Professional Documents
Culture Documents
Fesi Transtition
Uploaded by
Abie RexoMenOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Fesi Transtition
Uploaded by
Abie RexoMenCopyright:
Available Formats
Applied Surface Science 70/71 (1993) 578-582 North-Holland
applied surface science
Structural phase transition during heteroepitaxial growth of iron silicides on Si(lll)
J. A l v a r e z , A.L. Vflzquez d e P a r g a , J.J. H i n a r e j o s , J. d e la F i g u e r a , E . G . Michel, C. Ocal a n d R. M i r a n d a
Departamento de Fisica de la Materia Condensada, Universidad Autdnoma de Madrid, 28049 Madrid, Spain
Received 4 September 1992; accepted for publication 11 October 1992
We report here on a novel metal-semiconductor phase transition in FeSiz, induced by epitaxy on Si(111). The transition occurs at constant temperature as a function of the thickness of a silicide film with constant composition, epitaxially grown on Si(lll). The phase transition consists in a change of the silicide structure from y-FeSi2, a metallic phase with the highly symmetric fluorite structure, to/3-FeSi 2, a semiconducting phase with the low symmetry orthorhombic structure.
The study of metastable, crystalline phases has attracted much attention recently. This has been exemplified by the renewed interest in metastable magnetic films such as fcc Co [1] or bcc Co [2]. These phases have been stabilized at room temperature by epitaxial growth onto suitable substrates. Their magnetic properties are affected because the structural energy difference between the different phases is of the same order of magnitude as the magnetic energy difference. However, the difference in structural energy between compact structures such as fcc and hcp Co is rather small and both are metals. A more radical structural phase transition (e.g. from cubic diamond to distorted orthorhombic) may involve larger differences in energy, allowing for a more severe change in properties, for example from a metal to a semiconductor. One such case is the semiconductor Si which converts at high hydrostatic pressure (11.3-12.5 GPa) or close to plastic indentations into a metal with the/3-tin structure [31. Disilicides formed by the magnetic metals Co and Ni (CoSia, NiSi 2) crystallize in the fluorite structure [4]. One may expect that the third member of the family, namely FeSi 2, would also crystallize in the fluorite structure. The F e - S i bulk
phase diagram, however, shows that such a phase does not exist at ambient pressure. The electronic structure of an hypothetical phase with a FeSi 2 composition and a fluorite crystalline structure has been calculated by Christensen [5]. According to his calculation it would be a metallic compound with high density of states (DOS) at the Fermi level. Therefore, it would be prone to structural phase transitions diminishing the energy associated with a high DOS at E F. In fact, nature shows that the phase stable at R T and atmospheric pressure is /3-FeSi2, a semiconducting phase with an orthorhombic structure (a = 9.86 A, b = 7.79 ,~, c = 7.83 A), which may be considered as a distorted fluorite structure [6]. Therefore, its total energy must be lower than that of y-FeSi 2. The calculations indicate that Yo-FeSi2 would have a lattice constant of a = 5.389 A [5]. The low mismatch with the lattice parameter of Si (5.43 .~) suggests that the gamma phase could be stabilized by epitaxial growth on Si(111), specially if one considers that a large interfacial energy must be associated with the important lattice mismatch of/3-FeSi 2 with Si(lll). In fact, Onda et al. [7] identified for the first time y-FeSi2 as a thin film grown On Si(111). They reported an irreversible phase transition to the /3-phase in-
0169-4332/93/$06.00 1993 - Elsevier Science Publishers B.V. All rights reserved
J. Alvarez et al. / Heteroepitaxial growth o f iron silicides on Si(l l l)
579
