Professional Documents
Culture Documents
Propagation Techniques For Tropical Fruits PDF
Uploaded by
Brij Mohan SinghOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Propagation Techniques For Tropical Fruits PDF
Uploaded by
Brij Mohan SinghCopyright:
Available Formats
102
TROPICAL FRUITS IN ASIA: CONSERVATION AND USE
Propagation Techniques For Tropical Fruits
B.M.C. Reddy
Introduction
........................................................
103 103
Propogation Techniques . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . Mango, Citrus, Rambutan, Jackfruit, Litchi, Durian, Mangosteen References ........................................................
110
PROPAGATION TECHNIQUES FOR TROPICAL FRUITS
103
Propagation Techniques For Tropical Fruits
B.M.C. Reddy
Introduction The propagation of plants is of great importance in perennial/vegetatively propagated crops. When new kind of plants have to be conserved or propagated or exploited, we need to develop knowledge and technique to propagate them. Invention of propagation structures and discovery of root inducing chemicals, in addition to grafting and budding methods, have revolutionized the propagation and nursery procedures. An appropriate propagation technology can be selected for each kind of plant based on its growth, physiology, flowering and phenology. The propagation method can be primarily designed based on the mode of natural perpetuation like species with polyembryony can be propagated through seeds. Further, the general technique standardized for the species can be applied also to other species within the same genus with suitable modifications. This could be well explained within the family Anacardiaceae where mango and cashew both can be successfully multiplied by epicotyl grafting and soft wood grafting. This paper gives details on the propagation methods followed in some of the major and minor tropical fruits of the Asia-Pacific region, such as mango, citrus, rambutan, jackfruit, litchi, durian and mangosteen. Propagation Techniques Mango Mango is a cross pollinated and highly haterozygous fruit crop. Therefore, propagation by seeds (called stones in mango) lead to variability in the progeny and is a limitation for commercial orcharding, though desirable for breeding new varieties. Hence, vegetative methods of propagation are adopted for getting true-to-type plants. Seed propagation is still the chief method of multiplication of rootstocks.
Propagation of rootstocks Seed propagation
Rootstock plants are raised from stones. Most of the commercial varieties in India are monoembyronic but some varieties from South India are polyembryonic which give true-to-type seedlings from nucellar embryos (Sachar and Chopra 1957). Stones collected indiscriminately give poor germination but provide a lot of variation. Bolder stones give seedlings with more vigour irrespective of whether these were collected from grafted or seedling trees. The viability of mango stones is lost very quickly. Freshly collected stones from canning units give high germination but when these seeds are stocked or kept in sun for a large period, they lose germinability. The germination of mango stones is 80% when sown within a month of extraction but none thereafter. The viability of stones could be extended by storing them in charcoal powder (Teaotia and Singh 1971) and polythene bag (Singh and Kaisuwan 1971). Patil et al. (1986) reported 40% germination after 90 days of storage in polythene bags with charcoal. Although refrigerated storage had adverse effect on germination, seeds were successfully preserved for 12 months without any decline in viability and vigour after treatment with 1% hydroxyquinoline sulphate and storage in polythene bags of 100 guage at 150 C (Doijode 1989). The selected stones are spread on raised nursery beds and covered with leaf mould
104
TROPICAL FRUITS IN ASIA: CONSERVATION AND USE
or FYM for better germination. Germination starts within 12 - 15 days and continues up to 3 weeks. The germination is adversely affected if the stones are sown too deep or in pots or polybags. Seedlings are transplanted to pots or polybags as soon as they turn green and these are nursed and prepared for grafting. When polyembryonic seeds are sown, nucellar seedlings are separated within a month and planted separately.
Vegetative propagation
Propagation of rootstocks by vegetative methods is advocated to eliminate variation. These methods are not commonly used probably due to absence of standard rootstock type. (1) Cutting: The hardwood leafy cuttings are used for rooting. Cutting from younger trees rooted better than those from old trees (Mukherjee et al. 1966). Rooting as well as subsequent survival has been improved by using juvenile shoots, etiolation and ringing of shoots and application of IBA in lanolin paste on the ringed portion (Bid and Mukherjee 1972). A medium containing peat moss and sand (1:1) proved better and such cuttings have to be kept under mist for rooting. Use of bottom heat to provide a temperature of 30 to 200C (Reddy and Majumdar 1975) for Deshehari cuttings dipped in 5000 ppm IBA and planted in sphagnum moss and sand mixture resulted in 97 per cent rooting compared to only 15 per cent without bottom heat. Beneficial effect of bottom heat was observed by Rajan and Ram (1983), Sadhu and Bose (1986), and Reddy and Singh (1987). The synergism of phenols and flavonoids with IBA for root regeneration in cuttings has been demonstrated by Reddy and Majumdar (1975). Preplanting treatment with phenolic compounds such as hydroxybenzoic acid, coumaric acid and ferulic acid generally promoted auxininduced rooting in cuttings (Sadhu et al. 1978). (2) Air layering: Etiolation of shoots before layering resulted in more fibrous roots than without etiolation and application of IBA + NAA improved rooting (Bid and Mukherjee 1969). The success of air layers was markedly improved by use of growth regulators like 5000 to 20 000 ppm NAA and IBA in lanolin paste (Chhonkar and Singh 1972). (3) Stooling: Majumdar and Mukherjee (1961) described modified form of stool layering, wherein after about two years of growth, the mother plant is headed back at 10-12 cm above ground to induce emergence of several shoots below the cut end. Vigorous shoots are ringed and IBA (5000 ppm) in lanolin is applied on the ringed portion about a week before earthing up. Rooting takes place in 4 to 6 weeks and these are separated from the mother plant. The same mother plant can be utilized for about ten years to obtain genetically uniform rooted plants of a given clone. Clonal propagation of mango rootstocks by stooling has also been reported by Singh and Srivastava (1982). This is the easiest and fastest method for vegetative propagation to multiply mango rootstocks.
Propagation of scion plants
Mango plants in nurseries are raised by vegetative methods like budding and grafting.
Budding
Various budding methods, namely, patch forkert, shield, chip and T-budding are successful in mango. However, the success varies depending on geographical location and season. Since the buddings are likely to be damaged during transport, this method is not followed commercially.
Grafting
Inarching or approach grafting, veneer grafting, wedge or whip grafting, epicotyl grafting or stone grafting and softwood grafting are the most extensively used methods.
