Professional Documents
Culture Documents
01 Phet Reversible Reactions
Uploaded by
api-235688447Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
01 Phet Reversible Reactions
Uploaded by
api-235688447Copyright:
Available Formats
PHET REVERSIBLE REACTIONS BY DANIELLE POSEN
DIRECTIONS:
Download the Reversible Reactions sim from either the PhET website (linked on Edline) or from the classroom computer. Use the prompts below to prepare your write-up for your e-portfolio. You are expected to prepare a prose-style write-up that shows what you know. Include evidence such as data tables, screen shots, or even links to other websites to support your writing.
DISCUSS WHAT CHEMICAL EQUILIBRIUM IS, AND WHY IT IS CONSIDER ED A DYNAMIC P ROCESS.
In a chemical equilibrium there is a reverse and forward reaction. The products of the forward reaction can again react and form the reactants. Equilibrium is reached once the rate of the forward and reverse reactions are equal. The rate of the reaction however is in most cases not 0 but rather the rate of the forward and reverse are equal. These equilibriums are known as dynamic equilibriums in which the reactions never stop but both the reverse and forward reactions continue at equal rates.
COMPARE AND CONTRAST THE RELATIVE ABUNDANCIES OF REACTANTS (LEFT SIDE) AND PRODUCTS (RIGHT SIDE) AT DIFFERENT POINTS OF TIME.
1. Instructions: Investigate, using available tools in the simulation, the effect of a change in temperature, # of balls or activation energy on the Krg. Your investigation must include the following factors
EXPERIMENTAL QUESTIO N:
How does the adding of A particles affect the ratio of particles A and B after 1 minuet?
INDEPENDENT VARIABLE:
Increasing concentration of particle A
DEPENDENT VARIABLE
The ratio of particles
CONTROLLED VARIABLES
Temperature (particles will enter the system with a temperature of 500K) Activation energy (activation energy will remain at 20cm) Initial number of A and B particles (there will be an initially 5 A and B particles)
TABLE
Number of additional A particles 10 15 30
Trial 1 (A:B) 12:9 14:11 28:12
Trial 2 (A:B) 13:7 15:10 27:13
Trial 3 (A:B) 14: 6 18: 7 30:10
Average (A:B) 13:7 16:9 28:12
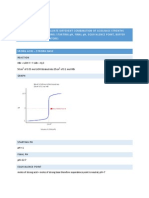
GRAPH
AVERAGE RATIO OF A AND B PARTICLES AFTER 1 MINUET
30 25 20 A particles 15 10 5 0 0 2 4 6 B Particles 8 10 12 14
CONCLUSIONS
The data illustrates that once additional A particles were added, which increased the concentration the reaction shifted to the opposite side of the additional particles. In this case additional A particles were added and as a result the equilibrium shifted to the B particles as the rate of the production of B particles increased. The reason for this increase is the idea of the collision theory. The addition of the A particles increased the frequency of collisions therefore more particles reacted and turned into B particles. The graph above shows a red line the average number of B particles exceeds the initial B particles added into the simulation therefore reinforcing Le chandeliers Principle that states an increase in concentration in one reaction will shift equilibrium toward the opposite reaction.
