Professional Documents
Culture Documents
Oxidation Ladder
Uploaded by
Satya KamOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Oxidation Ladder
Uploaded by
Satya KamCopyright:
Available Formats
"Master Organic Chemistry"
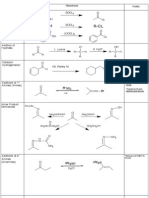
Summary Sheet - The Oxidation Ladder
Oxidation state of carbon
masterorganicchemistry.com June 2011. Version 1.0
Note - this sheet is not meant to be comprehensive. Your course may provide additional material, or may not cover some of the reactions shown here. Your course instructor is the final authority.
+4
Indicates oxidations Indicates reductions Neither oxidations nor reductions
CO2 Carbon dioxide
Notes: to keep things relatively simple, several common functional groups (amines, epoxides, ethers, and many more) have been omitted. All alkyl halides are drawn as chlorides ("Cl"). For Br and I, the corresponding reagent containing those atoms should be employed. Oxidation here is defined as: loss of a CH bond or gain of a CO bond (or equivalent) Reduction here is defined as: gain of a CH bond or loss of a CO bond (or equivalent)
H2O, acid ROH, acid O O Cl R NH3 or other amine O H2N Amide H2O, acid R P2O5 H2O, acid O C R RO Ester R
+2
LiAlH4
HO
SOCl2 H2O or NaOH
Carboxylic acid
Acid halide
Nitrile
R E D U C T I O N 0
O X I D A T I O N
amine, DCC O3, H2O H2CrO4 or H2O2
NH3 or other amine mCPBA RMgCl or RLi
H2CrO4 or KMnO4
DIBAL
BH3, H2O2 O H Aldehyde R Cl R Cl Dihalide (Vicinal) Cl2
H2O, H2SO4 or HgSO4, H2O, H2SO4 NaNH2 NaNH2 R Alkyne H2, Lindlar's catalyst HCl Cl Cl R Dihalide (Geminal) R Ketone O RMgCl or RLi
NaBH4 or PCC LiAlH4
O3, Zn (or DMS) H2SO4, heat BH3, H2O2 NaOH (SN2)
O3, Zn (or DMS) H2SO4, heat H2O, H2SO4 or Hg(OAc)2, H2O, then NaBH4 Cl R Alkyl halide (Secondary) NaOH (SN2) HCl
NaBH4 or LiAlH4
PCC or H2CrO4
HO
-2
Cl PCl3 or SOCl2
base (e.g. NaOEt)
HCl R Alkene base (e.g. NaOEt)
OH R Alcohol (Secondary)
OH R Alcohol (Tertiary)
Alcohol (Primary)
Alkyl halide (Primary)
Mg, then acid
Pd/C H2
Mg, then acid
Cl2, light
Cl2, light R Alkanes Omissions, Mistakes, Suggestions? james@writechem.com This sheet copyright 2011, James A. Ashenhurst masterorganicchemistry.com R
You might also like
- 1 Roh Carboxylic Acids: H CroDocument15 pages1 Roh Carboxylic Acids: H CroandrewwrobleNo ratings yet
- Reactions of Aldehydes and KetonesDocument1 pageReactions of Aldehydes and KetonesUday Prakash SahuNo ratings yet
- Organic Chemistry Fiitjee Flowcharts PDFDocument12 pagesOrganic Chemistry Fiitjee Flowcharts PDFAkshit Sharma50% (4)
- MOC Alcohol RXN Map PDFDocument2 pagesMOC Alcohol RXN Map PDFNickOoPandeyNo ratings yet
- Table of Common Functional GroupsDocument10 pagesTable of Common Functional GroupsAngelica Mae Lasam100% (1)
- AldehydesDocument21 pagesAldehydesNoor Farrah Wahida MuradNo ratings yet
- Chapter 15: Alcohols, Diols, and Thiols 15.1: Sources of Alcohols (Please Read)Document9 pagesChapter 15: Alcohols, Diols, and Thiols 15.1: Sources of Alcohols (Please Read)Rammohan VaidyanathanNo ratings yet
- 634566746179743750Document6 pages634566746179743750Abhijit SinghNo ratings yet
- Organic Chemistry All ReactionsDocument4 pagesOrganic Chemistry All ReactionsWaseem Alkakoz100% (4)
- Common Functional GroupsDocument1 pageCommon Functional Groupszeeshan876No ratings yet
- Functional Groups: Organic Chemistry EssentialsDocument8 pagesFunctional Groups: Organic Chemistry EssentialsJeremiah Paul Gotia HumiwatNo ratings yet
- Ald&Ketone IDocument41 pagesAld&Ketone IreinitavanyNo ratings yet
- Alcohol SummaryDocument3 pagesAlcohol SummarydanielmahsaNo ratings yet
- AminesphenolsDocument15 pagesAminesphenolsSivakumar PonnusamyNo ratings yet
- Synthetics Summary SheetDocument9 pagesSynthetics Summary SheetChris Pacis ÜNo ratings yet
- Alcohols, Diols, and ThiolsDocument3 pagesAlcohols, Diols, and ThiolsJeremy A. Baker100% (1)
- Aldehid Keton 08Document49 pagesAldehid Keton 08Mochamad Herdi NurzamanNo ratings yet
- A Review of Organic Reactions and Reagents For Chemistry 551Document38 pagesA Review of Organic Reactions and Reagents For Chemistry 551Cris WRNo ratings yet
- Alcohol Phenol & EtherDocument13 pagesAlcohol Phenol & EtherAbir DuttaNo ratings yet
- Organic ChemistryDocument1 pageOrganic ChemistryApril VirataNo ratings yet
- Alcohol, Phenols, Thiols, and EthersDocument17 pagesAlcohol, Phenols, Thiols, and EthersJohn Paul CuNo ratings yet