PROPAGATION TECHNIQUES FOR TROPICAL FRUITS
105
(1) Inarching: This is the oldest and still the chief technique employed in many mango growing regions. Although, it gives very high success, this method is costlier, cumbersome and requires larger bud wood and a long time to produce grafts. (2) Veneer grafting: In this method, mature scion of 3-4 months is grafted on 1 to 2 years old stock. Pre-curing the scion shoot by defoliation atleast 7 to 10 days prior to grafting is essential. However, in the coastal areas of Maharashtra, prior defoliation is not required (Gunjate et al. 1976). The period of veneer grafting differs from place to place owing to climatic variations. This technique is suitable for in situ grafting and for top working of seedling trees. (3) Wedge grafting: Mature precured scion shoot of 3 to 4 months old is wedge grafted on 1 to 2 year old stock. The success is high during the humid conditions and 90 to 100% success is obtained under greenhouse and mist chamber. This method is also used for in situ grafting and top working. (4) Epicotyl grafting or stone grafting: In this method, mango seedling of less than two weeks is wedge grafted with mature scion. For higher success and better growth, use of two stocks per scion is recommended. This is the commercial propagation method in Konkan region of Maharashtra and does not require pretreatment of scion (Gunjate et al. 1982). (5) Softwood grafting: In this technique, grafting is done with mature, precured scion on the emerging soft copper red shoot as described by Amin (1974). This technique was successful in establishing mango orchards in situ in drier tracts where mortality of nursery raised grafts is very heavy (Amin 1978). Citrus The sexual as well as asexual (vegetative) methods of propagation are used to raise plants of different species in citrus. The seedlings of acid lime and mandarins are still planted in southern and north-eastern region of India.
Seed propagation
The number of seeds per fruit varies between different citrus species and cultivars. Seeds obtained from healthy, virus-free old trees which have a pedigree performance of producing vigorous, uniform seedlings should be used.
Polyembryony
Seeds of most citrus are polyembryonic and thus nucellar seedlings are used both for raising uniform rootstocks as well as for direct planting, especially in acid lime and mandarins (Singh and Singh 1955). This also helps to raise healthy plants as citrus viruses are not transmitted through seed. Gopalakrishna and Kunte (1958) reported varietal differences in the degree of polyembryony in citrus. Motial (1963) observed that the number of embryos per seed was 4.7 in Jambhiri, 4.45 in Italian lemon and 4.25 in Galgal with a range of 1 to 11 in Citrus species. Galgal and Sadaphal had 90 and 95 per cent polyembryonic seeds respectively. Singh (1965) opined that constricted surface of seeds in Karna Khatta, Citrus jambhiri, C. aurantium and Poncirus trifoliata was associated with polyembryonic nature, except in P. trifoliata with polyembryony varying from 35.5 to 54.2 per cent. In citrus, deformed or uneven seeds were found to be usually polyembryonic (Singh 1971). Prasad and Ravishankar (1983) claimed 100 per cent polyembryony in Rusk Citrange, Trifoliate Orange and Swingle Citrumelo (93.3%), Troyer (86.6%), Carrizo (73.3%), but there was no relationship between the percentage of polyembryony and seed morphology.
106
TROPICAL FRUITS IN ASIA: CONSERVATION AND USE
Viability
Since Citrus seeds generally have no dormancy and if they are allowed to dry and the cotyledons separate, the seeds fail to germinate. Therefore, it is essential to sow the seeds immediately after extraction. The viability during storage varies depending upon species and storage conditions. Quick evaluation of seed viability could be done by tetrazolium test soaking the seeds in 1 % triphenyl tetrazolium bromide solution which strains the living tissue (Singh and Chopra 1962). Seed storage of Kagzi lime and Mosambi in charcoal powder at 50 per cent RH at room temperature was better than sealed polythene bags (Bajpai et al. 1963). Kumar and Majumdar (1973) reported that in C. aurantifolia, the seed germination was highest in freshly extracted seeds and declined as the storage period extended. Krishna and Shankar (1974) observed that in C. karna and Rusk Citrange, seeds viability was 100 per cent after 150 days storage in polythene bags with calcium chloride at 80C, whereas in case of C. jambhiri and C. limonia, it was 84 and 80 per cent respectively.
Raising seedlings
Citrus seeds are to be sown on raised and well manured beds at 2.5 cm apart in rows spaced at 15 cm. In commercial nurseries, sowing is done during mid August to February. Singh et al. (1970) observed that alkathene cover improved seedling growth of C. jambhiri, C. pseudolimon, C. limonia, C. megaloxycarpa and P. trifoliata over those in open. Many workers reported the usefulness of soaking the Citrus seeds in GA from 200 to 600 ppm to improve the rate of germination (Shant and Rao 1973; Mishra et al. 1982; Singh et al. 1989).
Vegetative propagation Cutting
Many species of Citrus can be successfully raised from cuttings and it is a very useful method of propagation especially when a species is desired to be colonally propagated on its own root system. However, there is wide variation in the rooting ability of different Citrus species. While limon and citron are easy to root, mandarin oranges, sour orange and grapefruit root with considerable difficulty (Sadhu 1986). Synthetic auxins (IBA, NAA and IAA) and phenolic compounds help in rooting of cuttings (Dhatt and Zora Singh 1993). However, age and physiological status of mother plant, type of wood, time of planting and media composition for planting of cuttings determine the extent of success (Gangwar and Singh 1965; Bajwa et al. 1977).
Air-layering
Air-layering is fairly common and is used to propagate pummelo, lime and sweet lime. Singh (1955) achieved appreciable improvement in the rooting of air-layers of sweet lime, grapefruit and sour lime by treating the cut surface with auxins (NAA + AAA both at 1 per cent). Shankar (1965) noted that certain rootstocks like C. karna, C. jambhiri, C. pennivesiculata, C. assamensis and C. megalocarpa can easily be raised from the air-layers even without treatment of growth regulators.
Budding
Budding is the most common and widely used method of vegetative propagation of Citrus. T-budding is probably the best method and is successful with many species of Citrus. Naik (1939) exhibited that bud take in Citrus spp. increased when wood was not removed from the bud. Stevenson (1947) reported that T-budding gave 87% success in Citrus species. In Punjab, the bud take of Kinnow on C. jambhiri was 98% with better subsequent growth when round buds without wood were used than by using angular buds with or without wood (Sharma and Sharma 1986). The time of budding affects bud
PROPAGATION TECHNIQUES FOR TROPICAL FRUITS
107
take and subsequent growth of plants. Experiments conducted at different places in India have shown that February and September-October are the best periods for budding in most Citrus species (Dhatt and Zora Singh 1993). Now polythene strips are commonly used as wrapping material instead of jute twine. Srivastava and Arya (1969) observed, while budding mosambi on C. karna, that 200 guage polythene strip was the best wrapping material. Delayed lopping of the rootstock till the bud has grown about 4-5 cm is usually practised by nurserymen. Rambutan The rambutan is one of the best known fruits of South-East Asia and is especially important in Thailand, Malaysia and Indonesia, but extends to the east as far as the Philippines.
