Professional Documents
Culture Documents
Metabolism Outline
Uploaded by
ChristinaFAUCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Metabolism Outline
Uploaded by
ChristinaFAUCopyright:
Available Formats
Review
for exam 2
Metabolism: Deni&on The totality of an organisms chemical reac3ons is called metabolism Types with examples: Catabolism and Anabolism *Catabolism: release energy by breaking down complex molecules to simpler compounds. A major pathway of catabolism is cellular respira3on, in which the sugar glucose and other organic fuels are broken down in the presence of oxygen to carbon dioxide and water. *Anabolism: consume energy to build complicated molecules from simpler ones; they are some3mes called biosynthe3c pathways. Examples of anabolism are the synthesis of an amino acid from simpler molecules and the synthesis of a protein from amino acids. Examples of exergonic, endergonic, spontaneous and non-spontaneous processes *spontaneous: energe3cally favorable A process that can occur without an input of energy is called a spontaneous process. explosion may be virtually instantaneous, while others, such as the rus3ng of an old car over 3me, are much slower. nonspontaneous: a process that cannot occur on its own, it will happen only if energy is added to the system. a bicycle cant work if you do not petal it. An exergonic reac3on proceeds with a net release of free energy An endergonic reac3on is one that absorbs free energyfrom its surroundings Gibbs equa&on and what does each term mean? G= H - T(S) This equa3on uses only proper3es of the system (the reac3on) itself: H symbolizes the change in the systems enthalpy (in biological systems, equivalent to total energy); S is the change in the systems entropy; and T is the absolute temperature in Kelvin (K) units State the two laws of thermodynamics rst law- Energy can be transferred and transformed, but it cannot be created or destroyed second law- Every energy transfer or transforma3on increases the entropy of the universe and during energy transfer, some amount of energy becomes unavailable, gets converted to heat. Bioenerge&cs: biology that concerns energy ow through living organisms Energy currency of the cell and its cycle Enzymes: biological catalyst
Lock and key, induced t with diagram Lock and key- ts perfectly induced t- ac3ve site changes Ac&va&on energy and lowering of reac&on temperature The eect of an enzyme on ac3va3on energy. Without aec3ng the free-energy change (G) for a reac3on, an enzyme speeds the reac3on by reducing its ac3va3on energy (EA). Above that temperature,however, the speed of the enzyma3c reac3on drops sharply. The thermal agita3on of the enzyme molecule disrupts the hydrogen bonds, ionic bonds, and other weak interac3ons that stabilize the ac3ve shape of the enzyme, and the protein molecule eventually denatures. Each enzyme has an op3mal temperature at which its reac3on rate is greatest. Without denaturing the enzyme, this temperature allows the greatest number of molecular collisions and the fastest conversion the reactants to product molecules. Most human enzymes have op3mal temperatures of about 3540C (close to human body temperature). The thermophilic bacteria that live in hot springs contain enzymes with op3mal temperatures 70C or higher Apoenzyme, Cofactor, coenzyme Many enzymes require nonprotein helpers for cataly3c ac3vity. These adjuncts, called cofactors, may be bound 3ghtly to the enzyme as permanent residents, or they may bind loosely and reversibly along with the substrate. The cofactors of some enzymes are inorganic, such as the metal atoms zinc, iron, and copper in ionic form. If the cofactor is an organic molecule, it is more specically called a coenzyme. Most vitamins are important in nutri3on because they act as coenzymes or raw materials from which coenzymes are made. Cofactors func3on in various ways, but in all cases where they. For a process to occur spontaneously, it must increase the entropy of the universe are used, they perform a crucial chemical func3on in catalysis. polypep3de or protein part of othe enzyme- apoenzyme
