Professional Documents
Culture Documents
CO2 Production
Uploaded by
Ghufran SaeedOriginal Description:
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
CO2 Production
Uploaded by
Ghufran SaeedCopyright:
Available Formats
PRODUCTON OF CARBON DOXDE FROM FUEL OL
PREFACE
Project report espouses the humble efforts of team
which is more inspired than equipped. It covers the design
aspects of Production of Carbon Dioxide from Fuel il!.
Carbon Dioxide is one of the best "nown gases and has
found a variet# of its applications. $et at present it stands among
notorious chemicals due to its harmful effects on environment.
%ajor uses of carbon dioxide are in urea manufacture& enhanced
oil recover#& as a heat transfer media& as an inert environment&
refrigerant and beverages. 'his project report deals with design
calculations of () ton per da# of carbon dioxide production rate
along with their relevant aspects such as instrumentation and
control& economic viabilit# and environmental aspects.
In fact the art and the practice of design cannot be
learned from the boo"s. 'he intuition and judgment necessar# to
appl# theor# to practice will onl# come from practical experience.
*e are obliged to our teachers and especiall# our advisor whose
intellectual interaction helped us broaden our vision about
engineering and life as well.
1
PRODUCTON OF CARBON DOXDE FROM FUEL OL
INTRODUCTION
Carbon dioxide is a colorless, odorless, incombustible gas, formed during
respiration, combustion, and organic decomposition and used in food
refrigeration, carbonated beverages, inert atmospheres, fire extinguishers, and
aerosols. Also called carbonic acid gas.
carbon dioxide, chemical compound, CO2, a colorless, odorless,
tasteless gas that is about one and one-half times as dense as air under ordinary
conditions of temperature and pressure. t does not burn, and under normal
conditions it is stable, inert and nontoxic. t will however support combustion of
magnesium to give magnesium oxide and carbon. Although it is not a poison, it
can cause death by suffocation if inhaled in large amounts. t is a fairly stable
compound but decomposes at very high temperatures into carbon and oxygen. t
is fairly soluble in water, one volume of it dissolving in an equal volume of water
at room temperature and pressure; the resultant weakly acidic aqueous solution
is called carbonic acid. The gas is easily liquefied by compression and cooling. f
liquid carbon dioxide is quickly decompressed it rapidly expands and some of it
evaporates, removing enough heat so that the rest of it cools into solid carbon
dioxide "snow. A standard test for the presence of carbon dioxide is its reaction
with limewater (a saturated water solution of calcium hydroxide) to form a milky-
white precipitate of calcium hydroxide.
History
The existence of carbon dioxide has been known since primitive times PLNY
wrote of lethal vapors from caverns in the first century A.D. t was first
recognized as a distinct gas, however, by VAN HELMONT (1577-1644), who
prepared the gas by various routes and demonstrated that it was the same
gas as that which issued from caverns and mines. He obtained it by fer-
2
PRODUCTON OF CARBON DOXDE FROM FUEL OL
mentation, by the action of acids on carbonates, and by the burning of
charcoal, and he studied many of its properties. Soon after HOFFMAN, while
investigating gas escaping from effervescent mineral waters, observed that
the gas was acidic by its effect on blue vegetable coloring materials. BLACK,
in 1757, showed that during respiration part of the atmospheric air was
chemically changed, and discovered the lethal effect such "fixed air" had on
animal life. CAVENDSH made various observations on the chemical properties
of "fixed air" and PRESTLEY, who lived near a brewery, made further
observations on the physical properties of the gas, finding that pressure
favored dissolution of the gas in water. The French chemist LAVOSER was the
first to prove the composition of "fixed air" by showing that it was produced
when carbon is heated in oxygen. The resultant gas, which he named
carbonic acid, was found to contain 23.5-28.9 parts by mass carbon and
71.1 -76.5 parts by mass oxygen.
The commercial exploitation of carbon dioxide began
with attempts to produce artificial mineral waters (solutions of CO
2
in water),
which were thought to have medicinal properties. The industrial development
of the gas really began, however, with the experiments of FARADAY on the
liquefaction of gases. He succeeded in liquefying CO
2
in a bent glass tube.
THLO RER repeated these experiments on a large scale. Two iron retorts
connected by a flexible coupling were used; the first contained a reaction
mixture of sodium hydrogen carbonate and sulfuric acid. This was agitated, and
the carbon dioxide thus produced was liquefied under pressure in the second
retort. Despite a spectacular explosion, which killed his assistant, THLO RER
succeeded in carrying out extensive experiments on the expansion, vapor
pressure, density, and enthalpy changes of the liquid CO
2
during evaporation.
He was also the first experimenter to produce solid carbon dioxide, which, on
the evaporation of liquid carbon dioxide, appeared as a white, flocculant, easily
3
PRODUCTON OF CARBON DOXDE FROM FUEL OL
compressible mass. He observed that it disappeared by slow evaporation
without first melting to form a liquid (i.e., it sublimed).
n 1845, FARADAY made larger quantities of liquid and
solid carbon dioxide by means of a hydraulic pump. He also used solid carbon
dioxide, in a mixture with ether, as a refrigerant for use in further gas liquefaction
studies. Soon after, NATTERER developed a mechanical compressor (a forerunner
of the modern multistage type), which was used to make liquid carbon di
oxide . As uses for the new liquid were proposed, compressors were
developed to produce greater quantities. The first factory for the production of
liquid carbon dioxide was established by RAYDT in Germany in 1884, and the
development of the industry continued as more uses were found . n particular,
its uses in ice-making and refrigeration and as a fire extinguisher became
important. Much carbon dioxide issued from the earth naturally in places such
as Germany. This could be used to make liquid CO
2
, but to avoid the high costs
of transporting cylinders of liquefied gas, new production methods were required.
The first facility was established in Berlin in 1889 to make carbon dioxide from
flue gases.
Since the turn of the century, a great many uses for
carbon dioxide have been identified, and several other methods of
manufacture have assumed commercial importance.
SOURCES
There are three principal commercial sources for carbon dioxide. High-purity
carbon dioxide is produced from some wells. The gas is obtained as a byproduct of
chemical manufacture, as in the fermentation of grain to make alcohol and the burning of
limestone to make lime. t is also manufactured directly by burning carbonaceous fuels.
!or commercial use it is a"ailable as a li#uid under high pressure in steel cylinders, as a
low-temperature li#uid at lower pressures, and as the solid dry ice.
$
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Properties
Name Carbon dioxide
Chemical Formula CO2
Appearance Colorless gas
Physical
Formula weight 44.0099 amu
Melting point Liquifies under high pressure
at 216 K (~57 C)
Boiling point sublimes at 195 K (~78 C)
Density 1.6 Mg/m
3
(solid)
1.98 kg/m
3
(gas at 298 K)
Solubility 0.145 g in 100 g water
Thermochemistry
AfH
0
gas ~393.52 kJ/mol
AfH
0
solid ? kJ/mol
S
0
gas, 100 kPa 213.79 J/(molK)
S
0
solid ? J/(molK)
Safety
ngestion May cause nausea, vomiting,
G hemorrhage.
nhalation Asphyxiant (suffocating),
causes hyperventilation. Repeated
exposure dangerous.
Skin Dry ice may cause frostbite.
Eyes Can lead to blindness.
Carbon dioxide is an atmospheric gas composed of one carbon and two
oxygen atoms. A very widely known chemical compound, it is frequently called by
its formula CO.
%
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Carbon dioxide results from the combustion of organic matter if sufficient
amounts of oxygen are present. t is also produced by various microorganisms
from fermentation and cellular respiration. Plants utilize carbon dioxide during
photosynthesis, using both the carbon and the oxygen to construct
carbohydrates. n addition, plants also release oxygen to the atmosphere which
is subsequently used for respiration by heterotrophic organisms, forming a cycle.
t is present in the Earth's atmosphere at a low concentration and acts as a
greenhouse gas. t is a major component of the carbon cycle.
CHE!IC"# "ND PH$SIC"# PROPERTIES
Carbon dioxide is a colorless gas which, when inhaled at high
concentrations (a dangerous activity due to the associated asphyxiation risk),
produces a sour taste in the mouth and stinging sensation in the nose and throat.
These effects result from the gas dissolving in the mucous membranes and
saliva, forming a weak solution of carbonic acid.
ts density at 25 C is 1.98 kg m
~3
, about 1.5 times that of air. The carbon
dioxide molecule (O=C=O) contains two double bonds and has a linear shape. t
has no electrical dipole. As it is fully oxidized, it is not very reactive and in
particular not flammable.
At temperatures below ~78 C, carbon dioxide condenses into a white
solid called dry ice. Liquid carbon dioxide forms only at pressures above 5.1 atm;
at atmospheric pressure, it passes directly between the gaseous and solid
phases in a process called sublimation.
Water will absorb its own volume of carbon dioxide, and more than this
under pressure. About 1% of the dissolved carbon dioxide turns into carbonic
acid. The carbonic acid in turn dissociates partly to form bicarbonate and
carbonate ions.
&
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Test For This Gas 'hen a lighted splint is inserted into a test tube containing this gas, it
is immediately extinguished, as carbon dioxide does not support combustion. To further
confirm that the gas is carbon dioxide, the gas may be bubbled into calcium hydroxide
solution. The calcium hydroxide turns milky due to the formation of calcium carbonate.
Uses
Liquid and solid carbon dioxide are important refrigerants, especially in the
food industry, where they are employed during the transportation and storage of
ice cream and other frozen foods.
Carbon dioxide is used to produce carbonated soft drinks and soda water.