Seed propagation
Propagation by seed has been the traditional practice and large number of trees now in production throughout South-East Asia originated from seed. This is not a desirable method since plants from seeds vary and many seedlings produce poor fruits. In addition, seedlings vary in sex. Male trees are practically useless, and female trees, unless well pollinated, do not bear well. Populations of seedlings of rambutan have yielded upto 67% males. Propagation from seed should only be used for the development of new varieties.
Viability
Seeds, when removed from the fruits, have a short life time and are recalcitrant (Chin 1975; Teng 1977). It is desirable to maintain them slightly moist and to plant them in 2 or 3 days. If the seeds are sown after 3 to 4 weeks, seeds usually do not germinate.
Raising seedling
Seeds can be sown in seed beds or in individual containers. The latter method is preferred as it eliminates the risk of transplanting and shows better establishment in the field.
Vegetative propagation
Vegetative propagation is indispensable for production of trees of high quality. These trees tend to come into fruiting at a younger age than seedlings. To preserve good, hermaphroditic varieties, vegetative propagation is necessary (Valmayor et al. 1961).
Air-layering
This is frequently used by home gardeners and small scale producers, but success rates are generally variable.
Grafting
Methods used include various forms of approach grafting and cleft grafting, which is particularly used in the Philippines. Top working of seedling or staminate trees and grafting with improved cultivars has been described.
Budding
In most of the South-East Asian countries, seedling stocks are budded by the modified Forkert method. Various other budding methods have been used such as shield or T-budding and patch budding with limited success. Jackfruit Seed propagation is an old method of multiplication which is planted in situ or raised
108
TROPICAL FRUITS IN ASIA: CONSERVATION AND USE
in pots. Now, vegetative propagation is the commercial method of jackfruit multiplication.
Seed propagation
This method is mainly used for the production of rootstocks. Soaking of seeds in NAA 25 ppm for 24 hours resulted in highest germination percentage as well as good seedling growth (Bose 1986). Shanmugavelu (1971) recorded 100 per cent germination by treating the seeds with GA3 at various concentrations upto 500 ppm compared with 80% in the untreated control.
Vegetative propagation Cutting
The highest percentage of rooting (84%) and survival (75%) was obtained in cuttings from invigorated and etiolated shoots treated with 5000 ppm IBA and kept under intermittent mist (Mukherjee and Chatterjee 1979). Dhua et al. (1983) recorded maximum rooting success (90%) in cutting taken from etiolated and ringed for 30 days and then treated with IBA at 3000 ppm plus ferulic acid at 2000 ppm. Etiolation and ringing for 15 days prior to planting also caused rooting in large number of cuttings which otherwise failed to strike roots.
Air-layering
Application of IAA, IBA and NAA significantly increased the percentage of rooting and IBA was most effective and produced large mass of roots in all the treated air - layers (Sen and Bose 1959). The beneficial effect of IBA on root formation was also recorded by Mukherjee and Chatterjee (1978).
Stooling
Success in stooling of one year old jackfruit plants was reported (Chatterjee and Mukherjee 1980 a). Soil was heaped upto 10 cm round the basal shoots for 15 - 20 days, after which it is removed. A ring of bark was taken from each etiolated shoot and IBA (5000 ppm) in lanolin was applied. At callus formation the shoots were again earthed up. About one month later, the rooted shoots were separated. This method gave an average of 75% rooted shoots of which 7% survived (Chatterjee and Mukherjee 1980 b).
Grafting
Though jackfruit was successfully propagated (84% success) by inarching on seedling rootstock (Srinivasan 1970), this method of propagation is cumbersome. Soft wood grafting is the commercial method of propagation and is 100% successful under high humid conditions of coastal areas. Wherever the conditions are not favaourable, the soft wood grafting was highly successful under intermittent mist.
Budding
Patch budding was very successful (100%) when done in middle of June while the success was less (90%) when performed in May or July (Bose 1986). Similar success with patch budding in June was obtained by Singh et al. (1982). Litchi
Seed propagation
This method is not desirable since the trees raised from seeds have long juvenile period, fail to produce true-to-type plants and often produce fruits of inferior quality. Propagation by seed is used mainly for rootstock propagation and breeding. The seeds of litchi have a very short viability, usually less than 5 days.
PROPAGATION TECHNIQUES FOR TROPICAL FRUITS
109
Vegetative propagation Cutting
Propagation by cuttings is possible, but the plants produced are less vigorous and this method is not generally recommended (Abutiate and Nakasone 1972; Kadman and Slor 1974; Paxton et al. 1978).
Air-layering
The main commercial method of propagation is by air-layering and rates of success may be as high as 95% under ideal conditions. Air-layered trees normally produce commercial crops after 3-6 years (Menzel 1991).
Grafting
Due to the limited and unpredictable activity of the cambium, which is related to the onset of leaf flushing, grafting is not always very successful. There is also possibility that some scion/stock combinations may be incompatible. However, approach grafting is generally successful. Side grafting has been reported to be successful up to 80% in Israel (Kadman and Slor 1974). Durian
Seed propagation
Seeds germinate within three days after sowing and there is no dormancy. Percentage of germination reduces drastically when seed moisture level depletes beyond 22%. Seedlings take longer time (8-15 years) for fruiting.
Vegetative propagation
Budding, grafting, air-layering or even cuttings are the vegetative propagation methods followed. However, whip grafting or patch budding are the most commercially used propagation methods. Better results of grafting/budding are obtained if rootstock of same species is used. Grafted or budded plants come to fruiting in less than 4-5 years. Mangosteen
Seed Propagation
The best known practice for the propagation of mangosteen is by seeds and when fresh seeds are sown, they germinate in 10 to 15 days. Since the seeds are of asexual origin, they produce trees identical to the mother. If the seed is dried or kept outside the fruit for several days before planting, germination is drastically reduced. However, the seeds can be maintained for 3 to 4 weeks within the fruit. The best way to carry seeds is by transporting the entire fruit. The size of the seeds is highly variable. The difference in seed weight brings variation not only in germination but also in later growth and survival. The ability to germinate and grow successfully is related to the amount of food stored in the seed. Therefore, it is recommended to establish new plantings only from the larger seeds, those that weigh 1 g or more.
Vegetative propagation
Many attempts have been made to develop stronger, more rapidly growing mangoseen trees with a short juvenile phase through cutting, air-layering, Forkert budding, approach grafting and cleft grafting but the results were not promising (Campbell 1966). All the propagation methods tried did not show any real advantage over the seed propagation method.
