 15% interest with an additional
year for construction. The objective function is given in qualitative form in Equation 1.
|
|
.
|
\
|
+
|
|
.
|
\
|
+
|
|
.
|
\
|
+
|
|
.
|
\
|
=
Utility Exchanger of
Value Present
Utility Compressor of
Value Present
s Compressor of
Cost Capital
Exchangers Heat of
Cost Capital
Objective (1)
CHEMCAD was treated as a black box function, and the optimization was performed according
to the following procedure:
1. Select an outlet pressure for each compressor.
2. Vary the outlet temperature from the heat exchangers to achieve 99.95% purity in the
final hydrogen product.
3. Evaluate the objective function.
4. Go back to step 1 until an optimum is identified.
The specifications supplied for heat exchangers and compressors in step 1 and 2 were subject to
the design limitations discussed above.
Cash Flows
This project is considered an addition to an existing process. No economics or cash flows related
to the current operations are considered, only incremental investments, costs, and revenues. The
tax rate is assumed to be 35%, and capital was depreciated using MACRS with a 9.5 year class
life and a 6 year recovery period. Since this project involves only installation of a few pieces of
equipment, it is assumed that construction can be completed in one year. The project life is
taken as 10 years with no working capital and no salvage value.
Created with novaPDF Printer (www.novaPDF.com). Please register to remove this message.
4
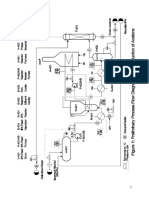
Process Design
Current Process
The current configuration comes from Turton et al.
[6]
and is presented in Figure 1. It is
discussed in detail in the previously submitted report
[3]
. An azeotropic mixture of IPA and water
is supplied to the process and combined with the recycle from the final separation column. This
mixture is vaporized then reacted in packed tubes. The reactor is heated by molten salt which
passes through a fired furnace. The exiting product mixture is cooled then refrigerated.
Figure 1: Process as currently installed. Reflux pumps, reboiler pumps, and accumulators are not
shown on the distillation columns. Data boxes show temperature in C and pressure in bar.
This refrigerated product mixture is flashed in a vessel. The overhead product passes through a
packed column which strips most of the acetone and IPA out of the gas with water, leaving a 90%
hydrogen stream. This hydrogen stream exits as a product stream. The bottom product of the
stripping column joins the liquid from the flash vessel and enters a second column for distillation.
The overhead product of this second column is condensed and leaves as pharmaceutical grade
liquid acetone. The bottom product of the second column is mostly IPA and water. This mixture
is sent to a third and final column where it is distilled again. The overhead product of the third
column is an azeotropic mixture of IPA and water which is condensed and recycled back to the
beginning of the process. The bottoms product of the third column is waste water potentially
containing trace amounts of organic solvents.
Improved Process
The new design proposes keeping all streams and equipment in the current process with the
addition of three compressors and two heat exchangers. The added equipment purifies the
hydrogen stream to 99.95% while recovering the acetone and water in this stream to be separated
by the second and third columns in the separation train. Figure 2 shows the proposed
configuration with the additions outlined in red. The hydrogen stream passes through three
compressors (C-401, C-402, C-403) in series to achieve a total compression ratio of 47:1.
Compressors C-401 and C-402 each have compression ratios of 4:1, and C-403 has a
compression ratio of 2.9:1 so that the largest compression ratio occurs at the lowest pressures.
This is less expensive than three equally-sized compressors. All three compressors are
Created with novaPDF Printer (www.novaPDF.com). Please register to remove this message.
5
reciprocating compressors with efficiencies of 80% as per heuristics
[6]
. In order to maintain a
stream factor of 0.9, a rotating spare compressor is included in the proposal. This spare is sized
to meet the demands of the largest compressor (C-403), so it can also do the work of either of the
smaller compressors. All the equipment in this expansion is made of carbon steel for consistency
with the existing equipment. Table 2 summarizes the specifications for the new compressors and
vessel V-405.
Figure 2: Proposed process configuration. The added equipment is outlined in a bold red
rectangle. All other process equipment and configuration is the same. Data boxes show pressure
in bar and temperature in C.
Table 2: Equipment specifications for new compressors and vessel.
Equipment C-401 C-402 C-403 A/B V-405
MOC Carbon Steel Carbon Steel Carbon Steel Carbon Steel
Fluid Power (kW) 54.5 84.7 93.3
Efficiency 80% 80% 80%
Type Reciprocating Reciprocating Reciprocating
Temperature (C) 185 406 650
Pressure In (bar) 1.5 6 24
Pressure Out (bar) 6 24 70
Diameter (m) 0.75
Height (m) 2.25
Orientation Vertical
Internals SS Demister
Pressure (bar) 70
In order to achieve the final purity of 99.95% hydrogen, the compressed vapor stream must be
cooled to 15 C. First, a small heat exchanger of 2.0 m
2
and a duty of -967 MJ/h cools the
vapor to 41 C using cooling water. This is the lowest temperature attainable with regular
cooling water while maintaining a 10 C approach for counter-current flow. To achieve the final
Created with novaPDF Printer (www.novaPDF.com). Please register to remove this message.