Traditionally, the carbonation in beer and sparkling wine comes about through
natural fermentation, but some manufacturers carbonate these beverages
artificially.
The leavening agents used in baking produce carbon dioxide to cause
dough to rise. Baker's yeast produces carbon dioxide by fermentation within the
dough, while chemical leaveners such as baking powder and baking soda
release carbon dioxide when heated or exposed to acids.
Carbon dioxide is often used as an inexpensive, non-flammable
pressurized gas. Life jackets often contain canisters of pressured carbon dioxide
for quick inflation. Steel capsules are also sold as supplies of compressed gas for
air guns, paintball markers, and for making seltzer. Rapid vaporization of liquid
CO2 is used for blasting in coal mines.
Carbon dioxide extinguishes flames, and some fire extinguishers,
especially those designed for electrical fires, contain liquid carbon dioxide under
pressure. Carbon dioxide also finds use as an atmosphere for welding, although
in the welding arc, it reacts to oxidize most metals. Use in the automotive
industry is common despite significant evidence that welds made in carbon
(
PRODUCTON OF CARBON DOXDE FROM FUEL OL
dioxide are brittler than those made in more inert atmospheres, and that such
weld joints deteriorate over time due to the formation of carbonic acid. t is used
as a welding gas primarily because it is much less expensive than more inert
gases such as argon or helium.
Liquid carbon dioxide is a good solvent for many organic compounds. t
has begun to attract attention in the pharmaceutical and other chemical
processing industries as a less toxic alternative to more traditional solvents such
as organochlorides.
Plants require carbon dioxide to conduct photosynthesis, and
greenhouses may enrich their atmospheres with additional CO2 to boost plant
growth. t has been proposed that carbon dioxide from power generation be
bubbled into ponds to grow algae that could then be converted into bio-diesel
fuel. High level of carbon dioxide in the atmosphere effectively exterminate many
pests. Greenhouses will raise the level of CO2 to 10,000 ppm (1%) for several
hours to eliminate pests such as whitefly, spider mites, and others.
n the theater, dry ice is used to produce fog as a special effect: when dry
ice added to water, the evaporating mixture of CO2 and cold humid air condenses
as a fog.
Dry ice is also used in cleaning: shooting tiny dry ice pellets at a surface
cools the dirt and causes it to pop off.
n medicine, up to 5% of carbon dioxide is added to pure oxygen used in
medicine, for stimulation of breathing after apnea and to stabilize the O2/CO2
balance in blood.
A common type of industrial gas laser, the carbon dioxide laser, uses
carbon dioxide as a medium.
)
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Carbon dioxide is commonly injected into or adjacent to producing oil
wells. t will act as both a pressurizing agent and when dissolved into the
underground crude oil will significantly reduce its viscosity, enabling the oil to flow
more rapidly through the earth to the removal well. n mature oil fields extensive
pipe networks are used to carry the carbon dioxide to the injection points.
%IO#O&$
Carbon dioxide is a waste product in organisms that obtain energy from
breaking down sugars or fats with oxygen as part of their metabolism, in a
process known as cellular respiration. This includes all plants, animals, many
fungi and some bacteria. n higher animals, the carbon dioxide travels in the
blood (where most of it is held in solution) from the body's tissues to the lungs
where it is exhaled.
Carbon dioxide content in fresh air is less than 1%, in exhaled air ca.
4.5%. When inhaled in high concentrations (about 5% by volume), it is toxic to
humans and other animals. Hemoglobin, the main molecule in red blood cells,
can bind both to oxygen and to carbon dioxide. f the CO2 concentration is too
high, then all hemoglobin is saturated with carbon dioxide and no oxygen
transport takes place (even if plenty of oxygen is in the air). As a result, people in
a poorly ventilated room will experience difficulty breathing due to accumulated
carbon dioxide, even before lack of oxygen becomes a problem. Carbon dioxide,
either as a gas or as dry ice, should be handled only in well ventilated areas.
OSHA limits carbon dioxide concentration in the workplace to 0.5% for
prolonged periods, or to 3% for brief exposures (up to ten minutes). OSHA
considers concentrations exceeding 4% as "immediately dangerous to life and
health." People who breathe 5% carbon dioxide for more than half an hour show
signs of acute hypercapnia, while breathing 710% carbon dioxide can produce
unconsciousness in only a few minutes.
*
PRODUCTON OF CARBON DOXDE FROM FUEL OL
According to a study by the USDA an average person's respiration
generates approximately 450 liters (roughly 900 grams) of carbon dioxide per
day.
Plants remove carbon dioxide from the atmosphere by photosynthesis,
which uses light energy to produce organic plant materials by combining carbon
dioxide and water. This releases free oxygen gas. Sometimes carbon dioxide gas
is pumped into greenhouses to promote plant growth. Plants also emit CO2
during respiration; but on balance they are net sinks of CO2.
"T!OSPHERE
Atmospheric CO2 concentrations, measured at Mauna Loa.
As of 2004, the earth's atmosphere is about 0.038% by volume (380 L/L
or ppmv) or 0.053% by weight CO2. This represents about 2.7 10
12
tones of
CO2. Due to the greater land area, and therefore greater plant life, in the northern
hemisphere as compared to the southern hemisphere, there is an annual
fluctuation of about 5 L/L, peaking in May and reaching a minimum in October
at the end of the northern hemisphere growing season, when the quantity of
biomass on the planet is greatest.
1+
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Despite its small concentration, CO2 is a very important component of
Earth's atmosphere, because it absorbs infrared radiation and enhances the
greenhouse effect.
The initial carbon dioxide in the atmosphere of the young Earth was
produced by volcanic activity; this was essential for a warm and stable climate
conducive to life. Volcanic activity now releases about 130 to 230 teragrams (145
million to 255 million short tons) of carbon dioxide each year. Volcanic releases
are about 1% of the amount which is released by human activities.
Global carbon dioxide emissions 17512000.
Since the start of the ndustrial Revolution, the atmospheric CO2
concentration has increased by approximately 110 L/L or about 40%, most of it
released since 1945. Monthly measurements taken at Mauna Loa since 1959
show an increase from 316 L/L in that year to 376 L/L in 2003, an overall
increase of 60 L/L during the 44-year history of the measurements. Burning
fossil fuels such as coal and petroleum is the leading cause of increased man-
made CO2; deforestation the second major cause. Various techniques have been
proposed for removing excess carbon dioxide from the atmosphere in carbon
dioxide sinks.
The Global Warming Theory (GWT) predicts that increased amounts of
CO2 in the atmosphere tend to enhance the greenhouse effect and thus
contribute to global warming.
11
PRODUCTON OF CARBON DOXDE FROM FUEL OL
'ariation in the past
CO2 concentrations over the last 400,000 years
The most direct method for measuring atmospheric carbon dioxide
concentrations for periods before direct sampling is to measure bubbles of air
(fluid or gas inclusions) trapped in the Antarctic or Greenland ice caps. The most
widely accepted of such studies come from a variety of Antarctic cores and
indicate that atmospheric CO2 levels were about 260280L/L immediately
before industrial emissions began and did not vary much from this level during
the preceding 10,000 years.
The longest ice core record comes from Vostok, Antarctica, where ice has
been sampled to a depth of 3,600 meters, corresponding to an age of 420,000
years before the present. During this time, the atmospheric carbon dioxide
concentration has varied between 180210 L/L during ice ages, increasing to
280300 L/L during warmer interglacial.
Some studies have disputed the claim of stable CO2 levels during the
present interglacial (the last 10 kyr). Based on an analysis of fossil leaves,
Wagner et al argued that CO2 levels during the period 7-10 kyr ago were
significantly higher (~300 L/L) and contained substantial variations that may be
correlated to climate variations. Others have disputed such claims, suggesting
they are more likely to reflect calibration problems than actual changes in CO2.
Relevant to this dispute is the observation that Greenland ice cores often report
12
PRODUCTON OF CARBON DOXDE FROM FUEL OL
higher and more variable CO2 values than similar measurements in Antarctica.
However, the groups responsible for such measurements (e.g. Smith et al)
believe the variations in Greenland cores result from in situ decomposition of
calcium carbonate dust found in the ice. When dust levels in Greenland cores are
low, as they nearly always are in Antarctic cores, the researchers report good
agreement between Antarctic and Greenland CO2 measurements.
On longer time scales, various proxy measurements have been used to
attempt to determine atmospheric carbon dioxide levels millions of years in the
past. These include boron and carbon isotope ratios in certain types of marine
sediments, and the number of stomata observed on fossil plant leaves. While
these measurements give much less precise estimates of carbon dioxide
concentration than ice cores, there is evidence for very high CO2 concentrations
(>3,000 L/L) between 600 and 400 Myr BP and between 200 and 150 Myr BP.
On long time-scales, atmospheric CO2 content is determined by the balance
among geochemical processes including organic carbon burial in sediments,
silicate rock weathering, and volcanism. The net effect of slight imbalances in the
carbon cycle over tens to hundreds of millions of years has been to reduce
atmospheric CO2. The rates of these processes are extremely slow, hence they
are of limited relevance to the atmospheric CO2 response to emissions over the
next hundred years. n more recent times, atmospheric CO2 concentration
continued to fall after about 60 Myr BP and there is geochemical evidence that
concentrations were <300 L/L by about 20 Myr BP. Low CO2 concentrations
may have been the stimulus that favored the evolution of C4 plants, which
increased greatly in abundance between 7 and 5 Myr BC. Although
contemporary CO2 concentrations were exceeded during earlier geological
epochs, present carbon dioxide levels are likely higher now than at any time
during the past 20 million years.