You might also like
- Oxidation-Reduction Potential of The Ferro-Ferricyanide System in Buffer SolutionsDocument7 pagesOxidation-Reduction Potential of The Ferro-Ferricyanide System in Buffer SolutionsscribedbioaNo ratings yet
- Asma Thesis 10-10-2019 Final FormDocument94 pagesAsma Thesis 10-10-2019 Final FormMuhammad Umer FaroqueNo ratings yet
- Comprehensive Notes On States of MatterDocument8 pagesComprehensive Notes On States of Matterma100% (1)
- Wine Glass Acoustics PDFDocument27 pagesWine Glass Acoustics PDFCarmen DraghiaNo ratings yet
- Two Coupled PendulumsDocument10 pagesTwo Coupled PendulumsBilal HaiderNo ratings yet
- Use of Alternative Energy Sources For The Initiation and Execution of Chemical Reactions and ProcessesDocument21 pagesUse of Alternative Energy Sources For The Initiation and Execution of Chemical Reactions and ProcessesNstm3No ratings yet
- Effect of Strain Rate Upon Plastic Flow of SteelDocument12 pagesEffect of Strain Rate Upon Plastic Flow of SteelLily MaNo ratings yet
- Williamson 2007 (I2)Document5 pagesWilliamson 2007 (I2)hongluc1991No ratings yet
- Investigating The Parameters of Circular OrbitsDocument4 pagesInvestigating The Parameters of Circular OrbitsAutumn HensleyNo ratings yet
- Classical Theory of Raman EffectDocument7 pagesClassical Theory of Raman EffectSudhaNo ratings yet
- FugacityDocument20 pagesFugacityFernando J. Correa DelgadoNo ratings yet
- 3.the Methods and Tools Used To Measure The Dynamic ForcesDocument3 pages3.the Methods and Tools Used To Measure The Dynamic ForcesМурад ГусейнзадеNo ratings yet
- GWR Observatory Superconducting Gravimeter - 2007!10!02Document36 pagesGWR Observatory Superconducting Gravimeter - 2007!10!02hppillaiNo ratings yet
- Ejercicios Pag 279.-280Document3 pagesEjercicios Pag 279.-280Kané Pamplona PamplonaNo ratings yet
- Air Pollution SpaceDocument78 pagesAir Pollution SpaceMohd IqbalNo ratings yet
- PHET Magnetism - Lab Grade: NameDocument4 pagesPHET Magnetism - Lab Grade: NameAyhan AbdulAzizNo ratings yet
- Calibration of A Vacuum Gauge by Comparison With A U-Tube ManometerDocument8 pagesCalibration of A Vacuum Gauge by Comparison With A U-Tube ManometerNazario Emil LintagNo ratings yet
- Cloud Formation PDFDocument7 pagesCloud Formation PDFAdonisNo ratings yet
- NMR Kinetics: Study of A Reversible Hydrolysis ReactionDocument8 pagesNMR Kinetics: Study of A Reversible Hydrolysis ReactionOldbooklover100% (2)
- Hall EffectDocument10 pagesHall EffectNidaul Muiz Aufa100% (1)
- SRO - Brydningsindeks I SukkerDocument19 pagesSRO - Brydningsindeks I SukkerFatima Al-Mosawi100% (1)
- Magnetic Field in SlinkyDocument4 pagesMagnetic Field in SlinkyJayvee GayosoNo ratings yet
- 5371 - Solutions To ProblemsDocument95 pages5371 - Solutions To ProblemsbatpatmanNo ratings yet
- ChronoamperometryDocument7 pagesChronoamperometrybettypaz89100% (1)
- Thermochemistry - Heat of Neutralization and Hess - S Law (Chemistry PDFDocument13 pagesThermochemistry - Heat of Neutralization and Hess - S Law (Chemistry PDFLiyana AthirahNo ratings yet