110
TROPICAL FRUITS IN ASIA: CONSERVATION AND USE
References
Abutiate, W.S., and H.Y. Nasasone. 1972. Studies of vegetative propagation of the lychee (Litchi chinensis Sonn.) with special reference to grafting. Ghana J. Agric. Sc. 51:202-212. Amin, R.S. 1974. Softwood grafting - A new technique for hardwood plants. Curr. Sci. 47:468-469. Amin, R.S. 1978. In situ softwood grafting in mango. Indian J. Hort. 35:105-108 Bajpai, P.N., R.K. Trivedi and A. Prasad. 1963. Storage of citrus seeds. Sci. & Cult. 29:45-46. Bajwa, G.S., Gurcharan Singh, A.S. Sandhu and H.N. Khajuria. 1977. Rooting of sweet lime (Citrus limettioides Tanaka) cutting as affected by the type of cut and indole butyric acid concentrations. Haryana J. Hort. Sci. 6:115-116. Bid, N.N. and S.K. Mukherjee. 1969. Varietal response to etiolation and growth regulator treatment in air layering of mango. Indian J Agric. Sci. 30:1013-1019. Bid, N.N. and S.K. Mukherjee. 1972. Studies into the effect of forced shoot etiolation and different media on the rootage of Mangifera indica L. cuttings. Acta Horticulturae 24:77-81. Bose, T.K. 1986. Jackfruit. Pp. 346-347 in Propagation of Tropical and Subtropical Horticultural Crops (T.K. Bose, S.K. Mitra and M.K. Sadhu, eds.). Naya Prokash, Calcutta, India. Campbell, Carl. W. 1966. Growing the mangosteen in sourthern Florida. Fla. State Hortic. Soc. 79:399-400. Chatterjee, B.K. and S.K. Mukherjee. 1980a. A note on the effect of leafy and non-leafy cuttings on rooting of jackfruit (Artocarpus heterophyllus Lam.). Progressive Hort. 11:49-51. Chatterjee, B.K. and S.K. Mukherjee. 1980b. Stooling potentiality of clones of jackfruit (Artocarpus heterophyllus Lam.). Sci. & Cult. 46:55-56. Chin, H.F. 1975. Germination and storage of rambutan (Nephelium lappaceum L.) seeds. Malaysian Agric. Res. 4:173-180. Chhonkar, V.S. and R.K. Singh. 1972. Propagation of Mangifera indica L. by air-layering. Acta Horticulturae 24:89-92. Dhatt, A.S. and Zora Singh. 1993. Propagation and rootstocks of citrus. Pp. 523-550. In Advances in Horticulture, Vol. 2 - Fruit Crops: Part 2 (K.L. Chadha and O.P. Pareek, eds.). Malhotra Publishing House, New Delhi. Dhua, R.S., S.K. Sen and T.K. Bose. 1983. Propagation of jackfruit (Artocarpus heterophyllus Lam.) by stem cuttings. Punjab Hort. J. 23:84-91. Doijode, S.D. 1989. Ex situ conservation of mango germplasm. 3rd International Mango Symposium, Darwin, Australia, 25-29 September 1989. Gangwar, R.P. and S.N. Singh. 1965. Effect of type of wood, season of planting and plant growth regulators on the propagation of sweet lime (Citrus limettioides Tom.) through stem cuttings. Trop. Agriculturist 121:55-62. Gopalakrishna, N. and Y.N. Kunte. 1958. Resume of research of Nagpur mandarin orange and need for its intensification in the development of citrus industry in the Vidarbha region. Poona. Agric. Coll. Mag. 49:19-25. Gunjate, R.T., A.S. Uradya and V.P. Limaye. 1976. Effect of season and defoliation of scion shoots on success in veneer grafting in Alphonso mango. J. Maharashtra Agric. Univ. 1:293-295. Gunjate, R.T., D.D. Dhakal and V.P. Limaye. 1982. Stone grafting on mango under the Konkan conditions. Indian J. Hort. 39:45-49. Kadman, A., and E. Slor. 1974. Experiments with propagation of lychee (Litchi chinensis) in Israel. Indian J. Hort. 31:28-33. Krishna, M.R.P. and V. Shankar. 1974. Studies on the longevity of citrus seeds under various storage conditions. Plant Sci. 6:103-104. Kumar, B.P. and A.M. Majumdar. 1973. A note on the viable period of kagzi lime seeds (Citrus aurantifolia L.). Lal Bagh Bull. 18:46-47. Majumdar, P.K. and S.K. Mukherjee. 1961. A note on the propagation of rootstocks in mango. Indian J. Hort. 18:167-168 Menzel, C.M. 1991. Litchi chinenesis Sonn. Pp. 191-195 in Plant Resources of South-East Asia. 2. Edible Fruits and Nuts (E.W.M. Verheij and R.E. Coronel, eds.). PUDOC. Wageningen, The Netherlands.