6
temperature of -15 C, a second heat exchanger with an area of 4.8 m
2
and a duty of -87 MJ/h
uses low temperature refrigerant supplied at -20 C. A 5 C approach with counter-current flow
is maintained as per heuristics for refrigeration
[6]
. Both these heat exchangers have very small
heat transfer areas because this is not a large stream. Table 3 summarizes the specifications for
the new exchangers.
Table 3: Specifications for new heat exchangers.
Equipment E-409 E-410
Duty (MJ/h) -967 -87
Area (m
2
) 2.0 4.8
Shell Side
Max Temp (C) 650 41
Pressure (barg) 70 70
Phase Condensing Vapor Condensing Vapor
MOC Carbon Steel Carbon Steel
Tube Side
Max Temp (C) 45 35
Pressure (bar) 4 4
Phase
Liquid
(cooling water)
Liquid
(refrigerant)
MOC Carbon Steel Carbon Steel
The cooled effluent from the second heat exchanger enters a flash vessel identical to vessel V-
402 in the existing plant. The vapor from this vessel is 70.1 kg/h of 99.95% hydrogen. The
liquid effluent of the vessel contains 2.5 kmol/h of acetone and 1.3 kmol/h of water. The acetone
and water mixture is throttled down adiabatically and mixed into the feed (stream 9) to tower T-
402 of the existing separation train. It is assumed that T-402 will require a proportional increase
in reboiler duty to separate this small incremental flow (less than a 6% increase in the feed rate),
but the column continues to recover all (99.6%) of this additional acetone into the condensed
overhead product. The increase in waste water flow will be negligible (approximately 3%).
Compared to the existing process, only streams in the separation train are changed, and these
changes are small (less than 10%). Table 4 shows stream tables with current operating
conditions and projected operating conditions for those streams which will be affected by the
expansion.
Created with novaPDF Printer (www.novaPDF.com). Please register to remove this message.
7
Table 4: Stream conditions for streams in Figures 1 (current) and 2 (new). Only streams which
are affected by the proposed expansion are shown.
7 9 11 12
Stream Number
Current New Current New Current New Current New
Temperature (C) 33 33 22 19.5 61 61 90 90
Pressure (bar) 1.5 1.5 1.63 1.63 1.5 1.5 1.4 1.4
Vapor Fraction 1.0 1.0 0.0 0.0 0.0 0.0 0.0 0.0
Total Flow
(kmol/h)
38.60 38.60 74.02 77.83 32.29 34.78 41.73 43.04
Hydrogen Flow
(kmol/h)
34.78 34.78 0.00 0.00
Acetone Flow
(kmol/h)
2.51 2.51 32.43 34.92 32.27 34.78 0.16 0.16
IPA Flow
(kmol/h)
0.02 0.02 3.84 3.86 0.02 0.02 3.82 3.84
Water Flow
(kmol/h)
1.29 1.29 37.75 39.04 37.75 39.04
Table 4 (continued)
15 16 17
Stream Number
Current New Current New Current New
Temperature (C) 109 109 NA -15 33 -15
Pressure (bar) 1.4 1.4 NA 70 1.5 70
Vapor Fraction 0.0 0.0 NA 0.0 1.0 1.0
Total Flow (kmol/h) 35.85 37.14 NA 3.81 38.60 34.79
Hydrogen Flow
(kmol/h)
NA 0.00 34.78 34.78
Acetone Flow
(kmol/h)
NA 2.50 2.51 0.01
IPA Flow (kmol/h) NA 0.02 0.02
Water Flow
(kmol/h)
35.85 37.14 NA 1.29 1.29
Created with novaPDF Printer (www.novaPDF.com). Please register to remove this message.
8
Results
This addition has an after-tax rate of return of 117% on the net profit. The total capital
investment of $1,460,100 and annual cost of manufacturing without depreciation (COM
d
) of
$2,805,500 result in an average revenue increase of $8,814,200 per year. This project will have
a payback period of less than 4.5 months after startup. The Appendix has the full spreadsheet
calculations from which these values and cash flows were obtained. The following figures and
tables summarize the economic results of the proposed expansion. Only incremental investment,
costs, and revenue are considered.
Table 5: Economic summary of major cash flows and rates of return.
Capital Investment Required $1,460,100
Incremental Annual Revenue $8,814,200
Incremental Annual Operating
Cost (COM
d
)
$2,805,500
Average Annual Net Profit $3,053,800
Before Tax Rate of Return 159%
After Tax Rate of Return 123%
After Tax Rate of Return on
Net Profit
117%
-$2
-$1
$0
$1
$2
$3
$4
$5
$6
$7
0 1 2 3 4 5 6 7 8 9 10 11
Year
C
a
s
h
F
l
o
w
(
M
i
l
l
i
o
n
s
)
Before Tax
After Tax
Figure 3: Discrete before and after tax cash flows for the life of the proposed project.
Created with novaPDF Printer (www.novaPDF.com). Please register to remove this message.
9
-$10
$0
$10
$20
$30
$40
$50
$60
$70
0 1 2 3 4 5 6 7 8 9 10 11
Year
C
a
s
h
F
l
o
w
(
M
i
l
l
i
o
n
s
)
Before Tax
After Tax
Figure 4: Cumulative before and after tax cash flows for the life of the proposed project.