duced by thermal annealing [7], proving that the fluorite structure of FeSi 2 was indeed metastable. Accordingly, y-FeSi 2 must lie at a local minimum of the total energy, higher, however, than the one corresponding to fl-FeSi 2. During epitaxial growth of y-FeSi2, each additional layer should add some excess energy (with respect to/3-FeSi 2) in such a way that this energy would surpass, for a given thickness, the interfacial strain energy gained. At this point the film must spontaneously convert into the more stable /3-FeSi 2 phase. We report on the first observation of this phase transition as a function of thickness at constant temperature. The experiments have been carried out in two different ultrahigh vacuum ( U H V ) chambers previously described [8,9]. As a part of an extensive research project, the F e - S i phase diagram has been explored [10] and several methods to prepare any desired iron silicide have been determined [8-14]. In particular, silicides can be grown by evaporating Fe on a heated Si substrate at a fixed temperature, selected to provide a given composition of the silicide [8,10]. The method has been termed reactive deposition epitaxy (RDE). We have demonstrated elsewhere that R D E at temperatures between 550 and 700C results in formation of compounds with FeSi 2 stoichiometry [8,12]. On the other hand, iron silicides can be p r e p a r e d by Fe evaporation at R T and annealing. This method is known as solid phase epitaxy (SPE) [13]. The u p p e r panel of fig. 1 shows the intensity ratio of F e 3 p / S i 2 p core levels measured with X-ray photoemission spectroscopy (XPS) during evaporation of Fe on S i ( l l l ) at 410C (open dots) and 665C (filled dots), respectively. The evaporation rate was in the range of 0.5-1 M L / 1 0 s. At 410C the ratio of intensities quickly saturates at a value of 0.45. Further deposition of Fe keeps the F e / S i ratio constant, indicating the formation of a stable compound with well-defined stoichiometry. The ratio of 0.45 proves that the compound formed is FeSi2, as concluded by comparison to previous calibrations [8,12]. A similar, albeit slower evolution is observed during evaporation at 665C. In spite of the fact that the chemical composition of the film does not change, the valence band DOS of the compound changes
0.5
o ' j
0.4
0.3
/.<-I
5 1~0 115 20 2'5 Iron coverage (ML) 30
0.2
0.1
3'5
4'0
.,
..,
".
.,
'.
".,
". %
,
4.5
-4
-3.5
-3
-2,5 -2 -1.5 -1 Binding Energy (eV)
-0.5
A ,".-.-.....-0 0.5
Fig. 1. T h e u p p e r p a n e l r e p r o d u c e s the r a t i o of i n t e n s i t i e s of
Fe3p and Si2p core levels (excited with MgKa radiation) during evaporation of Fe on Si(111) at 410C (open dots) and 665C (filled dots), respectively. The lower panel reproduces UPS spectra of the valence band measured at points A and B. They reflect the 2)<2-reconstructed fluorite phase (A), and the orthorhombic fl-FeSi2 phase (B), respectively.
abruptly at the thickness values indicated by the arrows. Angle-integrated ultraviolet photoemission spectroscopy (UPS) spectra taken just before (A) and after the transition (B) are reproduced in the lower panel of fig. 1. Before the transition, the spectrum (A) reflects the D O S of a metallic sample with emission at E r and a peaked maxim u m centered at 1.2 eV below E~. This spectrum is identical to the one found by us [10] and others [7] for the phase with the fluorite structure grown by SPE. The surface structure of y-FeSi 2 grown by SPE on S i ( l l l ) has been described in detail elsewhere [9]. A 2 2 surface reconstruction has been observed by means of low-energy electron
580
J. Alvarez et al. / Heteroepitaxial growth o f iron silicides on Si( l l l )