PROPAGATION TECHNIQUES FOR TROPICAL FRUITS
111
Mishra, R.S., S.B. Singh and D.N. Awasthi. 1982. Effect of plant growth regulators and ascorbic acid on germination and growth of Malta Common seedling (Citrus sinensis Osbeck) in Garhwal Hills. Prog. Hort. 14:165-168. Motial, V.S. 1963. Polyembryonic studies in some of the limes, lemons and other citrus rootstock varieties. Sci. & Cult. 29:79-84. Mukherjee, S.K., P.K. Majumdar and A.M. Goswami. 1966. Effect of the position of the shoots on the rooting of mango cuttings. Sci. & Cult. 32:377-378. Mukherjee, S.K. and B.K. Chatterjee. 1978. Effect of vigoration, etiolation and IBA on the rootage of jackfruit (Artocarpus heterophyllus Lam.) cutting. Sci. & Cult. 44:414-415. Mukherjee, S.K. and B.K. Chatterjee. 1979. Effect of forcing, etiolation and indole butryic acid on rooting of cuttings of Artocarpus heterophyllus Lam. Scientia Hort. 10:295-300. Naik, K.C. 1939. Some citrus nursery technique trials at the Fruit Research Station, Anantharajupet, Madras Presidency. Indian J. Agric. Sci. 9:651-673. Patil, R.D., R.T. Gunjate and M.J. Salvi. 1986. Effect of storage conditions on viability of mango stones. J. Maharashtra Agric. Univ. 11:362. Paxton, B.F., J. Saranah and K.R. Chapman. 1978. The propagation of lychee by cutting. Biennial Rep. Maroochy Hort. Res. Sta., Queensland, Australia 1:34. Prasad, M.V.N.V. and H. Ravishankar. 1983. Studies on polyembryony and seed morphology in some trifoliate orange hybrids. South Indian Hort. 31:101-103. Rajan, S. and S. Ram. 1983. Some factors affecting root regeneration in mango cuttings in mist and hot bed. Prog. Hort. 15:11-16. Reddy, Y.N. and P.K. Majumdar. 1975. Bottom heat - New technique for rooting hardwood cuttings of tropical fruits. Curr. Sci. 44:444-445. Reddy, K.M. and R.N. Singh. 1987. Propagation of mango by cutting. J. Res. APAU. 15:14-30. Sachar, R.C. and R.N. Chopra. 1957. A study of the endosperm and embryo in Mangifera. Indian J. Agric. Sci. 27:219-228. Sadhu, M.K., S. Bose and L. Shah. 1978. Auxin synergists in rooting of mango cuttings. Scientia Hort. 9:381-387. Sadhu, M.K. 1986. Pp. 124. in Propogation of Tropical and Sub-tropical Horticultural Crops (T.K. Bose, S.K. Mitra and M.K. Sadhu, eds.). Naya Prokash, Calcutta, India. Sadhu, M.K. and T.K. Bose. 1986. Effect of pretreatment of stock plants of mango with different growth regulating chemicals on rooting of cutting. Hort. Sci. 21:110-111. Shanmugavelu, K.G. 1971. Effect of plant growth regulators on jack (Artocarpus heterophyllus Lam.). Madras Agric. J. 58:97-103. Shankar, G. 1965. Response of some species of citrus to air layering. Allahabad Farmer 39:34-35. Shant, P.S. and S.N. Rao. 1973. Note on the effect of gibberellic acid on seed germination and seedling growth of acid lime (C. aurantifolia Swingle). Prog. Hort. 5:63-65. Sharma, K.K. and R.C. Sharma. 1986. Bud take and growth in Kinnow mandarin influenced by type of bud wood. Haryana J. Hort. Sci. 15:57-58. Singh, S.N. 1955. Studies on rootage of plants in relation to hormones V. Citrus fruits. Sci. & Cult. 21:206-208. Singh, L.B. and R.N. Singh. 1955. Studies in necellar seedlings of some common rootstocks for citrus. Indian J. Hort. 12:53-57. Singh, A. and D.P. Chopra. 1962. Quick evaluation of citrus seed viability by tetrazolium test. Indian J. Hort. 19:117-119. Singh, U.P. 1965. Identification of polyembryonic seeds of Citrus and Poncirus species with the help of some morphological characteristies. Indian J. Hort. 22:277-282. Singh, R., S.K. Saxena and V.P. Sharma. 1970. An improved method of raising citrus rootstocks. Punjab. Hort. J. 10:166-171. Singh, U.P. 1971. Nucellar embryony in citrus. Indian J. Hort. 28:117-122. Singh, S.M. and T. Kaisuwan. 1971. Effect of interval between extaction and sowing and container for storage on the germination of seedstock and growth of seedlings of mango. Allahabad Farmer 45:287-290.
112
TROPICAL FRUITS IN ASIA: CONSERVATION AND USE
Singh, U.R., I.C. Pandey, N.P. Upadhyay and R.S. Prasad. 1982. Propagation of jackfruit by budding. Punjab Hort. J. 22:103-105. Singh, N.P. and R.P. Srivastava. 1982. Clonal propagation of mango rootstock by stooling. Prog. Hort. 14:162-164. Singh, M., G.N. Singh, S.N. Singh and B.N. Singh. 1989. Effect of gibberellic acid on seed germination in Mosambi (Citrus sinensis Osbeck.). Haryana J. Hort. Sci. 18:29-33. Srivastava, K.P. and S.K. Arya. 1969. Effect of wrapping material and time of budding on percentage of bud take of Mosambi (Citrus sinensis Osbeck.). Allahabad Farmer 43:241-243. Stevenson, S.A. 1947. The technique of budding in citrus. Nagpur Agri. Coll. Mag. 21:60-66. Teaotia, S.S. and R.D. Singh. 1971. Studies on media for storage and germination of mango seed stones Mangifera indica L. Punjab Hort. J. 11:52-56. Teng, Y.T. 1977. Effect of drying on the viability of rambutan and durian seeds. MARDI Res Bul. Malaysia. 5(1):111-113. Valmayor, R.V., H.L. Valmayor and L.G. Gonzalez. 1961. Rambutan varieties and culture. Coll. Agric. Univ. Philipp. Tech. Bull. 7:11-15.
You might also like
- Coconut Production Technology-SereddyDocument71 pagesCoconut Production Technology-SereddyDr.Eswara Reddy SiddareddyNo ratings yet
- Neem Cultivation-457Document9 pagesNeem Cultivation-457mandaremNo ratings yet
- Quick Book On Fruit Tree GraftingDocument17 pagesQuick Book On Fruit Tree GraftingRochana100% (1)
- L7.oil CropsDocument22 pagesL7.oil CropsMohaajanan Ali100% (1)
- St. Augustine Orchid Society: Divisions. Sympodial Orchids With Pseudobulbs (Including Cattleyas, DendrobiumsDocument5 pagesSt. Augustine Orchid Society: Divisions. Sympodial Orchids With Pseudobulbs (Including Cattleyas, Dendrobiumscarlos arturo arias arangoNo ratings yet