Created with novaPDF Printer (www.novaPDF.com). Please register to remove this message.
10
Discussion
Profitability can be improved in two ways: reducing cost and increasing revenue. In this project,
the team chose to increase revenue. This decision was made based on an evaluation of the
potential profitability of each approach. In this case, the primary motivation was the lack of
incentive to reduce operating costs. Of the four major operating costs (raw materials, utilities,
waste treatment, and operating labor), raw material costs make up 92% of cost in the existing
design
[3]
. Because of effective separation and recycle, the overall mass balance of the system
has 99.9% conversion of IPA to acetone. This leaves no way to reduce raw material costs, so
only waste treatment, utilities, and operating labor can be optimized. Of these, waste treatment
is insignificant (less than $500/yr), and operating labor is essentially invariant without a major
redesign of the entire plant. Reducing utility usage leaves less than $1.4 million on the table for
improvement. Because even the best designed plant will obviously require some utilities, only a
fraction of this $1.4 million can be saved.
By contrast, 2.51 kmol/h of acetone is lost in the hydrogen stream. This acetone is potentially
worth $1.7 million per year. Additionally, this acetone contaminates the hydrogen stream so the
hydrogen can only be used as fuel gas. Hydrogen for heating purposes is worth 6% of industrial
grade hydrogen feedstocks. Preliminary calculations showed that if the hydrogen could be made
99.95% pure and sold as industrial hydrogen, the gas stream would be worth an additional $7.1
million annually. The combined potential for revenue from recovered acetone and purified
hydrogen is thus $8.8 million annually. It was believed that this entire potential revenue could
be realized, and the subsequent design work accomplishes this. By comparison, any fractional
reduction in utility costs is inconsequential. For this reason, no utility reduction projects were
explored.
In optimizing using the objective function, it became clear that for this project, compression
requires much larger capital and operating costs than refrigeration. The optimal design applies
the maximum cooling possible with just enough compression to achieve the required separation.
The cooling is limited by the temperature of the refrigerant, and this in turn sets the conditions
for the optimum design.
Future Projects
Although no utility reduction projects were explored, several opportunities for optimization were
identified. The design team suggests the following for future optimization projects:
- Use the reactor effluent to heat the reactor affluent. Exchangers E-401 and E-402
provide similar heat duties to heat then cool the process stream at a total utility cost of
$339,000/yr. It is unclear if one of these exchangers could be used or if a new exchanger
would be required. This straight-forward change was not pursued because of the small
saving potential.
- Use the furnace to provide heat to exchanger E-401 or possibly E-403. Direct energy
from natural gas is cheaper than steam, and these exchangers have an annual utility cost
of $585,000. To avoid using molten salt, it would probably be most economical if the
process fluid could pass directly through tubes inside the furnace. This was not pursued
because no information was available about the cost of such a change.
Created with novaPDF Printer (www.novaPDF.com). Please register to remove this message.
11
- Use a cheaper catalyst and/or molten salt. Little information was available about these
costs, but both must be replaced annually. If a cheaper material with equivalent
functionality could be found, this would be the simplest change possible.
- Use the gas stream leaving compressor C-403 to heat the reactor affluent. This could
save duty from both exchanger E-401 and E-409. This should probably be considered
before E-409 is installed, but the gas stream is small and could only supply a fraction of
the duty E-401 requires.
- Recover the waste water and recycle it as process water. The waste water contains only
trace process contaminants, and may be clean enough for recycle. This is a simple and
obvious change, but the total cost of fresh process water and waste treatment is less than
$1,000 annually
[3]
. The piping and pumping costs probably outweigh the benefits.
Created with novaPDF Printer (www.novaPDF.com). Please register to remove this message.
12
Conclusions
This report proposes an expansion to the existing acetone production facility. This expansion
purifies hydrogen and recovers more acetone. This separation is accomplished by compressing
and refrigerating the gaseous product stream. The most economic design involves minimizing
compression and maximizing refrigeration. Although there are many other opportunities for
optimization, the proposal in this report represents the largest, most profitable improvement
available.
This expansion is strongly recommended because the after tax net profit of $3.05 million per
year produces a 117% rate of return on a capital investment of $1.46 million. This project
should be undertaken immediately.
Created with novaPDF Printer (www.novaPDF.com). Please register to remove this message.
13
References
1. Energy Information Administration, US Department of Energy, February 19 (2007):
http://eia.doe.gov/ .
2. Forrest, W, Prices, supply getting more stable, Purchasing.com, April 6 (2006):
http://www.purchasing.com/article/CA6320292.html .
3. Frantz, E et al., Production of Acetone via the Dehydrogenation of Isopropyl Alcohol,
February 2007.
4. Hydrogen Analysis Resource Center, US Department of Energy, February 19 (2006):
http://hydrogen.pnl.gov/cocoon/morf/hydrogen .
5. Pyle, Walt. Hydrogen Purification. Home Power #67, October/November (1998).
6. Turton, R., et al., Analysis, Synthesis, and Design of Chemical Processes, 2
nd
ed. (Upper
Saddle River, New Jersey 2003).