En(ironmental Effects
13
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Other processes serve to increase significantly the level of carbon dioxide
in the atmosphere, in particular the combustion of fossil fuels. Since the
advent of the industrial revolution, the burning of oil, natural gas, and coal
has increased dramatically, and measurements of atmospheric carbon
dioxide levels over the last 25 years have shown that there is an annual
increase on the order of 1 mg/kg [27]. Although it is generally acknowledged
that atmospheric carbon dioxide levels are increasing, scientists disagree
as to the likely effects of such an increase [28]. One theory is based on the
ability of carbon dioxide to absorb and transmit radiation. Carbon dioxide is
transparent to the sun's incoming shortwave" radiation, but it absorbs the
long wave radiation radiating from the earth's surface. An increase in the
atmospheric carbon dioxide level could cause an increase in the earth's
temperature by this mechanism; known as the "greenhouse effect" (the
glass in a greenhouse performs the same function as the carbon dioxide).
The ultimate effects of this temperature rise might be radical changes in
climate and rainfall patterns and a rapid rise in the sea level caused by the
melting of polar ice. Other scientists argue that there may be other
contributing factors in the regulation of the earth's temperature and that the
carbon dioxide question should not be addressed in isolation.
PROCESS SE#ECTION
1$
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Comparison of a(ailable processes
Carbon Dioxide from "mmonia and Hydro)en Plants *Process &as CO
+
The process for the production of ammonia is outlined in
Figure 4. Desulfurization of the hydrocarbon feedstock (e.g., natural gas,
naphtha) is carried out before catalytic steam reforming of the hydrocarbon
to give a gaseous mixture of hydrogen, carbon dioxide, and carbon monox-
ide. Air is added and further steam reforming is effected in a gaseous mixture
that then also contains nitrogen. Because only hydrogen and nitrogen are
required to make ammonia, the carbon oxides are removed from the gas
stream. Most of the carbon monoxide is catalytically converted to carbon
dioxide, and the latter is removed by dissolution under pressure. n the
manufacture of hydrogen, the process is very similar, with the obvious
omission of the addition of the air. Thus, in both cases, carbon dioxide must
be removed before the process-gas stream can be used. Several methods
are available; all are based on the absorption of carbon dioxide into a
solution, under pressure, which flows countercurrent to the process-gas
stream. The absorbed gas is liberated in a separate vessel by raising the
temperature and lowering the pressure above the solution. Under the normal
process conditions, the solubility of carbon dioxide in water is too low for this
to be used as the absorbent. The solubility is enhanced by adding a solute to
the water so that chemical combination of carbon dioxide with the solution is
effected.
1%
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Potassi,m Carbonate Process
The potassium carbonate process is based on a reversible reaction.
K
2
CO
3
+H
2
O CO
2
+ 2KHCO
3
The rate of carbon dioxide absorption increases with decreasing
temperature, increasing partial pressure of the gas, and increasing
concentration of potassium carbonate solution. Figure 5 shows the usual
arrangement of absorber and regenerator. The absorption should take place
at a lower temperature than that of regeneration to maximize the amount of
gas removed and recovered. This is achieved by a two-stage absorption and
regeneration system. The solution containing carbon dioxide is pumped from
the base of the absorber to the top of the regeneration column, supplied with
a reboiler at its base. The solution is returned to the absorber from two
points on the regenerator. Part of the solution is withdrawn from an
1&
PRODUCTON OF CARBON DOXDE FROM FUEL OL
intermediate point and is only partially regenerated; this is pumped to an
intermediate point on the absorber. The remainder is withdrawn from the
base of the regenerator. t is more completely regenerated, and is pumped,
after it is cooled, to the top of the absorber. The carbon dioxide and water
vapor mixture released in the regenerator is cooled. The water condenses
out and the carbon dioxide is passed to the liquefaction plant.
Most commercial potassium carbonate systems also employ catalyst to
accelerate the rates of absorption and desorption of carbon dioxide.
(Corrosion inhibitors)are also added to the solution.
ETH"NO#"!INE PROCESS
The ethanolamine process, developed by the Girdler Corporation uses,
monoethanolamine instead of potassium carbonate. Monoethanolamine reacts
with carbon dioxide and water to form monoethanolamine carbonate.
Carbon Dioxide from -l,e &ases
Carbon dioxide is a component of all flue gases produced by the Complete
combustion of carbonaceous fuels. Typical concentrations of carbon dioxide in
such gases are 10- 18 vol%. The flue gases, after being cooled and cleaned by
passing through a water scrubber, are passed through an alkaline carbonate
solution or an amine solution which absorbs carbon dioxide.
The gas from the flue of the furnace is then fed to an absorption regeneration
system. The CO
2
is removed by using a concentrated aqueous solution of an
amine, which also contains corrosion inhibitors, and is liberated, by using
steam supplied from the enrichment boiler.
A further development in the removal of carbon dioxide from flue gases,
announced by Dow, involves a two-stage process. Caustic soda or soda ash is
1(
PRODUCTON OF CARBON DOXDE FROM FUEL OL
first used to remove sulfur dioxide, before the gas stream is passed through an
amine solution which removes the carbon dioxide. An unspecified solvent is
used, which, it is claimed, enables the attainment of two to three times the
carbon dioxide loading achievable with conventional systems.
Carbon Dioxide from -ermentation
Large quantities of carbon dioxide are generated by
fermentation processes, and up to 80 % of this gas may be recoverable.
Before being suitable for further use, however, the carbon dioxide must be
freed of the impurities in this method of manufacture, i.e., hydrogen sulfide,
sulfur dioxide, and various organic compounds "such as aldehydes, acids, and
higher alcohols and diols. Two general methods are available to purify
fermentation carbon dioxide. Both use water scrubbers to remove the bulk of
the entrained material. The impurities are then taken out by passing through
either an activated charcoal bed or solutions of potassium permanganate and
potassium dichromate. The first method relies on adsorption, whereas the
second involves chemical reactions. n the second case the gas must be
treated further downstream to remove oxidation products formed in the
earlier stages as well as any traces of the reagents used in the purification.
Carbon Dioxide from Carbonates
The production of carbon dioxide by the calcinations of calcium
carbonate and magnesium carbonate is no longer economically important.
Natural sources of gases containing very high concentrations of
carbon dioxide are present in various parts of the world. Often the gas is so
pure that it requires only drying before liquefaction. The main impurities
present in this source of carbon dioxide are methane and hydrogen sulfide.
These sources are becoming more important in areas where enhanced oil
recovery is being practiced.
1)
PRODUCTON OF CARBON DOXDE FROM FUEL OL
P,rification of Carbon Dioxide before #i.,efaction
Unlike carbon dioxide derived from fermentation processes, which
requires considerable purification as described, most of the gas from other
sources is very pure. t contains only trace amounts of foreign materials,
usually sulfur compounds. Because the ultimate use of the gas often involves
foodstuffs, these trace impurities must be removed since they often impart dis-
tinctive tastes and odors. This is achieved by the use of activated carbon.
Drying of the gas is also very important, because insufficiently dried material
tends, on liquefaction, to choke the lines in the plant. Activated alumina, silica
gel, or molecular sieve beds are used for drying. Most plants are multi-
streamed and are equipped with beds that can be reactivated by means of
hot carbon dioxide, nitrogen, air, or steam.
The level of foreign gases present in the carbon dioxide stream must be
minimized to reduce the energy required to liquefy and concentrate the
circulating gas. The solubility of gases in liquid carbon dioxide decreases
with decreasing temperature. f any are present, they separate out easily
during the liquefaction stage
#i.,efaction of Carbon Dioxide
The critical parameters of carbon dioxide, t
crit
= 31 C,
P
crit
=7.4 MPa, show that the gas may be liquefied at any temperature
between 31 C and its triple point (-56.6 C) by compression to the required
liquefaction pressure at that particular temperature and removal of the heat
of condensation. t is, therefore, possible to liquefy CO
2
near the critical
temperature by using a pressure of 7.4 MPa and water as the coolant, but
because it is often difficult to guarantee a constant water supply at
temperatures below 20 C, this method is less favored than one in which a
refrigerant is used. n this second method, the gas is compressed under 2-
4MPa before being cooled to subzero temperatures by means of a
refrigerant. The choice of refrigerant obviously is a function of the cost and
1*
PRODUCTON OF CARBON DOXDE FROM FUEL OL
availability of the likely materials at their point of use. The proximity of many
carbon dioxide liquefaction plants to ammonia production facilities means
that ammonia is often the preferred refrigeration medium. Fluorocarbons
and brine are used in some plants.
Raw carbon dioxide piped from the ammonia plant is cooled by water
and then passed to the suction side of three parallel, three-stage, electrically
driven reciprocating compressors. After compression under oil-free
conditions, the gas is scrubbed with water to remove traces of organic
material formed in catalytic stages of the purification train of the ammonia
process. Two further purification stages are then carried out, and traces of oil
and sulfur compounds are removed in an activated charcoal bed. The gas
then passes through one of a pair of parallel driers containing activated
alumina. The water content is reduced to mg/kg levels. (The alumina
absorbent is periodically regenerated by passing a countercurrent of warm,
dry carbon dioxide through the bed.) The dried, purified carbon dioxide is
then liquefied in a double-pass condenser, using liquid ammonia at -12 C as
refrigerant. The gaseous ammonia resulting from the transfer of heat is
passed back to the plant ammonia-gas main. The liquefied carbon dioxide is
passed down a stripping column in which entrained permanent gases
(hydrogen, nitrogen, and methane) are removed by a countercurrent flow of
purer carbon dioxide gas from recycle compressors. This stripping gas is the
flash gas from the storage vessels of liquid carbon dioxide. The carbon
dioxide from the top of the column, now rich in permanent gases, joins the
main gas stream from the compressors and goes on to the condenser.