diffraction ( L E E D ) and scanning tunneling microscopy (STM) [14]. The UPS spectrum (B) after the transition, reflects a semiconducting sample p-doped as grown. Accordingly, the Fermi level is fixed to the top of the valence band. Peaks are observed at 0.8, 1.3, and 1.6 eV. This spectrum has been identified as due to/3-FeSi 2, a semiconducting phase with an orthorhombic structure [8,101. The L E E D pattern observed during R D E at 665C (after the transition), however, indicates that the obtained fl-FeSi 2 film is polycrystalline. In fact, X-ray diffraction studies of thick films p r e p a r e d by R D E at 600C confirm their polycrystalline character and show that they contain /3-FeSi2(ll0) and (101) faces with rectangular unit cells of 7.79 .~ x 12.59 A and fl-FeS!2(100) and (010) faces with square 7.79 A 7.83 A unit cells. The fraction (100)/(110) increases with the deposition temperature. At 665C, the (100) variety is predominant, while at 410C the (110) variety is more abundant [16]. Fig. 2 shows STM images recorded after R D E deposition of Fe on S i ( l l l ) at 665C, which reveal the atomic arrangement of the film under these conditions. Most of the surface is covered by crystallites having the surface structure shown in fig. 2a. It consists of a complex arrangement of atoms forming stripes
hundreds of ,~ long and 30-50 ,~ wide. Crystallites showing this kind of structures correspond most probably to the fl-FeSi z phase. Their semiconducting character is indeed confirmed by the STM images reproduced in fig. 3, recorded at the same surface location with different sample bias (in magnitude and sign). T h e r e are clear changes in the aspect of the atomic protrusions visualized. This is in agreement with the general finding that semiconducting surfaces display STM features that change notably with the sample bias, since empty and filled dangling bonds are located on different spatial positions, not necessarily related to the atomic positions [15]. As exemplified by fig. 3, the images change in this case in such a complex fashion with tunneling bias that a detailed atomic model for the surface structure of the /3-FeSi z phase has not yet been obtained. Finally, it should be mentioned that the structural phase transition reported here exhibits the familiar p h e n o m e n o n of phase coexistence, indicating that it is a first-order metastable-to-stable phase transition, similar to the 1 x 1-5 x 1 transition of clean Ir(100) [17]. The phase coexistence is proved by the STM image reproduced in fig. 2b, which shows a small crystallite (200 .~ wide) displaying the characteristic hexagonal arrangement of Si adatoms with the 2 x 2 reconstruction
Fig. 2. (a) STM image of the surface of /3-FeSi2 grown by RDE at 665C. The size of the image is 250 A x 250 .~ and it was recorded at V= - 1.32 V. The grey scale covers 3.2 A from white to black; (b) STM imageo(200 A x 200 ,~) of a crystallite of 7-FeSi 2 coexistingwith/3-FeSi 2 on a terrace of Si(111). The grey scale covers 4.0 A. The bias was 1.69 V.
J. Alvarez et al. / Heteroepitaxial growth o f iron silicides on Si(l l l)
581
Fig. 3. Sequence of constant-current STM images of the same surface position for fl-FeSi 2 grown by R D E on Si(111). T h e arrow indicates a defect that may be used to guide the eye. T h e images were recorded with sample bias of (a) 2.85 V; (b) 1.56 V; (c) 0.64 V; (d) - 1.8 V; (e) - 1.2 V and (f) - 0.53 V, respectively. T h e size of the images is 140 ,~ x 140 ,~. T h e total grey scale covers 2.0 ,~.
582
J. Alvarez et al. / Heteroepitaxial growth o f iron silicides on Si( l l l )
of y-FeSi2(lll) [9]. It constitutes a minority representation of the fluorite phase. A hint of the atomistic mechanism of the phase transition (to be reported elsewhere) is given by the noticeable increase of longitudinal defects detected on top of these crystallites [15]. In conclusion we have demonstrated that 3'FeSi 2 is metastable and that an irreversible structural phase transition occurs to the/3 phase as a function of the film thickness. Previously, this transition had only been observed upon thermal annealing [7,16]. The structural phase transition produces a metal-semiconductor transition. It involves phase coexistence and leads to a polycrystalline phase. The driving force of the transition is the reduced bulk energy of the semiconducting phase. The countervailing energy cost is associated to the larger misfit of the /3 versus the 3' phase. Financial support by the CICYT MAT 910176-CE and by ESPRIT BRA 3026 are gratefully acknowledged.