Created with novaPDF Printer (www.novaPDF.com). Please register to remove this message.
14
Appendix
Table A.1: Capital investment
Heat Exchanger
Capacity
Bare Module
Price
Source
E-409 2 $11,800 CHEMCAD
E-410 4.8 $97,700 CHEMCAD
Compressor
C-401 56.5 $213,800 CHEMCAD
C-402 84.7 $275,600 CHEMCAD
C-403 93.3 $293,000 CHEMCAD
Vessel
V-405 0.75 x 2.25 $52,500 CAPCOST
Bare Module
Cost $1,237,400
Total Module
Cost $1,460,100
Table A.2: COM
d
, annual utility costs
Utilities
Heat Exchanger Utility
Utility
Duty
Utility
Unit
Utility
Price
Utility
Cost ($/yr)
E-409
Cooling
water
967 MJ/h $0.0004136 $3,150
E-410 Refrigerant 87 MJ/h $0.00922 $6,320
Compressor
C-401 Electricity 56.5
kW (actual
power)
$0.059 $26,280
C-402 Electricity 84.7
kW (actual
power)
$0.059 $39,400
C-403 Electricity 93.3
kW (actual
power)
$0.059 $43,400
Tower
T-402
Low
pressure
steam
210 MJ/h $0.0155 $25,610
Total $144,160
Created with novaPDF Printer (www.novaPDF.com). Please register to remove this message.
15
Table A.3: COM
d
, incremental annual operating labor
Operating Labor
Current
Design
Improved
Design
Pieces of Particulate
Handling Equipment
0 0
Pieces of Fluid
Handling Equipment
13 15
Number of
Operators per Shift
3.05 3.12
Number of
Operators Total
14 14
Incremental Labor Cost $0
Table A.4: COM
d
, annual incremental waste treatment costs
Waste Treatment
Extra Waste Water
Flow (m
3
/h)
0.0234
Tertiary Treatment
Cost ($/m
3
)
0.0942
Incremental Cost $17
Table A.5: COM
d
, incremental raw material costs
Raw Materials
Additional Raw
Materials
None
Incremental Cost $0
Table A.6: COM
d
, summary of total cost of manufacturing without depreciation
COM
d
Raw Materials $0
Utilities $144,160
Waste Treatment $17
Operating Labor $0
FCI $1,460,100
COM
d
$2,805,518
Created with novaPDF Printer (www.novaPDF.com). Please register to remove this message.
16
Table. A.7: Incremental revenue from more acetone recovery and purified hydrogen
Chemical
Flow
Rate
Current
Unit
Value
Improved
Unit
Value
Incremental
Revenue
Acetone
(kg)
145 $0 $1.46 $1,669,043
Hydrogen
(scf)
27530 $0.208 $3.50 $7,145,171
Total $8,814,214
Table A.8: Cash flows
Year Cost Revenue Depreciation Tax Before Tax
Discrete Cumulative
0 $1,460,100 $0 $0 $0 -$1,460,100 -$1,460,100
1 $0 $0 $0 $0 $0 -$1,460,100
2 $2,805,518 $8,814,000 $292,020 $2,000,762 $6,008,482 $4,548,382
3 $2,805,518 $8,814,000 $467,232 $1,939,437 $6,008,482 $10,556,864
4 $2,805,518 $8,814,000 $280,339 $2,004,850 $6,008,482 $16,565,345
5 $2,805,518 $8,814,000 $168,204 $2,044,097 $6,008,482 $22,573,827
6 $2,805,518 $8,814,000 $168,204 $2,044,097 $6,008,482 $28,582,309
7 $2,805,518 $8,814,000 $84,102 $2,073,533 $6,008,482 $34,590,791
8 $2,805,518 $8,814,000 $0 $2,102,969 $6,008,482 $40,599,273
9 $2,805,518 $8,814,000 $0 $2,102,969 $6,008,482 $46,607,755
10 $2,805,518 $8,814,000 $0 $2,102,969 $6,008,482 $52,616,236
11 $2,805,518 $8,814,000 $0 $2,102,969 $6,008,482 $58,624,718
Table A.8 (continued)
Year After Tax
Net Profit Discrete Cumulative
0 -$1,460,100 -$1,460,100 -$1,460,100
1 $0 $0 -$1,460,100
2 $3,715,700 $4,007,720 $2,547,620
3 $3,601,812 $4,069,044 $6,616,665
4 $3,723,293 $4,003,632 $10,620,296
5 $3,796,181 $3,964,384 $14,584,681
6 $3,796,181 $3,964,384 $18,549,065
7 $3,850,847 $3,934,949 $22,484,014
8 $3,905,513 $3,905,513 $26,389,527
9 $3,905,513 $3,905,513 $30,295,040
10 $3,905,513 $3,905,513 $34,200,554
11 $3,905,513 $3,905,513 $38,106,067
Created with novaPDF Printer (www.novaPDF.com). Please register to remove this message.