- Vapor-Liquid Equilibria For The Ternary System Methyl Acetate-Benzene-CyclohexaneDocument6 pagesVapor-Liquid Equilibria For The Ternary System Methyl Acetate-Benzene-CyclohexaneDaniel DominguezNo ratings yet
- Newton's Second Law of MotionDocument6 pagesNewton's Second Law of MotionCaitlin StrongNo ratings yet
- (Mech 2128) Lab RadiationDocument11 pages(Mech 2128) Lab RadiationUchio Akemi100% (1)
- IGCSE Equation List PhysicsDocument5 pagesIGCSE Equation List PhysicsJoseLinNo ratings yet
- Feasibility of Tapping Atmospheric Charge As A Power Source PDFDocument7 pagesFeasibility of Tapping Atmospheric Charge As A Power Source PDFRooakhNo ratings yet
- PCS-224 Lab ManualDocument7 pagesPCS-224 Lab ManualberryberryNo ratings yet
- Introductory Rotational Apparatus: Instruction Manual and Experiment Guide For The PASCO Scientific Model ME-9341Document40 pagesIntroductory Rotational Apparatus: Instruction Manual and Experiment Guide For The PASCO Scientific Model ME-9341Aldana Ayelén MercadoNo ratings yet
- Fluorescence Spectrophotometry: The Electronic Excited StateDocument4 pagesFluorescence Spectrophotometry: The Electronic Excited Stateadriana_obrNo ratings yet
- Rotating Reference FrameDocument6 pagesRotating Reference FrameCalvin SullivanNo ratings yet
- Gibbs PhaseDocument7 pagesGibbs PhaseAjeet KumarNo ratings yet
- 6-Franz Keldysh Effect and Quantum Confined Stark effect-28-Jul-2020Material - I - 28-Jul-2020 - 8-Franz - Keldysh - and - Stark - EffectsDocument13 pages6-Franz Keldysh Effect and Quantum Confined Stark effect-28-Jul-2020Material - I - 28-Jul-2020 - 8-Franz - Keldysh - and - Stark - EffectsAman Kumar TrivediNo ratings yet
- Chemical Kinetics SlidesDocument25 pagesChemical Kinetics SlidesShaurya100% (1)
- Exploring Thermo PhysicsDocument31 pagesExploring Thermo PhysicsclstewartNo ratings yet
- An A 24Document4 pagesAn A 24Doriz Jan OlanoNo ratings yet
- P1.7 Measurements of The Radiative Surface Forcing of ClimateDocument8 pagesP1.7 Measurements of The Radiative Surface Forcing of Climatemalfet1066No ratings yet
- Mohammed Muddassir 100814205 Synthesis and Conversion of Maleic Acid Report CompleteDocument4 pagesMohammed Muddassir 100814205 Synthesis and Conversion of Maleic Acid Report Completemohammed .muddassirNo ratings yet
- E.over.m RatioDocument8 pagesE.over.m Ratiobrain221304No ratings yet
- Module 1 Lec 2 - THERMODYNAMICS 2nd QTR SY1112 PDFDocument8 pagesModule 1 Lec 2 - THERMODYNAMICS 2nd QTR SY1112 PDFJason JohnsonNo ratings yet
- Experiment No. 1 CalorimetryDocument7 pagesExperiment No. 1 CalorimetryElah Mae Evangelista QuintilaNo ratings yet
- Rectification: Aims/ObjectivesDocument3 pagesRectification: Aims/ObjectivesTarun GoohuramNo ratings yet
- Zohdy, Eaton & Mabey - Application of Surface Geophysics To Ground-Water Investigations - USGSDocument63 pagesZohdy, Eaton & Mabey - Application of Surface Geophysics To Ground-Water Investigations - USGSSalman AkbarNo ratings yet
- Chemical Equilibrium .PresentationDocument17 pagesChemical Equilibrium .PresentationtalhawasimNo ratings yet
- Finite State Machine Design and VHDL Coding Techniques: Iuliana CHIUCHISAN, Alin Dan POTORAC, Adrian GRAURDocument6 pagesFinite State Machine Design and VHDL Coding Techniques: Iuliana CHIUCHISAN, Alin Dan POTORAC, Adrian GRAURjavedfmNo ratings yet