References
[1] C.M. Schneider, P. Bressler, P. Schuster, J. Kirschner, J.J. de Miguel and R. Miranda, Phys. Rev. Lett. 64 (1990) 1059. [2] G. Prinz, J. Magn. Magn. Mater. 100 (1991) 469. [3] See, for example: R.W. Cahn, Nature 357 (1992) 645. [4] H. von K~inel, R. Stalder, H. Sirringhaus, N. Onda and J. Henz, Appl. Surf. Sci. 53 (1991) 196. [5] N.E. Christensen, Phys. Rev. B 42 (1990) 7148. [6] M.C. Bost and J.E. Mahan, J. Appl. Phys. 58 (1985) 2696. [7] N. Onda et al., Helv. Phys. Acta 64 (1991) 197; N. Onda, J. Henz, E. Miiller, K. M~ider and H. von K[inel, Appl. Surf. Sci. 56-58 (1992) 421. [8] J. Alvarez, J.J. Hinarejos, E.G. Michel, G.R. Castro and R. Miranda, Phys. Rev. B 45 (1992) 14042. [9] A.L. V~izquez de Parga, J. de la Figuera, C. Ocal and R. Miranda, Europhys. Lett. 18 (1992) 595. [10] J. Alvarez, J.J. Hinarejos, E.G. Michel and R. Miranda, Surf. Sci. 287/288 (1993) 490. [11] J. Alvarez, J.J. Hinarejos, E.G. Michel, J.M. Gallego, A.L. Vfizquez de Parga, J. de la Figuera, C. Ocal and R. Miranda, Appl. Phys. Lett. 53 (1991) 99. [12] J. Alvarez, J.J. Hinarejos, E.G. Michel and R. Miranda, Surf. Sci. 269/270 (1992) 1011. [13] J.M. Gallego and R. Miranda, J. Appl. Phys. 69 (1991) 1377. [14] A.L. Vfizquez de Parga, J. de la Figuera, C. Ocal and R. Miranda, Ultramicroscopy 42-44 (1992) 845. [15] A.L. Vfizquez de Parga, PhD Thesis, Univ. Aut6noma de Madrid, 1992. [16] J. Derrien, private comunication. [17] K. Heinz, G. Schmidt, L. Hammer and K. Miiller, Phys. Rev. B 32 (1985) 6214.
You might also like
- Chapter 1. Materials For EngineeringDocument30 pagesChapter 1. Materials For EngineeringAbie RexoMenNo ratings yet
- Introduction To Welding: Welding Principles and ApplicationsDocument14 pagesIntroduction To Welding: Welding Principles and ApplicationsAbie RexoMenNo ratings yet
- Fusion Welding Process, Chapter 27Document24 pagesFusion Welding Process, Chapter 27Abie RexoMen100% (1)
- Final Automated Welding PresentationDocument26 pagesFinal Automated Welding PresentationAbie RexoMenNo ratings yet
- SRB Test InstructionsDocument1 pageSRB Test InstructionsAbie RexoMenNo ratings yet
- Chapter 2 Arc WeldingDocument39 pagesChapter 2 Arc WeldingAbie RexoMen0% (1)
- Metal AlloysDocument31 pagesMetal AlloysAbie RexoMenNo ratings yet
- Brazing: AG 221 - Metals and WeldingDocument18 pagesBrazing: AG 221 - Metals and WeldingAbie RexoMenNo ratings yet
- Vacuum Requirements For Steel DegassingDocument6 pagesVacuum Requirements For Steel DegassingAbie RexoMenNo ratings yet
- Saddle AnalysisDocument17 pagesSaddle AnalysisAntonio Perez Zornoza100% (1)
- Liquid Coatings For Girthwelds and Joints Proven Corrosion Protection Protection For PipelinesDocument15 pagesLiquid Coatings For Girthwelds and Joints Proven Corrosion Protection Protection For PipelinesAbie RexoMenNo ratings yet