17
Table A.9: Calculation used to find unit value of current hydrogen stream as fuel gas
Flow of
Hydrogen
(scfh)
Heating Value
of Hydrogen
(MJ/h)
Equivalent
Amount of Fuel
Gas (scfh)
Price of Fuel
Gas ($/100scf)
Value as
Fuel Gas
($/h)
Value as
Fuel Gas
($/100scf)
27530 8516 8212 0.696 57.15552 0.20761177
Table A.10: Table used to evaluate the object function. Outlet pressures from the final
compressor (green) and outlet temperature from the second exchanger (orange) were specified.
All other values are calculated by CHEMCAD or in the spreadsheet based on heuristics. The
present value of costs (the result of the objective function) is highlighted in blue.
Table A.10a: Compressor outlet pressure of 75 bar with 2 heat exchangers
E-1 E-2 C-1 C-2 C-3
Temperature
(
o
C)
665 -14 Pressure Out (bar) 6 24 75
Duty (MJ/h) -991 -85
Theoretical Power
(kW)
45.2 67.73 80.1
TLM (
o
C) 151 6.00 Efficiency 0.8 0.8 0.8
Area (m
2
) 2.1 4.63 Power Used (kW) 56.5 84.6625 100.125
Utility Price
($/GJ)
0.414 5.18
Utility Price
($/kWh)
0.059 0.059 0.059
Utility Cost
(yr
-1
)
$3,231 $3,468 Utility Cost (yr
-1
) $26,281 $39,381 $46,574
Present Value
of Cost
$16,217 $17,407
Present Value of
Utility Cost
$131,900 $197,645 $233,743
Capital $11,929 $96,495 Capital $213,778 $275,581 $306,346
Total Cost $1,501,042
Table A.10b: Compressor outlet pressure of 100 bar with two heat exchangers
E-1 E-2 C-1 C-2 C-3
Temperature
(
o
C)
729 -9 Pressure Out (bar) 6 24 100
Duty (MJ/h) -1099 -77
Theoretical Power
(kW)
45.2 67.73 103.82
TLM (
o
C) 163 8.25 Efficiency 0.8 0.8 0.8
Area (m
2
) 2.2 3.05 Power Used (kW) 56.5 84.6625 129.775
Utility Price
($/GJ)
0.414 5.18
Utility Price
($/kWh)
0.059 0.059 0.059
Utility Cost
(yr
-1
)
$3,584 $3,142 Utility Cost (yr
-1
) $26,281 $39,381 $60,366
Present Value
of Cost
$17,985 $15,769
Present Value of
Utility Cost
$131,900 $197,645 $302,961
Capital $12,087 $66,715 Capital $213,778 $275,881 $360,966
Total Cost $1,595,687
Created with novaPDF Printer (www.novaPDF.com). Please register to remove this message.
18
Table A.10c: Compressor outlet pressure of 85 bar and two heat exchangers
E-1 E-2 C-1 C-2 C-3
Temperature
(
o
C)
692 -12 Pressure Out (bar) 6 24 85
Duty (MJ/h) -1038 -82
Theoretical Power
(kW)
45.2 67.73 90.23
TLM (
o
C) 156 6.95 Efficiency 0.8 0.8 0.8
Area (m
2
) 2.2 3.85 Power Used (kW) 56.5 84.6625 112.7875
Utility Price
($/GJ)
0.414 5.18
Utility Price
($/kWh)
0.059 0.059 0.059
Utility Cost
(yr
-1
)
$3,385 $3,346 Utility Cost (yr
-1
) $26,281 $39,381 $52,464
Present Value
of Cost
$16,987 $16,793
Present Value of
Utility Cost
$131,900 $197,645 $263,304
Capital $12,087 $94,044 Capital $213,778 $275,881 $330,279
Total Cost $1,552,697
Table A.10d: Compressor outlet pressure of 70 bar with two heat exchangers (optimal)
E-1 E-2 C-1 C-2 C-3
Temperature
(
o
C)
650 -15 Pressure Out (bar) 6 24 70
Duty (MJ/h) -967 -87
Theoretical Power
(kW)
45.2 67.73 74.65
TLM (
o
C) 148 5.48 Efficiency 0.8 0.8 0.8
Area (m
2
) 2.1 5.18 Power Used (kW) 56.5 84.6625 93.3125
Utility Price
($/GJ)
0.414 5.18
Utility Price
($/kWh)
0.059 0.059 0.059
Utility Cost
(yr
-1
)
$3,153 $3,550 Utility Cost (yr
-1
) $26,281 $39,381 $43,405
Present Value
of Cost
$15,825 $17,817
Present Value of
Utility Cost
$131,900 $197,645 $217,839
Capital $12,087 $97,724 Capital $213,778 $275,881 $293,002
Total Cost $1,473,498
Created with novaPDF Printer (www.novaPDF.com). Please register to remove this message.
19
Table A.10e: Compressor outlet pressure of 70 bar with one heat exchanger
E-1 E-2 C-1 C-2 C-3
Temperature
(
o
C)
651 NA Pressure Out (bar) 6 24 70
Duty (MJ/h) -1052 NA
Theoretical Power
(kW)
45.2 67.7 74.7
TLM (
o
C) 127 NA Efficiency 0.8 0.8 0.8
Area (m
2
) 2.7 NA Power Used (kW) 56.5 84.625 93.375
Utility Price
($/GJ)
5.180 NA
Utility Price
($/kWh)
0.059 0.059 0.059
Utility Cost
(yr
-1
)
$42,963 NA Utility Cost (yr
-1
) $26,281 $39,364 $43,434
Present Value
of Cost
$215,620 NA
Present Value of
Utility Cost
$131,900 $197,558 $217,985
Capital $494,573 NA Capital $213,778 $275,881 $293,002
Total Cost $2,040,297
Created with novaPDF Printer (www.novaPDF.com). Please register to remove this message.