- Patterson 1956Document8 pagesPatterson 1956krishy19No ratings yet
- 08 Chapter3Document25 pages08 Chapter3Longdien AnngaiNo ratings yet
- Physical LimnologyDocument173 pagesPhysical LimnologyJuan Pablo Ramirez MonsalveNo ratings yet
- Analytical Chemistry-22 (Bulk Electrolysis) 2Document64 pagesAnalytical Chemistry-22 (Bulk Electrolysis) 2Fegalma EotNo ratings yet
- Lect 16 - 17 - Mechanism of Energy TransportDocument9 pagesLect 16 - 17 - Mechanism of Energy TransportSatyajit SamalNo ratings yet
- Negative Resistance DevicesDocument4 pagesNegative Resistance DevicesHasitha Gayan JayasundaraNo ratings yet
- Heat Capacity of Gases With Cobra3Document7 pagesHeat Capacity of Gases With Cobra3Jose GalvanNo ratings yet
- Transport PhenomenaDocument7 pagesTransport PhenomenaC. MNo ratings yet
- Calorimeter: From Wikipedia, The Free EncyclopediaDocument8 pagesCalorimeter: From Wikipedia, The Free EncyclopediaVictor Erdy SugionoNo ratings yet
- Alkalinity: Phenolphthalein and Total Alkalinity Method 8221 0 To 5000 MG/L As Caco Buret TitrationDocument6 pagesAlkalinity: Phenolphthalein and Total Alkalinity Method 8221 0 To 5000 MG/L As Caco Buret TitrationLita Peñafiel TumbacoNo ratings yet
- Equilibrium: Three Stooges in Chemical ReactionsDocument5 pagesEquilibrium: Three Stooges in Chemical ReactionsKhud SarNo ratings yet
- 1 Lab Handout PDFDocument5 pages1 Lab Handout PDFKhud SarNo ratings yet
- TitrationsDocument4 pagesTitrationsapi-235688447No ratings yet
- 01 Phet PH ScaleDocument2 pages01 Phet PH Scaleapi-235688447No ratings yet
- Venn Diagram of The Different Definitions For Acids and BasesDocument1 pageVenn Diagram of The Different Definitions For Acids and Basesapi-235688447No ratings yet
- Acids and Metals NewDocument4 pagesAcids and Metals Newapi-235688447No ratings yet
- 18 5Document3 pages18 5api-235688447No ratings yet
- 18.4 Acid-Base Titrations (HL)Document2 pages18.4 Acid-Base Titrations (HL)api-235688447No ratings yet
- 18.3 Salt Hydrolysis (HL)Document1 page18.3 Salt Hydrolysis (HL)api-235688447No ratings yet
- 8.3 Strong and Weak Acids & BasesDocument1 page8.3 Strong and Weak Acids & Basesapi-235688447No ratings yet
- Vapour Pressure LabDocument3 pagesVapour Pressure Labapi-235688447No ratings yet
- 18 2 BufferDocument2 pages18 2 Bufferapi-235688447No ratings yet
- 8.4 The PH ScaleDocument1 page8.4 The PH Scaleapi-235688447No ratings yet
- 18.1: Calculations Involving Acids & Bases (HL)Document6 pages18.1: Calculations Involving Acids & Bases (HL)api-235688447No ratings yet
- 8.2 Properties of Acids and BasesDocument1 page8.2 Properties of Acids and Basesapi-235688447No ratings yet
- 8 1Document3 pages8 1api-235688447No ratings yet
- 03 Haber and Contact ProcessesDocument2 pages03 Haber and Contact Processesapi-235688447No ratings yet
- Le Chateliers Principle Lab DanielleDocument4 pagesLe Chateliers Principle Lab Danielleapi-235688447No ratings yet
- SURFYNOL® 465 Surfactant: Nonionic Dynamic Wetting AgentDocument2 pagesSURFYNOL® 465 Surfactant: Nonionic Dynamic Wetting AgentJeidy Estefania Serrano MarquinNo ratings yet