Purging of the permanent gases is effected by means of a pressure-controlled
blow-off to the atmosphere. The pressure is lowered and the liquid carbon
dioxide is sent to storage tanks, from which both road and rail tankers may be
filled. The liquid may also be processed further to form dry ice.
2+
PRODUCTON OF CARBON DOXDE FROM FUEL OL
!ONOETH"NO#"!INE PROCESS
Process Description
A continuous scrubbing system is used to separate CO
2
from a gaseous stream. The system consists of two main elements, an
absorber, where CO
2
is absorbed into a sorbent and a regenerator (or
stripper), where CO
2
is released (in concentrated form) and the original
sorbent is recovered. Chemical absorption systems tend to be more
efficient than the other systems shown in Appendix A, as the process is
accompanied by a chemical reaction that enhances the overall mass
transfer from gas phase to liquid phase.
n a power plant application cooled flue gases flow vertically
upwards through the absorber countercurrent to the absorbent (MEA in a
water solution, with some additives). The MEA reacts chemically with the
CO
2
in the flue gases to form a weakly bonded compound (carbamate).
The scrubbed gases are then washed and vented to the atmosphere. The
CO
2
-rich solution leaves the absorber and passes through a heat
exchanger, then further heated in a reboiler using low-pressure steam. The
weakly bonded compound formed during absorption is broken
down by the application of heat, regenerating the sorbent, and producing a
concentrated CO
2
stream. The hot CO
2
lean sorbent is then returned to the
heat exchanger, where it is cooled, then sent back to the absorber. Some
fresh MEA is added make up for losses incurred in the process.
The CO
2
product is separated from the sorbent in a flash
separator, and then taken to the drying and compression unit. t is
compressed to very high pressures (about 2000 psig) so that it is liquefied
21
PRODUCTON OF CARBON DOXDE FROM FUEL OL
and easily transported to long distances to the designated storage or
disposal facility.
Process Chemistry
The process chemistry is complex, but the main reactions taking place are
CO "bsorption/
2 R-NH2 + CO2 R-NH3 + R-NH-COO
!E" Re)eneration:
R-NH-COO + R-NH3 + (Heat) CO2 + 2 R-NH2
Pure MEA (with R = HO-CH2CH2) is an "unhindered amine
that forms a weakly bonded intermediate called "carbamate that is fairly
stable. Only half a mole of CO2 is absorbed per mole of amine, as shown in
the CO2 absorption equation above. On application of heat, this carbamate
dissociates to give back CO2 and amine sorbent, as shown in the second
equation above. Since the carbamate formed during absorption is quite
stable, it takes lot of heat energy to break the bonds and to regenerate the
sorbent. For other "hindered amines (e.g., where R is a bulky group), the
carbamate formed is not stable, and an alternate reaction leads to
formation of bicarbonate ions and hence a higher theoretical capacity of
one mole of CO2 per mole of amine, as shown in the CO2 absorption
equation below
CO "bsorption/
R-NH2 + CO2 + H2O R-NH3 + HCO3
!E" Re)eneration/
HCO3 + R-NH3 + (less Heat) CO2 + H2O + R-NH2
22
PRODUCTON OF CARBON DOXDE FROM FUEL OL
The regeneration of these amines requires lesser amount of
heat energy as compared to the unhindered amines. But the CO2 uptake
rate of hindered amines is very low. Efforts are underway to formulate
better sorbent by combining favorable properties of these two groups of
amines.
Process E.,ipment
The CO2 capture and separation system consists of the
following capital equipment:
Quench tower:
The flue gases coming out of a power plant are quite hot. The
temperature of flue gas may be ranging from as low as 60 deg. C (in case
of coal-fired power plants with wet SO2 scrubbers) to more than 550 deg. C
(in case of natural gas fired simple cycle power plants). t is desirable to
cool down the flue gases to about 45-50 deg. C, in order to improve
absorption of CO2 into the amine sorbent (the absorption being an
exothermic process is favored by low temperatures), to minimize sorbent
losses (higher temperature may lead to sorbent losses due to evaporation
and degradation), and to avoid excessive loss of moisture with the exhaust
gases. n case of gas-fired power plants or majority of coal-fired power
plants that do not have wet scrubbers for SO2 removal, a direct contact
cooler has to be installed to bring down the temperature of the flue gas
stream to acceptable levels. n case of coal-fired power plant applications
that have a wet FGD (flue gas desulfurization) unit upstream of the amine
system,
The wet scrubber helps in substantial cooling of the flue
gases, and additional cooler may not be required.
-l,e )as blo0er:
23
PRODUCTON OF CARBON DOXDE FROM FUEL OL
The flue gas has to overcome a substantial pressure drop as
it passes through a very tall absorber column, countercurrent to the
sorbent flow. Hence the cooled flue gas has to be pressurized using a
blower before it enters the absorber.
"bsorber:
This is the vessel where the flue gas is made to contact with
the MEA-based sorbent, and some of the CO2 from the flue gas gets
dissolved in the sorbent. The column may be plate type or a packed one.
Most of the CO2 absorbers are packed columns using some kind of
polymer-based packing to provide large interfacial area.
Rich1lean cross heat exchan)er:
The CO2-loaded sorbent needs to be heated in
order to strip off CO2 and regenerate the sorbent. On the other hand, the
regenerated (lean) sorbent coming out of the regenerator has to be cooled
down before it could be circulated back to the absorber column. Hence
these two sorbent streams are passed through a cross heat exchanger,
where the rich (CO2- loaded) sorbent gets heated and the lean
(regenerated) sorbent gets cooled.
Re)enerator:
This is the column where the weak intermediate compound
(carbamate) formed between the MEA-based sorbent and dissolved CO2 is
broken down with the application of heat and CO2 gets separated from the
sorbent to leave reusable sorbent behind. n case of unhindered amines
like MEA, the carbamate formed is stable and it takes large amount of
2$
PRODUCTON OF CARBON DOXDE FROM FUEL OL
energy to dissociate. t also consists of a flash separator where CO2 is
separated from most of the moisture and evaporated sorbent, to give a
fairly rich CO2 stream.
Reboiler:
The regenerator is connected with a reboiler which is basically a
heat exchanger where low-pressure steam extracted from the power plant
is used to heat the loaded sorbent.
!E" reclaimer:
Presence of acid gas impurities (SO2, SO3, NO2 and HCl)
in the flue gas leads to formation of heat stable salts in the sorbent stream,
which can not be dissociated even on application of heat. n order to avoid
accumulation of these salts in the sorbent stream and to recover some of
this lost MEA sorbent, a part of the sorbent stream is periodically distilled
in this vessel. Addition of caustic helps in freeing of some of the MEA. The
recovered MEA is taken back to the sorbent stream while the bottom
sludge (reclaimer waste) is sent for proper disposal.
Sorbent cooler:
The regenerated sorbent has to be further cooled down
even after passing through the rich/lean cross heat exchanger using a
cooler, so that the sorbent temperature is brought back to acceptable level
(about 40 deg C). Also, in order to make up for the sorbent losses, a small
quantity of fresh MEA sorbent has to be added to the sorbent stream. So,
the sorbent processing area primarily consists of sorbent cooler, MEA
storage tank, and a mixer. t also consists of an activated carbon bed filter
that adsorbs impurities (degradation products of MEA) from the sorbent
stream.
2%
PRODUCTON OF CARBON DOXDE FROM FUEL OL
CO dryin) and compression ,nit:
The CO2 product may have to be
carried to very long distances via pipelines. Hence it is desirable that it
does not contain any moisture in order to avoid corrosion in the pipelines.
Also, it has to be compressed to very high pressures so that it gets
liquefied and can overcome the pressure losses during the pipeline
transport. The multistage compression unit with inter-stage cooling and
drying yields a final CO2 product at the specified pressure (about 2000
psig) that contains moisture and other impurities (e.g. N2) at acceptable
levels.
Limitations of the MEA Process
Although MEA-based absorption process is the most suitable
technology available for capture of CO2 from power plant flue gases, it has
its own drawbacks. e.g corrosion and loss of solvent.