You might also like
- Liquid Membranes: Principles and Applications in Chemical Separations and Wastewater TreatmentFrom EverandLiquid Membranes: Principles and Applications in Chemical Separations and Wastewater TreatmentNo ratings yet
- Project 4 Acrylic AcidDocument16 pagesProject 4 Acrylic AcidN193746100% (3)
- B2 Group 1..acetone Production PDFDocument21 pagesB2 Group 1..acetone Production PDFElif TaşdövenNo ratings yet
- Acetone Production Plant DesignDocument25 pagesAcetone Production Plant DesignSerdar Arıcan100% (1)
- Methane Oxidation To Acetic AcidDocument31 pagesMethane Oxidation To Acetic AcidАндрей КолесниковNo ratings yet
- Plant Design Project FinalDocument221 pagesPlant Design Project FinalYasser AshourNo ratings yet
- Project 6 - Ethylene Oxide PDFDocument13 pagesProject 6 - Ethylene Oxide PDFStephanie Hawkins100% (1)
- Production of Ethylene OxideDocument22 pagesProduction of Ethylene OxideShahabuddin Khan Niazi100% (1)
- CPE639 Mini Project - Production of Acetonitrile Using Fluidized Bed Reactor PDFDocument41 pagesCPE639 Mini Project - Production of Acetonitrile Using Fluidized Bed Reactor PDFnoorNo ratings yet
- Production of Methanol From MethaneDocument3 pagesProduction of Methanol From MethanemuhamadtarmiziNo ratings yet
- Material and Energy BalanceDocument9 pagesMaterial and Energy BalanceSana100% (1)
- Design of Acetone HYSYSDocument6 pagesDesign of Acetone HYSYSlockas222100% (1)
- Production of AcetaldehydeDocument80 pagesProduction of Acetaldehydeyinka omojesuNo ratings yet
- AcetoneDocument7 pagesAcetoneGeorgiana AndreeaNo ratings yet
- Engineers Guide - Cumene Peroxidation Process For Phenol ProductionDocument2 pagesEngineers Guide - Cumene Peroxidation Process For Phenol ProductionEdrian A. Mañalong100% (1)
- Assignment Aspen PlusDocument8 pagesAssignment Aspen PlusVinayak PathakNo ratings yet
- Allyl Chloride Production PDFDocument4 pagesAllyl Chloride Production PDFmarisolNo ratings yet
- Report 0Document19 pagesReport 0Joseph OrjiNo ratings yet
- Ethylene 2520oxide Energy 2520 BalanceDocument9 pagesEthylene 2520oxide Energy 2520 BalanceDick El RinoceronteNo ratings yet
- Ethylene Glycol Production PDFDocument2 pagesEthylene Glycol Production PDFMohamedNo ratings yet
- 5 6251216941030047774Document41 pages5 6251216941030047774Salihah AbdullahNo ratings yet
- Ethylene Glycol ProductionDocument3 pagesEthylene Glycol ProductionQuang NguyễnNo ratings yet
- Simulation Using PFR (Aspen Plus)Document10 pagesSimulation Using PFR (Aspen Plus)Cik Tiem NgagimanNo ratings yet
- MATERIAL BALANCE Distillation ColumnDocument9 pagesMATERIAL BALANCE Distillation ColumnRajeev Kumar DohareNo ratings yet
- Cyclohexane ReportDocument5 pagesCyclohexane ReportLuffy RajNo ratings yet
- Finalp 1Document47 pagesFinalp 1Imtiaz Hussain100% (1)
- Assignment 2 FinalDocument12 pagesAssignment 2 FinalRobin Kwan100% (1)
- Industrial Catalytic Processes-Phenol Production: Robert J. SchmidtDocument15 pagesIndustrial Catalytic Processes-Phenol Production: Robert J. SchmidtUzair WahidNo ratings yet
- Manfacture OF: Cyclo HexaneDocument91 pagesManfacture OF: Cyclo HexaneNikhil Kumar Chennuri100% (4)
- Design 002H AmmoniaSynthesis ClosedLoopDocument15 pagesDesign 002H AmmoniaSynthesis ClosedLoopNicandroGonzalesNo ratings yet
- GAS ABSORPTION - ReportDocument6 pagesGAS ABSORPTION - Reportgzairene8762No ratings yet
- Mini Project Full PDFDocument37 pagesMini Project Full PDFMohamad El KheirNo ratings yet
- TOURTON-páginas-37-124-73-88 PDFDocument16 pagesTOURTON-páginas-37-124-73-88 PDFJesús David González CañasNo ratings yet
- Separation of Ammonia and WaterDocument5 pagesSeparation of Ammonia and WaterJamie MaloneNo ratings yet
- Process Simulation of Ethanol Production From Biomass Gasification and Syngas Fermentation PDFDocument33 pagesProcess Simulation of Ethanol Production From Biomass Gasification and Syngas Fermentation PDFramesh pokhrelNo ratings yet