- Blending - IBCDocument2 pagesBlending - IBCRoyNo ratings yet
- DR TMMP (Quantum)Document50 pagesDR TMMP (Quantum)Tmmp SmileNo ratings yet
- Light Metals 2012Document5 pagesLight Metals 2012Sinan YıldızNo ratings yet
- Cambridge IGCSE: Physics 0625/11Document16 pagesCambridge IGCSE: Physics 0625/11Ridley Valley TutorialNo ratings yet
- Gunnebo Offshore 2011 JanuaryDocument28 pagesGunnebo Offshore 2011 JanuaryDavid Wise-MannNo ratings yet
- Qac D 072 Kao Fabric Softener 6 ConcentrationDocument2 pagesQac D 072 Kao Fabric Softener 6 ConcentrationChemist Technologist0% (1)
- Design and Construction of Raceways and Other Flow-Through SystemsDocument9 pagesDesign and Construction of Raceways and Other Flow-Through SystemsFlorentina SuduNo ratings yet
- NCERT - Complete Geography PDFDocument579 pagesNCERT - Complete Geography PDFsarav.karthikNo ratings yet
- Ekaland & Vultac® Range For Rubber Application: P P P PDocument1 pageEkaland & Vultac® Range For Rubber Application: P P P Pjulius hasan33No ratings yet
- And Reactivity in Chemistry and How These Are Also ManDocument354 pagesAnd Reactivity in Chemistry and How These Are Also Manluiz13eduardoNo ratings yet
- NE2571 Farmer DataSheet 10092018Document1 pageNE2571 Farmer DataSheet 10092018Gonzalo Suarez SanchezNo ratings yet
- Rohaizat JMCWM10.1007 - s10163 017 0672 7Document10 pagesRohaizat JMCWM10.1007 - s10163 017 0672 7Hadi Iz'aanNo ratings yet
- TBR Bio2 OptDocument495 pagesTBR Bio2 OptTheodore Marghitu100% (1)
- NMR of Hydrogen (AQA A2 Chemistry) PART 1 OF 2 TOPICSDocument1 pageNMR of Hydrogen (AQA A2 Chemistry) PART 1 OF 2 TOPICSzaheeraNo ratings yet
- 3a Lattice Vibrations PDFDocument18 pages3a Lattice Vibrations PDFGanesh NegiNo ratings yet
- Phys 230 Winter 2010 - Chapter 23Document32 pagesPhys 230 Winter 2010 - Chapter 23almaty138gymNo ratings yet
- Liquid Crystals Seminar SMDocument7 pagesLiquid Crystals Seminar SMSmriti100% (1)
- MIT2 25F13 Couet and PoisDocument3 pagesMIT2 25F13 Couet and Poisugoala brightNo ratings yet
- ImrulDocument5 pagesImrulJobaer ShaonNo ratings yet
- M705-GRN360-MZ: High-Reliability, High-Preheat Resistance Lead-Free Solder PasteDocument12 pagesM705-GRN360-MZ: High-Reliability, High-Preheat Resistance Lead-Free Solder PasteAlejandro Hernandez de DiosNo ratings yet
- Candy Bar TectonicsDocument2 pagesCandy Bar Tectonicspandrea112285No ratings yet
- Statistical Thermodynamics of Rubber ElasticityDocument8 pagesStatistical Thermodynamics of Rubber Elasticitychiuchan888No ratings yet
- Nature of ResistanceDocument28 pagesNature of Resistancelocutor100% (1)
- Solution: A 160 200 B 300 C 100 D 140 E 160Document2 pagesSolution: A 160 200 B 300 C 100 D 140 E 160A.Kh.SNo ratings yet
- M Alkalinity and P AlkalinityDocument4 pagesM Alkalinity and P AlkalinityiastraNo ratings yet
- Chem2 Laboratory Manual MLS LA1 7 PrelimDocument52 pagesChem2 Laboratory Manual MLS LA1 7 Prelimsampong mga dalereNo ratings yet
- Formulating For Extruding AbateDocument11 pagesFormulating For Extruding Abatehossny100% (1)
- UNIT 1 Introduction To BiopharmaceuticsDocument208 pagesUNIT 1 Introduction To BiopharmaceuticsMamta Pant100% (5)