- Conifer GraftingDocument8 pagesConifer GraftingMisha Mrkich100% (1)
- Fruit and Nut ProductionDocument46 pagesFruit and Nut ProductionDananjaya RanasingheNo ratings yet
- Super Moringa EbookDocument43 pagesSuper Moringa Ebookanteparts100% (1)
- PapayaDocument12 pagesPapayaAssefa FikaduNo ratings yet
- Home Gardening: Bob Westerfield UGA Extension HorticulturistDocument20 pagesHome Gardening: Bob Westerfield UGA Extension HorticulturistKandice LedfordNo ratings yet
- Rootstock SynopsisDocument4 pagesRootstock SynopsisRusty SunshineNo ratings yet
- Greenhouse Salad CropsDocument64 pagesGreenhouse Salad CropsmadmossyNo ratings yet
- Pruning Fruit TreesDocument2 pagesPruning Fruit TreesMohammad Tayyab KhanNo ratings yet
- Economic Botany PresentationDocument16 pagesEconomic Botany Presentationjames allenNo ratings yet
- Plant Propagation: Adapted From Texas Master Gardener Manual Curtis W. Smith, NMSU Extension Horticulture SpecialistDocument19 pagesPlant Propagation: Adapted From Texas Master Gardener Manual Curtis W. Smith, NMSU Extension Horticulture SpecialistZachary BartonNo ratings yet
- Cucamelon Guide PDFDocument3 pagesCucamelon Guide PDFDeni AndaniiNo ratings yet
- Produce Organic Concoctions and Extracts - 2Document51 pagesProduce Organic Concoctions and Extracts - 2Allen Jade PateñaNo ratings yet
- Propagation Techniques in PepperDocument4 pagesPropagation Techniques in PepperDr.Eswara Reddy SiddareddyNo ratings yet
- How To Grow Carrots: Detailed CultivationDocument1 pageHow To Grow Carrots: Detailed CultivationAll Good Things Organic Seeds100% (1)
- BPI Orchid GuideDocument10 pagesBPI Orchid GuideHenry James SisonNo ratings yet
- Haygrove Garden Polytunnels Brochure 2011Document20 pagesHaygrove Garden Polytunnels Brochure 2011anon_550491808No ratings yet
- Sahaja Seeds - Order FormDocument33 pagesSahaja Seeds - Order Formmalayakumar67% (3)
- CITRUS PRUNING Presentation PDFDocument63 pagesCITRUS PRUNING Presentation PDFbassam el alyNo ratings yet
- Artificial Vegetative Propagation For Fruit Tree NurseryDocument27 pagesArtificial Vegetative Propagation For Fruit Tree NurseryDerrick Yson (Mangga Han)No ratings yet
- Sansevieria Cylindrica: Family: AsparagaceaeDocument4 pagesSansevieria Cylindrica: Family: AsparagaceaeEmmanuel Veloz TenebrosoNo ratings yet
- How To Grow ApplesDocument4 pagesHow To Grow ApplesOllamhaAnneNo ratings yet
- Pruning & Training The OrchardDocument14 pagesPruning & Training The OrchardFlorin DanNo ratings yet
- CarobDocument92 pagesCarobJaga Deepan100% (1)
- Basics For Pruning Trees 10Document2 pagesBasics For Pruning Trees 10Mcedisi NkomazanaNo ratings yet
- The Cost of Producing Potted Orchids Hawaiian Agricultural ProductsDocument4 pagesThe Cost of Producing Potted Orchids Hawaiian Agricultural ProductsStefana LnNo ratings yet
- Put More Fruit On Your TreesDocument7 pagesPut More Fruit On Your TreesRosalie Tilos OritoNo ratings yet
- Production of Various Concoctions and ExtractsDocument20 pagesProduction of Various Concoctions and Extractsbaitan dadigan100% (1)
- Cyclamen For The Temperate GardenDocument15 pagesCyclamen For The Temperate GardenDennis CareyNo ratings yet
- Peppers in The Garden PDFDocument4 pagesPeppers in The Garden PDFTafara GwarindaNo ratings yet
- How To Grow Mango Tree in PotDocument5 pagesHow To Grow Mango Tree in Potsupermanedit100% (1)
- Kigelia Africana IntDocument2 pagesKigelia Africana IntMia Audina MiyanoshitaNo ratings yet
- Garden Journal 2011: Natural Products For Your Organic Home, Lawn, Garden, Farm and PetsDocument25 pagesGarden Journal 2011: Natural Products For Your Organic Home, Lawn, Garden, Farm and PetsAnne Marie DuBois Cullon100% (1)
- Pruning Ornamental Trees and Shrubs: Department of HorticultureDocument12 pagesPruning Ornamental Trees and Shrubs: Department of HorticulturegheorghiudNo ratings yet
- Major Pests of ChilliDocument6 pagesMajor Pests of ChilliNuramin Fitri Bin AminuddinNo ratings yet
- ARBICO Garden Journal 2018Document25 pagesARBICO Garden Journal 2018А.ЈМитревскиNo ratings yet
- Strawberry GrowingDocument3 pagesStrawberry GrowingjbfrostjrNo ratings yet
- Annona SquamosaDocument5 pagesAnnona SquamosaPhan Thanh TrangNo ratings yet
- Gardening For HoneybeesDocument5 pagesGardening For HoneybeesIndiana Public Media NewsNo ratings yet
- Fragrant BookDocument28 pagesFragrant BookHO YOKE YENG MoeNo ratings yet
- WatermelonDocument3 pagesWatermelonmashitiva100% (3)
- Floral Structure, Breeding and Pollination Mechanism During Seed Production in BarleyDocument23 pagesFloral Structure, Breeding and Pollination Mechanism During Seed Production in BarleyJai Prakash GuptaNo ratings yet
- Fruit Trees: Planting and Care of Young Trees: PublicationDocument5 pagesFruit Trees: Planting and Care of Young Trees: PublicationGarudaOzoNo ratings yet
- Book Cultivation CardamomDocument46 pagesBook Cultivation CardamomRamesh GowdaNo ratings yet
- Vegetable GuideDocument16 pagesVegetable GuideRaquelito Belmonte Cenal100% (2)
- Saffron Manual For AfghanistanDocument20 pagesSaffron Manual For Afghanistanksm256100% (1)
- Principles of Vegetative PropagationDocument5 pagesPrinciples of Vegetative PropagationJensenNo ratings yet
- Ammonium Nitrogen and Acidic Water For Xerophytic Plant Grow1 PDFDocument6 pagesAmmonium Nitrogen and Acidic Water For Xerophytic Plant Grow1 PDFCamilo LemusNo ratings yet
- Grafting and Budding Nursery Crop PlantsDocument20 pagesGrafting and Budding Nursery Crop PlantsArt Del R SalongaNo ratings yet
- Container Gardening ManualDocument12 pagesContainer Gardening ManualApeuDerropNo ratings yet