- Organo Sulphur Compounds by Dr. Savita DesaiDocument3 pagesOrgano Sulphur Compounds by Dr. Savita DesaiNickOoPandeyNo ratings yet
- Typical Hydrogen and Carbon NMR Chemical ShiftsDocument2 pagesTypical Hydrogen and Carbon NMR Chemical ShiftsErica CouzensNo ratings yet
- Aldehyde Ketone and AcidDocument15 pagesAldehyde Ketone and AcidAbir DuttaNo ratings yet
- Aldehid Keton 08Document48 pagesAldehid Keton 08Priagung SetyawanNo ratings yet
- Organic Mind MapDocument37 pagesOrganic Mind Mapkamalia8980% (5)
- Ald&Ketone IDocument41 pagesAld&Ketone IAbhay NarvekarNo ratings yet
- Conversion of organic compounds reagentsDocument4 pagesConversion of organic compounds reagentsJleodennis RajNo ratings yet
- Organic reactions and conditionsDocument3 pagesOrganic reactions and conditionsAvrinoxNo ratings yet
- OrganicDocument15 pagesOrganicI am madNo ratings yet
- Functional GroupsDocument1 pageFunctional GroupsStan SmithNo ratings yet
- Orgo Reaction SheetDocument9 pagesOrgo Reaction SheetKyle Broflovski100% (1)
- O C SPA S A S: Rganic Hemistry Kill UmmaryDocument6 pagesO C SPA S A S: Rganic Hemistry Kill Ummaryhasan_j688675No ratings yet
- Reagent and The Reactions They CauseDocument3 pagesReagent and The Reactions They CauseChip Timmons100% (8)
- Mod 4 Revision Guide 10 Synthetic RoutesDocument2 pagesMod 4 Revision Guide 10 Synthetic RoutesdufraiscNo ratings yet
- CHEM 215 F12 Chapter 13 Notes UMICHDocument13 pagesCHEM 215 F12 Chapter 13 Notes UMICHRoxanne IlaganNo ratings yet
- Ald&KetoneDocument41 pagesAld&KetoneFeng SpencerNo ratings yet
- CH 16Document102 pagesCH 16Amer KhanNo ratings yet
- FUNCTIONAL GROUP INTERCONVERSIONS GUIDEDocument6 pagesFUNCTIONAL GROUP INTERCONVERSIONS GUIDEJulia MaramatNo ratings yet
- Acfrogcxqpd3dqml GQHMZQ B0c089di81vpcrvgphrwcu4gh Bzezvshldjt4clxztdrke4cieuxds1wlvk6scla 0byn2rmeu4btdaq8ckybm0chweegnztu7u2olcnli2lia5txmf386nyikuDocument6 pagesAcfrogcxqpd3dqml GQHMZQ B0c089di81vpcrvgphrwcu4gh Bzezvshldjt4clxztdrke4cieuxds1wlvk6scla 0byn2rmeu4btdaq8ckybm0chweegnztu7u2olcnli2lia5txmf386nyikuAchal ParekhNo ratings yet
- Tablo A - 1: 884 TermodinamikDocument1 pageTablo A - 1: 884 TermodinamikAlberto GutierrezNo ratings yet
- Undergraduate organic reactions summaryDocument41 pagesUndergraduate organic reactions summaryKathyNo ratings yet
- Key Functional GroupsDocument24 pagesKey Functional GroupsThuan Tăng NguyenNo ratings yet
- Ald&ketone IDocument41 pagesAld&ketone Iasney2512No ratings yet
- C10K Carbonyl Chemistry EmailDocument37 pagesC10K Carbonyl Chemistry EmailMatthew яeject'z BennettNo ratings yet
- Bab 19. Asam AminoDocument58 pagesBab 19. Asam AminoSisca Ayu VerawatiNo ratings yet
- ALDEHYDE AND KETONE REACTIONSDocument4 pagesALDEHYDE AND KETONE REACTIONSBILL RUSSO100% (4)
- Protecting Groups StabilityDocument7 pagesProtecting Groups StabilityKeng Goy PlungpongpanNo ratings yet
- Appendix Homologous Series Functional Group General Formula Structural Formula Iupac Nomenclature ExampleDocument2 pagesAppendix Homologous Series Functional Group General Formula Structural Formula Iupac Nomenclature ExampleThivyah AhilanNo ratings yet
- R-Cooh, R-Co H,: À Ant À VinegarDocument43 pagesR-Cooh, R-Co H,: À Ant À VinegarArvin MarasiganNo ratings yet
- Reactions and Interconversions of Organic Functional GroupsDocument3 pagesReactions and Interconversions of Organic Functional Groupsmichelsonyip100% (1)
- CH 17Document18 pagesCH 17MirjanaNo ratings yet
- Acids, Derivatives and NitrilesDocument23 pagesAcids, Derivatives and NitrilesLuqman HakimNo ratings yet
- Monohydric Alcohols Their Ethers and Esters Sulphur Analogues Nitrogen Derivatives Organometallic Compounds: A Modern Comprehensive TreatiseFrom EverandMonohydric Alcohols Their Ethers and Esters Sulphur Analogues Nitrogen Derivatives Organometallic Compounds: A Modern Comprehensive TreatiseNo ratings yet
- Practice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersFrom EverandPractice Makes Perfect in Chemistry: Acids, Bases, and Salts with AnswersNo ratings yet
- Aliphatic Compounds: A Modern Comprehensive TreatiseFrom EverandAliphatic Compounds: A Modern Comprehensive TreatiseNo ratings yet
- Schaum's Easy Outline of Organic Chemistry, Second EditionFrom EverandSchaum's Easy Outline of Organic Chemistry, Second EditionRating: 3.5 out of 5 stars3.5/5 (2)