2&
PRODUCTON OF CARBON DOXDE FROM FUEL OL
!aterial and
Ener)y %alance
2(
PRODUCTON OF CARBON DOXDE FROM FUEL OL
%asis/
1hr operation
CO
prod,ced/ 234 5)1hr
Overall Balance:
Product =417 kg/hr CO
2
Raw material: Flue gas from combustion of fuel oil
Fuel oil Burnt =134 kg/hr
C in fuel oil =1340.873
=117 kg/hr
12 kg C produces CO2 = 44 kg
117 kg produces CO2 =
11(
12
$$
=429 kg/hr
Recovery efficiency =
, 1++
$2*
$1(
=97.2%
-,rnace
Reactions
2 2
CO O C +
O H O H
2 2
2 $ +
2 2
SO O S +
2 2 2 2 2 NO O N +
-,el oil composition
C 87.30%
H 12.60%
O 0.04%
N 0.01%
2)
PRODUCTON OF CARBON DOXDE FROM FUEL OL
S 0.22%
Ash 0.01%
total 100%
Quantity Burnt=134 kg/hr
"ir
Oxygen Required = O2 required for reactions 1, 2, 3
O
2
required for reaction 1 =
12
)(3 . + 13$
kg mol
=9.75 kg-mol
O
2
required for reaction 2 =
2 2
12& . + 13$
kg-mol
=4.22 kg-mol
O
2
required for reaction 3 =
32
++22 . + 13$
=0.01 kg-mol
Oxygen present in fuel oil =
2
+++$ . + 13$
kg-mol
=0.027 kg-mol
Total O
2
required =9.75+4.22+0.01-0.027
=14.04 kg-mol
20% excess air is supplied
Therefore O
2
supplied =(1+0.2) 14.04
=16.85 kg-mol
Air supplied =
21 . +
)% . 1&
=80.2 kg-mol
N
2
supplied =80.2-16.85
=63.35 kg-mol
2*
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Humidity 0.008kg-mol/kg-mol air
Total water with air =0.008 2 . )+
=0.64 kg-mol
-l,e &as Composition
Flue gas
COMPONEN
T
MOL% KG-
MOL
KG
CO
2
11.48% 9.8 430
H
2
O 10.89% 9.3 166.7
N
2
74.33% 63.2 1768
O
2
3.29% 2.8 90
SO
2
0.01% 0.01 0.6
NO
2
300ppm 0.02 0.7
Total 100.00% 85.0 2456
Energy Balance of furnace:
Heat liberated by fuel:
=5.610
9
J/hr
Heat Absorbed by process fluid:
t mC
p
=4.2410
9
J
Heat in flue gases at 470
o
C:
=1.35610
9
J/hr
Heat Balance
Heat Liberated = Heat utilized+ Heat in flue Gases
6,ench To0er
Entering:
From bottom;
3+
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Flue gas =2456 kg/hr with composition as given above.
From top;
Cooling water=15900 kg/hr
SO2 removed in quench tower =0.900.09
=0.008 kg-mol
SO2 still in flue gas =0.009-0.008
=0.001 kg-mol
CO
2
removed =
$$ 1)
++% . + 1%+++
=0.1 kg mol
Flue gas leaving quench tower at a dew point of 48
o
C
Humidity =0.118 kg-mol/kg-mol dry air
Bone Dry flue gas =85-9.3
=75.7 kg mol
So water with quenched flue gas =0.11875.7
=8.9 kg mol
Therefore water removed =9.3-8.9
=0.4 kg mol
Water Entering =15900kg/hr
CO
2
Solubility at quench temperature and pressure
=400mL/kg water
CO
2
Dissolved into Quench water
=5 kg/hr
After Quench tower
comp mol% kg-mole kg
CO
2
11.42% 9.7 425
H
2
O 10.60% 9.0 161
31
PRODUCTON OF CARBON DOXDE FROM FUEL OL
N
2
74.67% 63.2 1769
O
2
3.31% 2.8 90
SO
2
0.00% 0.0 0
total 100.00% 84.6 2446
%alance/
Material entering =15900+2456
=18356 kg/hr
Material leaving =2446+ (15900+4.5+5+0.5)
=18356 kg/hr
Therefore,
Material in = Material out
Energy Balance:
Heat in =1.35610
9
+ 1.9810
9
=3.33610
9
J/hr
Heat out =3.1810
9
+1.5610
8
=3.33610
9
J/hr
"%SOR%ER
Flue gas Entering
Absorption Efficiency: 98% of CO
2
in flue gas is absorbed in MEA
n Flue gas leaving unabsorbed CO
2
=0.02 x 9.7
=0.194 kg-mol
32
PRODUCTON OF CARBON DOXDE FROM FUEL OL
CO
2
absorbed =9.7-0.194
= 9.48 kg-mol
Solvent rate=?
Lean amine loading = 0.20 mole CO
2
/ mol MEA
Rich amine loading = 0.40 mole CO
2
/ mol MEA
From CO
2
balance
CO
2
entering = CO
2
leaving
0.20M+9.51= 0.40M
So MEA required for the desired absorption is
M =
( ) 2+ . + $+ . +
%1 . *
= 47.55 kg-mol MEA
= 47.5561=2900 kg
But as 20 wt % solution is used so the solution quantity is
=
2+ . +
2*++
=14500 kg/hr solution
With 80% water =0.8014500
=11600 kg
=645 kg mol
Total Solvent flow rate =692 kg mol/hr
With average molecular weight
=14500/692
=20.95 kg/kg-mol
Flue gas Entering
= 85kg mol/hr
Flue gas leaving from top:
CO
2
absorbed = 0.989.7
=9.5 kg mol /hr
33
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Unabsorbed flue gas (sweet gas)
=85-9.5
= 75.5 kg mol /hr
Enterin) the "bsorber
% Kg-mol/hr kg
CO
2
11.42% 9.7 425
H
2
O 10.60% 9.0 161
N
2
74.67% 63.2 1769
O
2
3.31% 2.8 90
SO
2
0.00% 0.0 0
total 100.00% 84.6 2446
Unabsorbed &as Kg-mol kg
CO
2
0.26% 0.2 9
H
2
O 11.94% 9.0 161
N
2
84.08% 63.2 1769
O
2
3.73% 2.8 90
SO
2
0.00% 0.0 0
total 100.00% 75.2 2029
3$
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Balance
Material entering =14855+2446
=17300 kg/hr
Material leaving =15273+2029
=17300 kg/hr
Therefore
SO#'ENT
Rich kg-mol mol% 5) wt %
MEA 47.4 6.7% 2889 18.92%
H
2
O 641.5 90.6% 11548 75.61%
CO
2
19.0 2.68% 836 5.47%
total 707.9 100.0% 3748 100.00%
#ean
MEA 47.4 6.9% 2889 19.82%
H
2
O 641.5 91.7% 11548 77.30%
CO
2
9.5 1.40% 418 2.88%
total 698.4 100.0% 32977 100.00%
3%
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Material in = Material out
STRIPPER
Enterin)
Rich Amine ( CO
2
+amine solution)
=707 kg-mol
Top product contains 34% CO
2
Total top product flow rate =
3$ . +
% . *
=27.94 kg-mol
Stripped Prod,ct Composition
Equilibrium pressure of CO
2
at top conditions is
=59 kPa
Now from ternary VLE of CO
2
, H
2
O and MEA
yi=
P
xiPisat
CO
2
in stripped product
y
1
=59/173
=34.1%
Also from VLE
y
i
= x
i
Pisat
/ P
total
For water at 105
o
C,
P
sat
=120kPa
y
2
= 65.3 %
And for Mono-ethanolamine
P
sat
=11 kPa
y
3
= 0.6 %
Therefore top product is
=
3$ . +
% . *
= 27.94 glom
3&
PRODUCTON OF CARBON DOXDE FROM FUEL OL
CO
2
=9.5 kg-mol
H
2
O =0.6527.94
=18.2 kg-mol
MEA =0.00627.94
=0.2 kg-mol
Bottom Lean Amine
CO
2
=16.6-9.5
=7.1
H
2
O =643-18.2
=624.8 kg-mol
MEA =47.4-0.2
=47.2 kg-mol
Total =7.1+624.8+47.2
=679.1 kg mol
Material entering =707.9 kg-mol/hr
=15233 kg/hr
Material leaving =Leaving as lean solvent +Leaving as top product
=679.1+27.94
=707.04kg-mol/hr
=15230kg/hr
Balance
Material entering = Material Leaving
--
3(
PRODUCTON OF CARBON DOXDE FROM FUEL OL
DESI&NIN& O- -URN"CE
STEPS
C"#CU#"TE TOT"# HE"T RE6UIRED
C"#CU#"TE "!OUNT O- -UE# RE6UIRED
R"DI"TION SECTION C"#CU#"TION
HE"T DUT$
NU!%ER O- TU%E RE6UIRED
R"DI"TION SECTION "RE"
CON'ECTION SECTION C"#CU#"TION
HE"T DUT$
NU!%ER O- TU%ES RE6UIRED
CON'ECTION SECTION "RE"
3)
PRODUCTON OF CARBON DOXDE FROM FUEL OL
D"T" RE6UIRED
Lower heating value of fuel=18200 Btu/lb
=4.2210
9
J/kg
Properties of process fluid i.e.(Oil)
Viscosity
$
1+ $
=
Pa.s
Density
% . (11 =
kg/m
3
Thermal capacity +(*& . + = k W/m.K
Heat Capacity
*3 . 2 =
p
c
kJ/kg.K
C"#CU#"TION O- "!OUNT O- -UE# %URNT
Fuel required is found out by the formula
Fuel consumption m
F
=
fuel of value heateing lower
releasd Heat Net - -
But here we calculate it from material balance of flue gases produced
Composition of fuel oil No. 2 is
C 87.30%
H 12.60%
O 0.04%
N 0.01%
S0.22%
Ash <0.01%
Total 100%
Flue Gas flow rate =91kg-mol
3*
PRODUCTON OF CARBON DOXDE FROM FUEL OL
CO
2
composition= 10.73%
C Balance gives amount of fuel consumed
Fluegas
m
F
=
1+(3 . +
12
)(3 . +
Fuel rate =
)(3 . +
12 *1 1+(3 . +
=134 kg
C"#CU#"TE TOT"# HE"T RE6UIRED
Net Heat released=Lower heating value Amount of Fuel burnt
=4.2210
7
J/kg134 kg/hr
Q
F
=5.610
9
J/hr
Taking efficiency of furnace 75%
Heat Utilized =0.755.610
9
J
=4.2410
9
J
R"DI"TION SECTION C"#CU#"TION
1) HEAT DUTY
2) NUMBER OF TUBE REQURED
3) RADATON SECTON AREA
R"DI"TION SECTION HE"T DUT$
The cheapest overall cost will be obtained if the radiant heat
absorption rate is made as high as practicable. Normally modern
heaters will handle about 70-75% of the total duty in the radiant
section.