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5795)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1091)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- FM Definition & Function (Full)Document15 pagesFM Definition & Function (Full)Nurul Arafah IshakNo ratings yet
- Transferring A 38DLP - FTP Format File Using 2GB MicroSD CardDocument5 pagesTransferring A 38DLP - FTP Format File Using 2GB MicroSD CardBoulHich BoulHichNo ratings yet
- Trade Life Cycle1Document2 pagesTrade Life Cycle1ezefatNo ratings yet
- Paytm Software Requirement Specification SrsDocument14 pagesPaytm Software Requirement Specification Srscandy ramosNo ratings yet
- Ancient's TimelineDocument103 pagesAncient's TimelineVarga NándorNo ratings yet
- Quick StartDocument82 pagesQuick StartMạnh Hùng NguyễnNo ratings yet
- PresuppositionDocument8 pagesPresuppositionWIWID SUTANTI ALFAJRINNo ratings yet
- KaphDocument7 pagesKaphFrater MagusNo ratings yet
- 1st PU Physics Model QP 1 PDFDocument14 pages1st PU Physics Model QP 1 PDFPrasad C M71% (7)
- Induction Heating For ForgingDocument8 pagesInduction Heating For ForgingFahad NaseemNo ratings yet
- New Technology: Single Sign On (SSO) Employee PortalDocument6 pagesNew Technology: Single Sign On (SSO) Employee PortalDerek HuiNo ratings yet
- The Monitor HypothesisDocument6 pagesThe Monitor HypothesisJosé Henríquez GalánNo ratings yet
- Soil Survey Mapping - LectureNotes2Document132 pagesSoil Survey Mapping - LectureNotes2Udaya KumarNo ratings yet
- Risk Assessment For HouseDocument3 pagesRisk Assessment For HouseaishahkhanNo ratings yet
- Kingdom of SiththarsDocument7 pagesKingdom of Siththarsutcm77No ratings yet
- BIC3313-Internet Payment Systems-Assignment 1Document2 pagesBIC3313-Internet Payment Systems-Assignment 1Borotho MolemoNo ratings yet
- Controversies On The Origin of Life PDFDocument10 pagesControversies On The Origin of Life PDFMarvin OlidNo ratings yet
- يَا نَبِي سَلَام عَلَيْكَDocument1 pageيَا نَبِي سَلَام عَلَيْكَAcengMNasruddinNo ratings yet
- 100 Ab Workouts by DarebeeDocument40 pages100 Ab Workouts by DarebeeTurtle HermitNo ratings yet
- Using Bapi in LSMWDocument17 pagesUsing Bapi in LSMWapi-3731371100% (2)
- SAMI-using FibermetDocument15 pagesSAMI-using FibermetAnonymous MwAZh7ripLNo ratings yet
- Record & Field Mapping For Fusion ReportsDocument6 pagesRecord & Field Mapping For Fusion ReportsRajiNo ratings yet
- Semantics and GrammarDocument12 pagesSemantics and GrammarYara TarekNo ratings yet
- Chemicals Zetag MSDS Inverse Emulsion Zetag 8857 FSB - 0510Document6 pagesChemicals Zetag MSDS Inverse Emulsion Zetag 8857 FSB - 0510PromagEnviro.comNo ratings yet
- COBOL SyllalbusDocument10 pagesCOBOL SyllalbusKiranNo ratings yet
- Maths 1º ESODocument206 pagesMaths 1º ESONuria Martinez GomezNo ratings yet
- Heli FlexDocument5 pagesHeli Flexdmepdo100% (2)
- DisksDocument3 pagesDisksYogesh C GangadharanNo ratings yet
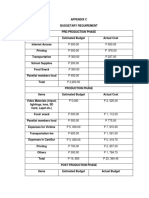
- Other AppendicesDocument9 pagesOther Appendicesbeverly bombitaNo ratings yet