- 1.3 Process Flow Diagram 1.3.1 Description of Process Flow DiagramDocument12 pages1.3 Process Flow Diagram 1.3.1 Description of Process Flow DiagramSyahirun NissaNo ratings yet
- 2 Ethyl 2520hexanol Methods 2520of 2520 ProductionDocument10 pages2 Ethyl 2520hexanol Methods 2520of 2520 Productionapi-3714811No ratings yet
- Production of IsopropanolDocument9 pagesProduction of IsopropanolJohanNo ratings yet
- FORMALDEHYDE Cost EstimationDocument5 pagesFORMALDEHYDE Cost EstimationPradeep Munna100% (2)
- Acrolein Design ProjectDocument13 pagesAcrolein Design ProjectPeter McCormack100% (1)
- Wasteless Economic Method of Production of Phenol and AcetoneDocument14 pagesWasteless Economic Method of Production of Phenol and AcetoneSiswand BIn Mohd AliNo ratings yet
- Excess Methanol RecoveryDocument6 pagesExcess Methanol RecoverySteven Putra HalimNo ratings yet
- Production of Maleic Anhydride PresentationDocument15 pagesProduction of Maleic Anhydride PresentationNqobile LowakwaMkhize100% (1)
- PFD Acetone From Isopropyl AlcoholDocument1 pagePFD Acetone From Isopropyl AlcoholNabila Rizki AmaliaNo ratings yet
- Batch Distillation of Water-Methanol SystemDocument78 pagesBatch Distillation of Water-Methanol Systemsatadruc50% (4)
- For Hysys UsersDocument5 pagesFor Hysys UsersZohaib RanaNo ratings yet
- Benzene To CyclohexaneDocument16 pagesBenzene To Cyclohexanekalwani20No ratings yet
- Research Framework Siti Nur Farhanis Dialysate EffluentDocument2 pagesResearch Framework Siti Nur Farhanis Dialysate EffluentChannis ExoNo ratings yet
- Flash Distillation ProblemDocument2 pagesFlash Distillation ProblemprudhvifireNo ratings yet
- The Dehydrogenation of Isopropanol To Form Hydrogen GasDocument31 pagesThe Dehydrogenation of Isopropanol To Form Hydrogen GasEdidiong AsuquoNo ratings yet
- Plant Design EthylbenzeneDocument43 pagesPlant Design EthylbenzeneDex JH100% (2)
- UntitledDocument12 pagesUntitledapi-256504985No ratings yet
- Distillation Model Rev1Document9 pagesDistillation Model Rev1mehul1094167% (3)
- Ethylene and Acetylene Plant PDFDocument405 pagesEthylene and Acetylene Plant PDFاحمد الدلالNo ratings yet
- Separations and Reaction Engineering Design Project Production of AmmoniaDocument10 pagesSeparations and Reaction Engineering Design Project Production of AmmoniaRyan WahyudiNo ratings yet
- Allyl3 LECHO FLUIDocument9 pagesAllyl3 LECHO FLUIJoha BetancurNo ratings yet
- Section 5: Process IntensificationDocument23 pagesSection 5: Process IntensificationPrince EugoNo ratings yet
- Final Project ReportDocument15 pagesFinal Project Reportwhãts brøNo ratings yet
- Recompression and Determining The New Steam EconomyDocument2 pagesRecompression and Determining The New Steam EconomyArpit GuptaNo ratings yet
- Che 456 Spring 2003 Major 2 Drying Oil Production: ConstraintsDocument7 pagesChe 456 Spring 2003 Major 2 Drying Oil Production: ConstraintstonbaldinNo ratings yet
- Sterilization PDFDocument21 pagesSterilization PDFshahed IasirNo ratings yet
- Cleaning Disinfecting and Sterilizing Plastics PDFDocument2 pagesCleaning Disinfecting and Sterilizing Plastics PDFshahed IasirNo ratings yet
- SWW2010 Presentation21026 VPopov PDFDocument57 pagesSWW2010 Presentation21026 VPopov PDFshahed IasirNo ratings yet
- Oilref PDFDocument37 pagesOilref PDFshahed IasirNo ratings yet
- FS CFPPDocument19 pagesFS CFPPrgbyunNo ratings yet
- Process Simulation Modeling Design For Soybean Oil Extraction Using Liquid Propane PDFDocument154 pagesProcess Simulation Modeling Design For Soybean Oil Extraction Using Liquid Propane PDFshahed IasirNo ratings yet
- CD4063Document25 pagesCD4063Mir HassanNo ratings yet
- USB Interfacing and Real Time Data Plotting With MATLABDocument9 pagesUSB Interfacing and Real Time Data Plotting With MATLABthietdaucongNo ratings yet
- ChE Thoughts - Vol-03, No-1 PDFDocument52 pagesChE Thoughts - Vol-03, No-1 PDFshahed IasirNo ratings yet
- Enviroment INDEXDocument1 pageEnviroment INDEXshahed IasirNo ratings yet
- IELTS US Recognition Form 2012Document1 pageIELTS US Recognition Form 2012shahed IasirNo ratings yet