$+
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Assumed 70% of total heat is absorbed in the radiation section. So
radiation section heat duty (Q
R
) is
Q
R
=0.70Q
=2.9710
9
J/hr
=(281500 Btu/hr)
Assumed average heat flux
= 25000 W/m
2
K
CP
A
Q
= 2 average flux
= 2 25000
= 50000 W/m
2
K
Assumed Overall Exchange factor
f = 0.5
f A
CP
= 110
5
W/m
2
K
TU%E SUR-"CE TE!PER"TURE
nlet temperature of Fuel oil = 25
o
C
nlet temperature of fluid in tube = 25
o
C
Out let temperature of fluid from the tubes = 250C
Average temperature of fluid T
F
=137.5C
Average tube wall temperature T
t
= T
F
+56
T
s
=193.5
o
C
Temperature of the flue gas leaving the radiant section (T
G
) from fig
19.14 kern
T
G
= 815
o
C
ENER&$ %"#"NCE
$1
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Entering air temperature = 30C
Enthalpy of entering air H
Air
= 25.1kJ/kg
Q
Air
=H
Air
m
air
Q
Air
is the sensible heat in combustion air above 25
o
C
Q
Air
= 57800 kJ/hr
Q
W
= 2% of Q
F
Q
W
is the heat loss through furnace walls, (1 to 10% of Q
F
, 2% is a
good design figure)
Where Q
F
is the heat liberated by fuel
i.e. Q
F
= Qn
Q
W
= 0.02 5.610
9
Q
W
= 1.1210
8
J/hr
CO!%USTION &"SES
Considering the representative reactions occurring
C + O
2
CO
2
4H+ O
2
2H
2
O
S + O
2
SO
2
N + O
2
NO
2
Therefore total oxygen required will be the sum of that required by
each reaction and the excess oxygen
Oxygen Consumed = 14 kg-mol/hr
As oxygen is supplied in 20% excess so
Oxygen supplied = 14 1.20
= 16.8 kg-mol/hr
Oxygen in flue gas = 16.8 14
= 2.8kg-mol/hr
Nitrogen in flue gas = 16.28
21 . +
(* . +
$2
PRODUCTON OF CARBON DOXDE FROM FUEL OL
= 61.24 kg-mol/hr
CO
2
in flue gases = 9.8 kg-mol/hr
H
2
O in flue gases = 9.3kg-mol/hr
At T
G
= 1088 K the enthalpy of different gases is shown
in the table
Name of gas Enthalpy
(kJ/kg-mol)
N
25472
CO
39802
H
O 31011
O
26940
Q
N2
= 25472 61.24
= 1.5610
9
J/hr
Q
O2
= 26940 2.8
= 7.5410
8
J/hr
Q
H2O
= 31011 9.3
= 2.8810
8
J/hr
Q
CO2
= 39802 9.8
= 3.910
8
J/hr
Q
G
= q
N2
+ q
O2
+ q
H2O
+ q
CO2
=1.5610
9
+7.5410
8
+2.8810
8
+ 3.910
8
=2.9910
9
J/hr
Q
G
= 2.9910
6
kJ/hr
Now Net heat load
$3
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Q
R
= Q
F
+ Q
A
Q
W
Q
G
Q
R
=5.610
9
+ 57800 1.1210
8
2.9910
9
Q
R
= 2.49810
9
J/hr
(t is close to actual heat load i.e. 2.97 10
9
J/hr)
C"#CU#"TION O- NU!%ER O- TU%ES
OD of tubes = 4.5 in
D of tubes = 4.026 in
Centre to centre distance = 8 in
Assume tube length =10.5ft
Surface area =1.178 ft
2
/ft
Flow area =0.088 ft
2
Surface area per tubes A
S
=1.178 10.5
=12.37 ft
2
=1.15m
2
Flux per tube =ass.flux A
S
=250001.15
Qtube =1.0310
8
J/hr
As our furnace is vertical tube single layer
No of tubes =
Qtube
Q
=
)
*
1+ +3 . 1
1+ 2.*(
N
tube
= 28 tubes
E6UI'"#ENT CO#D P#"NE SUR-"CE
A
CP
A
CP
per tube = (length of each tube) (C C distance)
= 10.5 8/12
$$
PRODUCTON OF CARBON DOXDE FROM FUEL OL
A
CP
per tube = 7 ft
2
(Effectiveness factor)
Ratio =
tubes of ! O
ce dis C C
.
tan
Ratio = 1.8
From Fig 19.11 Kern
= 0.917
A
CP
/tube =0.9177 ft
2
=6.42 ft
2
Taking 2 shield tubes, also for shield tubes =1
A
CP
for shield tubes =712
=14 ft
2
A
CP
for other tubes =266.42 ft
2
=166.9 ft
2
Total A
CP
=166.9 +14
=180.9 ft
2
RE-R"CTOR$ SUR-"CE
Length of tube =10.5ft
Tube in one row =12
Height (H
R
) = (12+1) (C C distance)
= (12+1) 8/12
=8.67ft=9ft
Width (W
R
) = (shield tubes+1) (arc tubes+1) (C C distance)
= (2+1) (2+1) 8/12
=4ft
End walls =2H
R
W
R
=294
$%
PRODUCTON OF CARBON DOXDE FROM FUEL OL
=72 ft
2
Side walls =2LH
R
=210.59 ft
2
=189 ft
2
Floor and arch =2LW
R
=210.54 ft
2
=84 ft
2
A
RT
= Total area of radiant section
A
RT
=72+189+84 ft
2
=345ft
2
So refractory surface (A
R
) is
A
R
= A
RT
- A
CP
=345-180.9 ft
2
=164.1ft
2
Radiant surface (A
Rt
) is
A
Rt
=
F"#$
Q
A
Rt
=
)+++
2(*&+++
A
Rt
=349 ft
2
Volume of radiant section
V
R
=LW
R
H
R
=10.549 ft
2
=378 ft
3
*1 . +
* . 1)+
1&$
= =
CP
A
A
!ean beam len)th
For dimension 1-1-2 mean beam length is
$&
PRODUCTON OF CARBON DOXDE FROM FUEL OL
L=
3
3
2
ume Furancevol
(table 19.1 kern)
L=
3
3()
3
2
L=4.82ft
Emissi(ity of &as/ E
&
Partial pressure of CO
2
and H
2
O is
P =0.23atm
PL=0.234.82
=1.11atm-ft
From graph emissivity of the gas (Cg) at PL=1.22 and T
G
= 1500F is
Cg=0.33 (Fig. 1.8)
So Over all exchange factor (f) is
f = 0.48
(Which is very close to true assumed value of f = 0.5)
Now
* . 1)+ $) . +
2)1%+++
=
f A
Q
CP
=32419 Btu/hr ft
2
Using
f A
CP
value of T
G
is
T
G
=1520
o
F
(Which is close to actual value T
G
=1500
o
F. Hence we can consider
our calculation and assumptions correct.)
Actual flux :
At T
G
=1500
o
F and for 20% excess air from graph
Qn
Qg
=0.43
Radiant zone heat balance is
$(
PRODUCTON OF CARBON DOXDE FROM FUEL OL
=
Qn
Qg
Qn
Qw
Qn
Q
1
Q
R
=Qn (1-0.02-0.43)
Q
R
=3.1x10
9
J/hr
=2938000Btu/hr
F"#$
A
Q
t
=
=8419 Btu/hr/ft
2
(This is close to assumed value of 8000 Btu/hr/ft
2
)
CON'ECTION SECTION C"#CU#"TION
1) HEAT DUTY
2) NUMBER OF TUBE REQURED
3) CONVECTON SECTON AREA
CON'ECTION SECTION HE"T DUT$
The convection section heat duty (Q
C
) is
(Q
C
) =Q-Q
R
=4.2410
9
-2.49 10
9
J
=1.7510
9
J
Enthalpy Q
S
of the stack gas, given by the overall heat balance
=
Qn
Q
Qn
Q
Qn
Qw
Qn
Q
C S
1
=
Qn
Q
S
(1-0.02-0.76)
=0.22
Now the stack temperature T
S
at
=
Qn
Q
S
0.22 and for 20% excess air
from graph is
T
S
=880
o
F
= 470
o
C
$)
PRODUCTON OF CARBON DOXDE FROM FUEL OL
The crossover temperature of the fluid=140
o
C
TE!PER"TURE DI--ERENCE
Hot fluid(flue
gas)
Cold
fluid(CH4+S)
Difference
816
o
C Higher
temp.
140
o
C 676
o
C
470
o
C Lower
temp.
25
o
C 445
o
C
=
$$%
&(&
ln
$$% &(&
"%&!
LMTD=552
o
C
Gross width (G.W) = (No. of shield tubes) (C C distance)
=28/12
=1.33ft
Free width (F.W) = (G.W)-(No. of shield tubes) (tube OD)
=1.33-24.5/12
=0.583ft
Free area (F.A) = (F.W) (tube length)
=0.58310.5
=6.125 ft
2
Flue gas mass rate G
F
is given by material balance to be
=2470 kg/hr
=1.51 lb/sec
Mass velocity
$*
PRODUCTON OF CARBON DOXDE FROM FUEL OL
G =
A F
'
F
.