- 52 Profiles On Agroecology: Zero Budget Natural Farming in IndiaDocument4 pages52 Profiles On Agroecology: Zero Budget Natural Farming in Indiakeshav shishya100% (1)
- Ghar Ka Manzar Shadi Kerne Ke Baad Kya Ho Ga PDFDocument5 pagesGhar Ka Manzar Shadi Kerne Ke Baad Kya Ho Ga PDFBrij Mohan SinghNo ratings yet
- Fitness Numbers - CERG - NTNU PDFDocument3 pagesFitness Numbers - CERG - NTNU PDFBrij Mohan SinghNo ratings yet
- More About HRmax - NTNU PDFDocument4 pagesMore About HRmax - NTNU PDFBrij Mohan SinghNo ratings yet
- HR Calculator - CERG - NTNU PDFDocument3 pagesHR Calculator - CERG - NTNU PDFBrij Mohan SinghNo ratings yet
- Gurus, Godmen and Good People PDFDocument10 pagesGurus, Godmen and Good People PDFBrij Mohan SinghNo ratings yet
- Swastika!: by Brian W. AldissDocument6 pagesSwastika!: by Brian W. AldissBrij Mohan SinghNo ratings yet
- Cultures of Meristems and CalliDocument4 pagesCultures of Meristems and CalliBrij Mohan SinghNo ratings yet
- From Cells To Embryos To Rooted Plantlets in A Mist BioreactorDocument9 pagesFrom Cells To Embryos To Rooted Plantlets in A Mist BioreactorBrij Mohan SinghNo ratings yet
- DactylorhizaDocument7 pagesDactylorhizaBrij Mohan SinghNo ratings yet
- Cattleya OrchidsDocument4 pagesCattleya OrchidsBrij Mohan SinghNo ratings yet
- NiazboDocument18 pagesNiazboBrij Mohan SinghNo ratings yet
- Cattleya OrchidsDocument4 pagesCattleya OrchidsBrij Mohan SinghNo ratings yet
- Method For Large Scale ProductionDocument9 pagesMethod For Large Scale ProductionMargie HallNo ratings yet
- Fungiculture (Manual Small Scale)Document86 pagesFungiculture (Manual Small Scale)Dedy Lesmana86% (7)
- Tissue Culture of Himalayan Orchids-ReviewDocument5 pagesTissue Culture of Himalayan Orchids-ReviewBrij Mohan SinghNo ratings yet
- Sequence Comparison Method of Dna PredictionDocument18 pagesSequence Comparison Method of Dna PredictionbwwcomNo ratings yet
- Dactylorhiza PaperDocument6 pagesDactylorhiza PaperBrij Mohan SinghNo ratings yet
- Ab 497 e 01Document20 pagesAb 497 e 01Brij Mohan SinghNo ratings yet
- Bioinformatics: Mark Gerstein, Yale University Bioinfo - Mbb.yale - Edu/mbb452aDocument21 pagesBioinformatics: Mark Gerstein, Yale University Bioinfo - Mbb.yale - Edu/mbb452azuhalNo ratings yet
- Dactylorhiza PaperDocument6 pagesDactylorhiza PaperBrij Mohan SinghNo ratings yet
- Organic Cultivation of GanodermaDocument0 pagesOrganic Cultivation of GanodermaBrij Mohan SinghNo ratings yet
- Amaryllis - University of FloridaDocument5 pagesAmaryllis - University of FloridaBrij Mohan SinghNo ratings yet
- Ab 497 e 02Document10 pagesAb 497 e 02Brij Mohan SinghNo ratings yet
- Genetically Modified Organisms in Food and Agriculture: Where Are We? Where Are We Going?Document7 pagesGenetically Modified Organisms in Food and Agriculture: Where Are We? Where Are We Going?Brij Mohan SinghNo ratings yet
- The Benefits and Ethical Issues Behind Using Genetically Modified Organisms in AgricultureDocument5 pagesThe Benefits and Ethical Issues Behind Using Genetically Modified Organisms in Agriculturebrijmohansingh401124No ratings yet
- Reishi MushroomDocument5 pagesReishi MushroomBrij Mohan SinghNo ratings yet
- Care and Handling of Button MushroomsDocument7 pagesCare and Handling of Button MushroomsBrij Mohan Singh100% (1)
- Indiana - GMO & EquityDocument28 pagesIndiana - GMO & EquityBrij Mohan SinghNo ratings yet
- Organic Cultivation of GanodermaDocument0 pagesOrganic Cultivation of GanodermaBrij Mohan SinghNo ratings yet
- A-Z of English Idioms: 150 Most Common Expressions: Quick IntroDocument16 pagesA-Z of English Idioms: 150 Most Common Expressions: Quick IntroDaphnie Joy De la CruzNo ratings yet
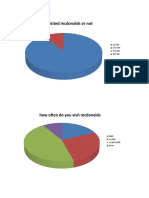
- Analysis Mcdonalds SurveyDocument8 pagesAnalysis Mcdonalds SurveySanshu KhuranaNo ratings yet
- Rheological Properties of Whey Protein Isolate Stabilized EmulsionsDocument6 pagesRheological Properties of Whey Protein Isolate Stabilized EmulsionscunmaikhanhNo ratings yet
- Modul 2 Add Maths 2016 (JPPP)Document18 pagesModul 2 Add Maths 2016 (JPPP)Tang Sok MinNo ratings yet
- 6C Uh BingDocument7 pages6C Uh BingMulik AzizahNo ratings yet
- List of Harmful E NumbersDocument33 pagesList of Harmful E Numberslumidee89No ratings yet
- Design & Layout of Foodservice FacilitiesDocument33 pagesDesign & Layout of Foodservice Facilitiesmanishpandey1972100% (2)
- Servicio Nacional de Aprendizaje Sena Worksheet No 1 - EnglishDocument14 pagesServicio Nacional de Aprendizaje Sena Worksheet No 1 - EnglishNicole MartínezNo ratings yet
- BioZone Training - Application N ProjectsDocument72 pagesBioZone Training - Application N Projectsthaitruong26No ratings yet
- Frozen ProductsDocument14 pagesFrozen ProductsSteffi ClaroNo ratings yet
- NCP About Fluid IntakeDocument5 pagesNCP About Fluid IntakeWild Rose100% (1)
- Dpr-Srikanth Reddy - Polyhouse With SubsidyDocument23 pagesDpr-Srikanth Reddy - Polyhouse With SubsidyGenesisGenesis75% (4)
- Fertilizers Storage and Handling: Indian Perspective: Fertilizer & AgricultureDocument4 pagesFertilizers Storage and Handling: Indian Perspective: Fertilizer & AgriculturenovinabsNo ratings yet
- Country Report Aceh Tengah RegencyDocument4 pagesCountry Report Aceh Tengah RegencyZahara ZakariaNo ratings yet
- Globetrotter Botswana Travel PackDocument25 pagesGlobetrotter Botswana Travel PackMapStudioNo ratings yet
- bài tập tiếng anhDocument27 pagesbài tập tiếng anh11A4 LTT-Hồ Gia BảoNo ratings yet
- Hyundai Global Snap On Epc 09 2021 Spare Parts CatalogDocument22 pagesHyundai Global Snap On Epc 09 2021 Spare Parts Catalogwalterwatts010985wqa100% (137)
- Reproductive Performance and Blood Constituents of Damascus Goats As Affected by YeastDocument18 pagesReproductive Performance and Blood Constituents of Damascus Goats As Affected by YeastRaj CellaNo ratings yet