- Fertilizer IntroDocument50 pagesFertilizer Introshahed IasirNo ratings yet
- ElasticityDocument7 pagesElasticityshahed IasirNo ratings yet
- Pressure Relief Devices Scott OstrowskiDocument82 pagesPressure Relief Devices Scott OstrowskiAffify AfifyNo ratings yet
- Sonaia ThobaniDocument10 pagesSonaia Thobanishahed IasirNo ratings yet
- Advanced Simulation Case Using HysysDocument232 pagesAdvanced Simulation Case Using HysysridhajamelNo ratings yet
- Roll: - 200306040 Group: - A2Document6 pagesRoll: - 200306040 Group: - A2shahed IasirNo ratings yet
- B. Inggris X - 7Document8 pagesB. Inggris X - 7KabardiantoNo ratings yet
- Rights of Parents in IslamDocument11 pagesRights of Parents in Islamstoneage989100% (2)
- Operating Instructions: HTL-PHP Air Torque PumpDocument38 pagesOperating Instructions: HTL-PHP Air Torque PumpvankarpNo ratings yet
- C. Robert Mesle (Auth.) - John Hick's Theodicy - A Process Humanist Critique-Palgrave Macmillan UK (1991)Document168 pagesC. Robert Mesle (Auth.) - John Hick's Theodicy - A Process Humanist Critique-Palgrave Macmillan UK (1991)Nelson100% (3)
- I Pmtea 2020 HandoutDocument94 pagesI Pmtea 2020 HandoutAbhijeet Dutta100% (1)
- Case Study - Montana Mountain BikingDocument6 pagesCase Study - Montana Mountain Bikingbonny MishNo ratings yet
- The Reason: B. FlowsDocument4 pagesThe Reason: B. FlowsAryanti UrsullahNo ratings yet
- Healthymagination at Ge Healthcare SystemsDocument5 pagesHealthymagination at Ge Healthcare SystemsPrashant Pratap Singh100% (1)
- SurveyingDocument26 pagesSurveyingDenise Ann Cuenca25% (4)
- RN42Document26 pagesRN42tenminute1000No ratings yet
- Arbans Complete Conservatory Method For Trumpet Arbans Complete ConservatoryDocument33 pagesArbans Complete Conservatory Method For Trumpet Arbans Complete ConservatoryRicardo SoldadoNo ratings yet
- Music 9 Q3 Mod4 Musical Elements of Given Romantic Period PiecesDocument19 pagesMusic 9 Q3 Mod4 Musical Elements of Given Romantic Period PiecesFinn Daniel Omayao100% (1)
- Neet Question Paper 2019 Code r3Document27 pagesNeet Question Paper 2019 Code r3Deev SoniNo ratings yet
- Income Tax and VATDocument498 pagesIncome Tax and VATshankar k.c.100% (2)
- Clint Freeman ResumeDocument2 pagesClint Freeman ResumeClint Tiberius FreemanNo ratings yet
- Transparency and Digitalization in The Public Administration of RomaniaDocument8 pagesTransparency and Digitalization in The Public Administration of RomaniaMădălina MarincaşNo ratings yet
- ST3 ManualDocument48 pagesST3 ManualRon FosterNo ratings yet
- John Wren-Lewis - NDEDocument7 pagesJohn Wren-Lewis - NDEpointandspaceNo ratings yet
- Fortigate Firewall Version 4 OSDocument122 pagesFortigate Firewall Version 4 OSSam Mani Jacob DNo ratings yet
- 300u Specs Diodo 300 Amps. 25 Dolares RadiosurtidoraDocument6 pages300u Specs Diodo 300 Amps. 25 Dolares RadiosurtidorarepelindNo ratings yet
- Honda IzyDocument16 pagesHonda IzyTerry FordNo ratings yet
- DNA Vs RNA - Introduction and Differences Between DNA and RNADocument10 pagesDNA Vs RNA - Introduction and Differences Between DNA and RNAKienlevyNo ratings yet
- Lesson 1 Q3 Figure Life DrawingDocument10 pagesLesson 1 Q3 Figure Life DrawingCAHAPNo ratings yet
- Days Papers 2001Document341 pagesDays Papers 2001jorgefeitoza_hotmailNo ratings yet
- Vocabulary FceDocument17 pagesVocabulary Fceivaan94No ratings yet
- Second Conditional Conversation QuestionsDocument2 pagesSecond Conditional Conversation QuestionsEdith Salomé PinosNo ratings yet
- ET4254 Communications and Networking 1 - Tutorial Sheet 3 Short QuestionsDocument5 pagesET4254 Communications and Networking 1 - Tutorial Sheet 3 Short QuestionsMichael LeungNo ratings yet
- Artificial Intelligence Practical 1Document5 pagesArtificial Intelligence Practical 1sadani1989No ratings yet
- Sheltered 2 Item Recycle ListDocument5 pagesSheltered 2 Item Recycle ListRachel GNo ratings yet
- Motivation Theories Description and CriticismDocument14 pagesMotivation Theories Description and CriticismAhmed Elgazzar89% (18)