=
12% . &
%1 . 1
=0.246 lb/sec ft
2
Overall heat transfer coefficient U
C
in convection section is
U
C
= (a+bG+cG
2
) (4.5/d)
0.25
Where
d is the tube outside diameter (in)
G is the flue gas mass velocity
a=2.461-0.759Z+1.625Z
2
b=0.7675+21.373Z-9.6625Z
2
c=9.7938-30.809Z+14.333Z
2
Z=T
F
/1000 average out side film temperature
By putting the values
T
F
= (407+25+552)/2
=492
o
C
=918
o
F
Z =918/1000
=0.918
a=3.134
b=12.245
c=-6.410
Hence
U
C
=5.76 Btu/hrft
2
-
o
F
=32.7068 W/m.K
So the area of convection section A
C
is
"%&! #
Q
A
C
C
C
=
%%2 3&++ (+( . 32
1+ (% . 1
*
=
C
A
A
C
=26.9m
2
=290ft
2
No, of tubes
% . 1+ 1() . 1
2*+
=
=23.4
Say 24 tubes
Height (H
C
) = (10+1) (C C distance)
%+
PRODUCTON OF CARBON DOXDE FROM FUEL OL
=118/12
=7.3ft
Width (W
C
) = (shield tubes+1) (C C distance)
= (4+1) 8/12
=3.3ft
Volume of convection section (V
C
)
V
C
=LW
C
H
C
=10.53.37.3 ft
3
=253 ft
3
Total volume of the furnace (V)
V
T
=V
R
+V
C
=378+253
=631 ft
3
= 17.9 m
%1
PRODUCTON OF CARBON DOXDE FROM FUEL OL
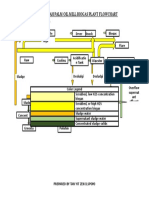
DESI&N O- 6UENCHIN& TO:ER/
Heat D,ty/
Flue Gas Flow Rate:
m
o
= 2440 kg/hr
Avg c
p
= 1.123 kJ/kg.k
T
h
= 470
o
C
T
c
= 50
o
C
Q
o
= m
o
c
p
AT
= m
o
c
p
(T
h
-T
c
)
= 1.2 x 10
6
kJ/hr
So, Water Required
T
w,in
= 30
o
C
T
w,out
= 48
o
C
m
o
= Q
o
/(c
p
* AT)
= 15900 kg/hr
Re.,ired To0er Cross Section "rea/
Assuming Liquid Loading Maximum = 10000 kg/hr.m
2
Req. Tower Cross Section Will Be = 15900/10000
=1.59 m
2
So, Ground Dimensions
%2
PRODUCTON OF CARBON DOXDE FROM FUEL OL
One Length = (1.56)
1/2
=1.25 m
= 1.25 m x 1.25 m Square
Hei)ht Calc,lation
haV/L = } (dt/LMTD)
LMTD = (490-48)-(50-30)/(ln(490-48)/(50-30))
=139
o
C
dt =48-30=18
o
C
haV/L =
"%&!
t
=18/139
= 0.13
Approx L
e
= 0.98 (fig: 17.4 Kern)
So, n
d
= (0.13*4.186)/(0.98*1.12)
= 0.50
There fore
Z = n
d
*L*(L
e
*C)/ha4.1
= 0.50* 2050*(0.98*0.28)/(24.94.1)
=2.76 ft
= 0.84 m
Approx = 1.0 m
For Safety
%3
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Specification Sheet
Flue Gas Flow Rate 2440kg/hr
Flue gas
temperature
470
o
C
Heat Duty 1.2*10
6
kJ/hr
Cooling Water
Required
15900kg/hr
Quench Tower
Ground Dimensions
1.25m x 1.25m
Square
Height of Tower 1.0 m
%$
PRODUCTON OF CARBON DOXDE FROM FUEL OL
"%SOR%ER DESI&N
&as absorption/
Gas Absorption is defined as "the removal of one or more
components from a mixture of gases into a suitable solvent.
P,rpose/
The above examples show that absorption of the gas in the
liquid can be treated as a physical process, when chemical reaction having
no appreciable effect. However, a chemical reaction may occur which may
affect the actual rate of absorption.
Types of absorption
There are two types of absorption;
Chemical absorption
Physical Absorption
Physical absorption:
Removal of one component from a mixture of gases
by using a suitable solvent is called physical absorption.
Chemical "bsorption/
Absorption followed by a chemical reaction in liquid
phase is often used to get more complete removal of a solute from a gas
mixture. For example, a dilute acid solution can be removed to scrub
ammonia from gas streams, and basic solutions are used to remove
carbon dioxide and other acid gases.
Here reaction in the liquid phase reduces the equilibrium partial
pressure of solute over the solutions, which greatly increase the driving
force for the mass transfer.
"bsorption col,mns/
There are two types of absorption columns i.e;
Packed columns
Plate columns
Characteristics of pac5ed and plate col,mns
Pac5ed col,mns/
Necessary to assure good liquid distribution
%%
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Not suitable for very low liquid rates
Handling corrosive, toxic and flammable liquids due to small hold up
Low pressure drop
nternal construction is simple
More contact time is available for the mass transfer.
Liquid holdup is appreciably lower, suitable for toxic or flammable
liquids
Plate col,mns
Handle large quantity of liquid and gas flow rates
Efficiency can be predicted more efficiently
Necessitates the use of cooling coils and side streams withdrawal
Easier to make provision for cleaning when the liquid cause fouling
:hy pac5ed col,mn is selected/
On the basis of the, above discussion packed column is selected for
our process since
packed tower usually provide more contact time as compared to
plate column
Low pressure drop
Packed column is suitable for foaming system
Following steps are considered in the designing of packed columns
Performance of material Balance
Performance of Energy Balance
The Operating conditions
Select the type, size and material of packing
Determine the diameter (capacity) of the column..
Determine the pressure drop.
Calculation of the %age flooding velocity.
Calculation of the Number of Transfer Unit and Height of the
packing and thus the height of the column.
Selection of the column internals
Operatin) conditions
20% by wt. mono ethanolamine solution is used.
Lean amine loading is 0.2molCO
2
/mol MEA
Rich amine loading is 0.4molCO
2
/molMEA
Flue gas entering temperature is 50 0C
%&
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Lean amine entering at 40 0C
Flue gas leaving absorber at 45 0C
Rich amine leaving at 510C
Absorption takes place at 50 0C
Material balance Over the Absorber
%(
Unabsorbed
gas
2+2*kg.hr
/0
2
1+.2&,
H
2
0111.*$,
2
2
1)$.+),
0
2
13.(3,
Rich solvent
1%2(3kg.hr
34512)*+kg
H
2
0111%&3kg
/0
2
1)33kg
Flue gas In
2$$&kg.hr
/0
2
111.$2,
H
2
011+.&+,
2
2
1($.&(,
0
2
13.31,
Lean solvent
1$%3+kg.hr
34512(%%kg
H
2
0111%%$kg
/0
2
1$1&kg
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Monoethanolamine Material Balance
Flue Gas Material Balance
Component #ea(in) rich "mine
*5)1hr+
Enterin) lean "mine
*5)1hr+
MEA 2889 2877
CO
2
836 418
H
2
O 11548 11219
Total 15273 14530
%)
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Ener)y %alance o(er the "bsorber
Basis: one hour operation
Reference temperature = 40
o
C
Heat of absorption = 72 kJ/mol CO
2
CO
2
absorbed = 9.48 kg-mol /hr
The energy balance equation is given as
(Heat flow rate)in - ( heat flow rate)out = rate of accumulation of heat
Rate of accumulation = zero
Total heat of absorption = 682560.0kJ/hr
(mCp AT)
FGin
+( mCpAT)
LAin
+(AH)
Abs
= (mCpAT)
RAout
+ (mCpAT)
FGout
Mass flow rate of flue gas in = 2446 kg/hr
Mass flow rate of flue gas out = 2029 kg/hr
Mass flow rate of lean amine in = 14530 kg/hr
Mass flow rate of rich amine out = 15273 kg/hr
Temperature of flue gas in = 55
o
C
Temperature of flue gas out = 45
o
C
Temperature of lean amine in = 40
o
C
Temperature of rich amine out = T = ?
o
C
Compone
nt
Compositio
n
*;+
-l,e )as
enterin)
*5)1hr+
CO
2
11.42 425
N
2
74.67 1769
H
2
O 10.60 161
O
2
3.31 90
Total 100.00 2446
Componen
t
Compositio
n
*;+
-l,e )as
lea(in)
*5)1hr+
CO
2
0.26 9
N
2
84.08 1769
H
2
O 11.94 161
O
2
3.73 90
Total 100.00 2029
%*
PRODUCTON OF CARBON DOXDE FROM FUEL OL
2446(1.45)(55-40) + 14530(4.20)(40-40) + 682560.0 = 2029(1.16)(45-40)
+ 15273(4.53)(T-40)
Hence the temperature of amine leaving = 51
o
C
Pac5in) selection
Metal pall ring is selected because
t gives lower pressure drop than rachig ring
Lower HTU and higher flooding limit.
Good liquid distribution and high capacity
As preliminary estimate of diameter is greater than 0.9m
Recommended design value is 1 to 2 in for diameter equal to 1.0m
hence the selected size is 32 mm.