You might also like
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Test Bank For Biochemistry 1st Edition Roger L Miesfeld Megan M McevoyDocument20 pagesTest Bank For Biochemistry 1st Edition Roger L Miesfeld Megan M McevoyJohn Creel100% (38)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- CHE 1120 E Lecture Syllabus Fall 20Document14 pagesCHE 1120 E Lecture Syllabus Fall 20Christopher MassoneNo ratings yet
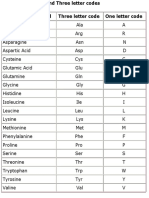
- Amino Acid CodesDocument1 pageAmino Acid Codesmorzan ortizNo ratings yet
- 8.1 WorksheetDocument7 pages8.1 WorksheetNoor ssNo ratings yet
- ATP and NADPH,-Potosynthesis and RespirationDocument9 pagesATP and NADPH,-Potosynthesis and RespirationNicole Geba SamonteNo ratings yet
- Arraina. Aquaculture Nutrient RequirementsDocument20 pagesArraina. Aquaculture Nutrient RequirementsJohan TheronNo ratings yet
- Beneficial Effects of Green Tea: A Literature ReviewDocument9 pagesBeneficial Effects of Green Tea: A Literature ReviewYoussef FaragNo ratings yet
- BIOKIMIADocument38 pagesBIOKIMIAmaulidianurasifaNo ratings yet
- SEED GERMINATION - ReportDocument5 pagesSEED GERMINATION - ReportpriyanktiwariNo ratings yet
- BIOLOGYDocument11 pagesBIOLOGYCarl PavicoNo ratings yet
- SmofKabiven Peripheral PM ENG 020218 022122Document58 pagesSmofKabiven Peripheral PM ENG 020218 022122Myrill Shane OtadoyNo ratings yet
- Seafood Enzymes Utilization and Influence On Postharvest Seafood Quality PDFDocument689 pagesSeafood Enzymes Utilization and Influence On Postharvest Seafood Quality PDFkamal100% (1)
- Module 3 Earth & Life ScienceDocument16 pagesModule 3 Earth & Life ScienceNicole Mae SumaltaNo ratings yet
- Photosynthesis (Handouts)Document5 pagesPhotosynthesis (Handouts)Jelea MagallanesNo ratings yet
- Lactic Acid BacteriaDocument280 pagesLactic Acid BacteriaNicolas CalderonNo ratings yet
- Exercise 8-Life ScienceDocument2 pagesExercise 8-Life ScienceKarinaNo ratings yet
- Activities To Develop Pre-TaskDocument6 pagesActivities To Develop Pre-Taskeduardo arenasNo ratings yet
- Unit 2B Guided Notes 2022Document6 pagesUnit 2B Guided Notes 2022Victoria WarrenNo ratings yet
- 99 Day Study Schedule For USMLE Step 1 PDFDocument27 pages99 Day Study Schedule For USMLE Step 1 PDFAhmad JaleelNo ratings yet
- Quantification of Starch in Plant Tissues: ProtocolDocument4 pagesQuantification of Starch in Plant Tissues: ProtocolSharif M Mizanur RahmanNo ratings yet
- Biology Concepts and Connections Campbell 6th Edition Test BankDocument25 pagesBiology Concepts and Connections Campbell 6th Edition Test BankRichard RunnelsNo ratings yet
- Examination Content Guideline CDocument5 pagesExamination Content Guideline CNHNo ratings yet
- Bio 4212examDocument5 pagesBio 4212examAsheNo ratings yet
- Ephoderin BiosynthesisDocument4 pagesEphoderin BiosynthesisHina AftabNo ratings yet
- Physiological Adaptations To HIIT PDFDocument16 pagesPhysiological Adaptations To HIIT PDFRoberto CornejoNo ratings yet
- Chapter 16 The Citric Acid Cycle: Multiple Choice QuestionsDocument14 pagesChapter 16 The Citric Acid Cycle: Multiple Choice QuestionshielNo ratings yet
- 6BBL0325 2 TheoriesofAgeingRandomDamageDocument10 pages6BBL0325 2 TheoriesofAgeingRandomDamageVaidehi KatariaNo ratings yet
- BIOENERGETICS Trans - IncDocument3 pagesBIOENERGETICS Trans - IncChino Paolo SamsonNo ratings yet
- Patient Bill: Test Name Reporting Date & Time Rate SRDocument1 pagePatient Bill: Test Name Reporting Date & Time Rate SRShahab KhanNo ratings yet
- Biology Campbell 10e Druk Samenvatting-OriginalDocument340 pagesBiology Campbell 10e Druk Samenvatting-Originalnaddah.hawasiNo ratings yet