- TurkeysDocument2 pagesTurkeysVegan FutureNo ratings yet
- Lecheroons (No Oven Needed) : I Bought It in SM Supermarket (Baking Supply Section)Document8 pagesLecheroons (No Oven Needed) : I Bought It in SM Supermarket (Baking Supply Section)Van Aero VacioNo ratings yet
- Ôn Tập Thi Học Kì 2 Anh Văn 8 Theo DangDocument12 pagesÔn Tập Thi Học Kì 2 Anh Văn 8 Theo DangVan DoNo ratings yet
- Arby'S Menu: Limited Time Offers Chicken Friends of MeatDocument1 pageArby'S Menu: Limited Time Offers Chicken Friends of MeatMNo ratings yet
- Filipino Food Court Interior DesignDocument2 pagesFilipino Food Court Interior DesignJohn Paul S. DucusinNo ratings yet
- ChicoryDocument9 pagesChicorySkriikkNo ratings yet
- Writing Practice My Day Worksheet PDFDocument2 pagesWriting Practice My Day Worksheet PDFnazhuda0% (1)
- Basic English Grammar - Have, Has, Had - Lesson NotesDocument4 pagesBasic English Grammar - Have, Has, Had - Lesson NotesAkhil P SudhakaranNo ratings yet
- Beginner Unit 11a (2) (2) ResueltoDocument3 pagesBeginner Unit 11a (2) (2) ResueltoCarlos Fabian LopezNo ratings yet
- English - II FinalDocument31 pagesEnglish - II FinalNaeem Akhtar0% (1)
- Procedure Text How To Make Ice CreamDocument6 pagesProcedure Text How To Make Ice CreamIrma Triyani Yahya100% (1)
- Research-Plan Aloe Vera ExtractDocument42 pagesResearch-Plan Aloe Vera ExtractKrishan Carlos CredoNo ratings yet
- ChatGPT Money Machine 2024 - The Ultimate Chatbot Cheat Sheet to Go From Clueless Noob to Prompt Prodigy Fast! Complete AI Beginner’s Course to Catch the GPT Gold Rush Before It Leaves You BehindFrom EverandChatGPT Money Machine 2024 - The Ultimate Chatbot Cheat Sheet to Go From Clueless Noob to Prompt Prodigy Fast! Complete AI Beginner’s Course to Catch the GPT Gold Rush Before It Leaves You BehindNo ratings yet
- The Fabric of Civilization: How Textiles Made the WorldFrom EverandThe Fabric of Civilization: How Textiles Made the WorldRating: 4.5 out of 5 stars4.5/5 (58)
- Hero Found: The Greatest POW Escape of the Vietnam WarFrom EverandHero Found: The Greatest POW Escape of the Vietnam WarRating: 4 out of 5 stars4/5 (19)
- Sully: The Untold Story Behind the Miracle on the HudsonFrom EverandSully: The Untold Story Behind the Miracle on the HudsonRating: 4 out of 5 stars4/5 (103)
- The End of Craving: Recovering the Lost Wisdom of Eating WellFrom EverandThe End of Craving: Recovering the Lost Wisdom of Eating WellRating: 4.5 out of 5 stars4.5/5 (81)
- Transformed: Moving to the Product Operating ModelFrom EverandTransformed: Moving to the Product Operating ModelRating: 4 out of 5 stars4/5 (1)
- Reality+: Virtual Worlds and the Problems of PhilosophyFrom EverandReality+: Virtual Worlds and the Problems of PhilosophyRating: 4 out of 5 stars4/5 (24)
- Faster: How a Jewish Driver, an American Heiress, and a Legendary Car Beat Hitler's BestFrom EverandFaster: How a Jewish Driver, an American Heiress, and a Legendary Car Beat Hitler's BestRating: 4 out of 5 stars4/5 (28)
- Pale Blue Dot: A Vision of the Human Future in SpaceFrom EverandPale Blue Dot: A Vision of the Human Future in SpaceRating: 4.5 out of 5 stars4.5/5 (588)
- The Intel Trinity: How Robert Noyce, Gordon Moore, and Andy Grove Built the World's Most Important CompanyFrom EverandThe Intel Trinity: How Robert Noyce, Gordon Moore, and Andy Grove Built the World's Most Important CompanyNo ratings yet
- Packing for Mars: The Curious Science of Life in the VoidFrom EverandPacking for Mars: The Curious Science of Life in the VoidRating: 4 out of 5 stars4/5 (1395)
- The Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaFrom EverandThe Beekeeper's Lament: How One Man and Half a Billion Honey Bees Help Feed AmericaNo ratings yet
- How to Build a Car: The Autobiography of the World’s Greatest Formula 1 DesignerFrom EverandHow to Build a Car: The Autobiography of the World’s Greatest Formula 1 DesignerRating: 4.5 out of 5 stars4.5/5 (54)
- A Place of My Own: The Architecture of DaydreamsFrom EverandA Place of My Own: The Architecture of DaydreamsRating: 4 out of 5 stars4/5 (242)
- The Future of Geography: How the Competition in Space Will Change Our WorldFrom EverandThe Future of Geography: How the Competition in Space Will Change Our WorldRating: 4 out of 5 stars4/5 (5)
- How to Build a Car: The Autobiography of the World’s Greatest Formula 1 DesignerFrom EverandHow to Build a Car: The Autobiography of the World’s Greatest Formula 1 DesignerRating: 4.5 out of 5 stars4.5/5 (122)
- The Weather Machine: A Journey Inside the ForecastFrom EverandThe Weather Machine: A Journey Inside the ForecastRating: 3.5 out of 5 stars3.5/5 (31)
- Broken Money: Why Our Financial System is Failing Us and How We Can Make it BetterFrom EverandBroken Money: Why Our Financial System is Failing Us and How We Can Make it BetterRating: 5 out of 5 stars5/5 (3)
- The Things We Make: The Unknown History of Invention from Cathedrals to Soda CansFrom EverandThe Things We Make: The Unknown History of Invention from Cathedrals to Soda CansNo ratings yet
- Dirt to Soil: One Family’s Journey into Regenerative AgricultureFrom EverandDirt to Soil: One Family’s Journey into Regenerative AgricultureRating: 5 out of 5 stars5/5 (125)
- The Technology Trap: Capital, Labor, and Power in the Age of AutomationFrom EverandThe Technology Trap: Capital, Labor, and Power in the Age of AutomationRating: 4.5 out of 5 stars4.5/5 (46)
- Data-ism: The Revolution Transforming Decision Making, Consumer Behavior, and Almost Everything ElseFrom EverandData-ism: The Revolution Transforming Decision Making, Consumer Behavior, and Almost Everything ElseRating: 3.5 out of 5 stars3.5/5 (12)