DI"!ETER <D= C"#CU#"TION/
The recommended design values for pressure drop in absorbers
(and strippers) expressed in mm of water per m of packing may
range from:
15 to 50 mmH
2
O/m packing
So we assume the pressure drop of 25 mm of water per m of
packing
&+
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Molar flow rate of gas in = G = 0.679kg/sec
Molar flow rate of solvent out = L = 4.24kg/sec
p
G
= 1.299kg/m3.
p
L
= 999kg/m3
Viscosity of liquid = 0.00856 pa-sec
Viscosity of gas = 0.0000178 pa-sec
Surface tension of liquid = 0.0455 N/m
Because the rough estimate for dia is 3 feet
So
Packing size = 0.0.032 m
Now by the help of graph calculations are made as;
L
w
*/V
w
* (p
G
/p
L
)
0.5
versus K4
&1
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Where
K4 = (13.1(V
w
*)2F
p
(
L
/p
L
)
0.1
/ {p
V
(p
L
-p
V
)}
Now
F
L
= L*
w
/V*
w
(pv/p
L
)
= 4.24/0.679(1.299/999)1/2
= 0.22
Recommended value for pressure drop is 25 mmH
2
O
From graph the ordinate for sobar (25 mmH
2
O) is
K
4
= 0.8
K`
4
at flooding
K
4
= 2.2
Now check for %age flooding velocity
= (0.80/2.2)1/2 100
= 60.12 %
Hence operating velocity is 60% of the flooding velocity and satisfactory for
design
K
4
= (13.1(V
w
*)2F
p
*(
L
/p
L
)
0.1
/ {p
V
(p
L
-p
V
)}
Putting the value in Eq. and calculating for the Gas mass velocity
F
p
= 160
V
w
* = \ K
4
p
V
(p
L
-p
V
)/ 13.12F
p
*(
L
/p
L
)
0.1
V
w
* = 1.189 kg/m
2
-sec
But
G = 0.679kg/sec
Hence Area of cross section = G/ V
w
*
= 0.679/1.189
Area = A = 0.673 m
2
Diameter = \ (4A/3.1416)
= 0.925 m = 3.03 ft
Round off it to 3.0 ft.
Then
Adjusted area = A = 0.915 m
&2
PRODUCTON OF CARBON DOXDE FROM FUEL OL
And the adjusted values of
V
w
* = 1.19 kg/m
2
-sec
K
4
= 0.80
Hence the AP = 27 mmH
2
O/ m packing (from graph)
-loodin) (elocity
As K`
4
= 2.2
And
V
w
* = \ 2.21.299(9991.299)/13.1(160)(.00856/999)0.1
Vw* = 1.97kg/m2-sec
Calc,lation of Hei)ht of col,mn
Number of Transfer Unit/
As the chemical absorption here and the reaction
in the liquid phase reuses the equilibrium partial pressure of solute over
the solution which greatly increase the driving force for mass transfer so
equilibrium partial pressure is zero and N
oy
can be calculated for y* =0
N
OG
= ln y
2
/y
1
As
y
2
= 11.42 and y
1
= 0.26
N
OG
= 4
Height of the transfer unit
Applying Onda method
First of all the effective area is calculated as
a
w
/a = 1- exp[(o
c
/o
L
)
0.75
(L*
w
/
a
L
)
0.1
(L*
w
2
a/ p
L
2
g)
-0.05
(L*
w
2
/ p
L
o
L
a)
0.2
&3
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Calculated effective area for the mass transfer
a
w
= 86.2 m
2
/m
3
Now kg and k
L
are calculated by using Onda`s correlations
Hence,
kgRT/aDv = K
5
(V
w
*/a
v
)
0.7
(
v
/ p
V
Dv)
1/3
(ad
P
)
-2.0
K
5
= 5.23 for packing size above 15 mm
Dv =1.92 10
-6
kg = 4.37 10
-4
kg-mol/m
2
-bar-sec
And
k
L
(p
L
/
L
g)
1/3
= 0.0051(L
w
*/a
w
L
)
2/3
(
L
/p
L
D
L
)
-1/2
(adp)
0.4
D
L
= 1.656 10
-6
k
L
= 1.43610
-4
m/sec
Total concentration of leaving amine
C
T
= density of amine/mol.wt.
= 999/21.57
= 46.31 kmol/m
3
As
P
T
= 1.01325 bar
Now
H
G
= G
m
/ k
g
a
w
P
G
m
= 0.0357 kg-mol/m
2
-sec
H
G
= 0.937 m
And
H
L
= L
m
/k
L
a
w
C
T
L
m
= 0.298 kg-mol/m2-sec
H
L
= 0.519 m
Now overall height of transfer unit
H
OG
= Hg + m.G
m
/L
m
H
L
m.G
m
/L
m
= 0.6 - 0.85is Optimum range given by Colburn
H
OG
= 0.937 +0.85(0.519)
= 1.378 m
&$
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Total height of packing:
Z
t
= N
OG
H
OG
Z
t
= 4 1.378
Z
t
= 5.52 m
Providing the 15% of the packing free space for liquid gas separation
above and below the packing which is 2 meter
So,
Total height of the Column is
Z
T
= 5.52 + 2 = 7.52 meter
Total pressure drop in the absorber
As calculated value is 27 mmH
2
O/m packing
APt = 27 mmH
2
O/m packing 5.52
= 149.0 mm H
2
O
= 1.48 Kpa
As total height of packing is 18.10ft so no need of redistribution and
accounting for pressure drop for Distributor and Support plate
AP
s
= 0.3 in H
2
O 2
= 0.6 inch H
2
O
= 0.15 Kpa
Hence the total pressure drop
AP
T
= 1.63 Kpa
Support plate and Distributor:
Support must be strong to carry the weight of reasonable height of
packing, poorly designed support will give high pressure drop
The best choice is the "Gas-njection type support.
&%
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Trough-type liquid distributor is selected because the dia of column
is in the range of three ft.
Design Data
Density of flue gas = 1.299 kg/m3
Density mono ethanolamine = 999 kg/m3
Viscosity of flue gas = 1.78 10
-5
pa=sec
Viscosity of mono ethanolamine = 8.56 10
-3
pa-sec
Surface tension of mono ethanolamine
Diffusivity of MEA into CO
2
=
D
L
= 1.656 10
-6
Diffusivity of CO
2
in MEA =
D
V
= 1.92 10
-6
Surface tension of MEA solution = 45.53 10
-3
N/m
Molecular Wt. of MEA = 28.90 kg/ kg-mol
Molecular Wt. of Flue gas = 21.57 kg /kg-mol
Specification sheet
dentification: Absorber
tem number: 03
Number required: 01
Function:
"To absorb CO
2
gas from Flue gas into 20% by wt. Mono-
ethanolamine solution.
Operation: Continuous
Operating pressure 101.325 kPa
Operating Temperature 50 oC
Height of transfer unit 1.37 m
Number of transfer units 4
Height of packed section 5.52 m
Total height of the column 7.52 m
nside diameter 0.915 m
Flooding Mass Velocity 1.97 kg/m2-sec
Pressure drop 1.65 kPa
CO
2
Removal Efficiency 98%
Material of construction Carbon Steel
&&
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Col,mn Internals
Type of packing Pall Rings
Size of packing 0.032 m
Material of packing Metal
Packing Support Gas-injection Type
Liquid distributor Weir Trough Type
DESI&N O- HE"T E>CH"N&ER
INTRODUCTION
A heat exchanger is a heat-transfer devise that is used for transfer of
internal thermal energy between two or more fluids available at different
temperatures. n most heat exchangers, the fluids are separated by a
heat-transfer surface, and ideally they do not mix. Heat exchangers are
used in the process, power, petroleum, transportation, air conditioning,
refrigeration, cryogenic, heat recovery, alternate fuels, and other
industries. Common examples of heat exchangers familiar to us in day-
to-day use are automobile radiators, condensers, evaporators, air pre-
heaters, and oil coolers.
n our project a number of heat exchangers are used . Here we will
discuss heat exchanger used as
&(
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Condenser
Reboiler
Cooler
All of these are shell and tube heat exchangers.
Selection &,ide To Heat Exchan)er Types
Type
Si)nificant
feat,re
"pplications best
s,ited
#imitations
"pproxim
ate
relati(e
cost in
carbon
steel
constr,cti
on
Fixed tube
sheet
Both tube sheets
fixed to shell.
Condensers;
liquid-liquid; gas-
gas; gas-liquid;
cooling and
heating, horizontal
or vertical,
reboiling.
Temperature
difference at
extremes of about
200
o
F Due to
differential
expansion.
1.0
Floating
head or
tubesheet
(removabl
e and
nonremov
able
bundles)
One tubesheet
"floats in shell or
with shell, tube
bundle may or may
not be removable
from shell, but
back cover can be
removed to expose
High temperature
differentials, above
about 200
o
F
extremes; dirty
fluids requiring
cleaning of inside
as well as outside
of shell, horizontal
nternal gaskets
offer danger of
leaking.
Corrosiveness of
fluids on shell-side
floating parts.
Usually confined to
horizontal units.
1.28
&)
PRODUCTON OF CARBON DOXDE FROM FUEL OL
tube ends. or vertical.
U-tube;
U-Bundle
Only one tube
sheet required.
Tubes bent in U-
shape. Bundle is
removable.
High temperature
differentials, which
might require
provision for
expansion in fixed
tube units. Easily
cleaned conditions
on both tube and
shell side.
Bends must be
carefully made, or
mechanical
damage and
danger of rupture
can result. Tube
side velocities can
cause erosion of
inside of bends.
Fluid should be
free of suspended
particles.
0.9-1.1
Double
pipe
Each tube has own
shell forming
annular space for
shell side fluid.
Usually use
externally finned
tube.
Relatively small
transfer area
service, or in
banks for larger
applications.
Especially suited
for high pressures
in tube (greater
than 400 psig).
Services suitable
for finned tube.
Piping-up a large
number often
requires cost and
space.
0.8-1.4
Pipe coil
Pipe coil for
submersion in coil-
box of water or
sprayed with water
is simplest type of
exchanger.
Condensing, or
relatively low heat
loads on sensible
transfer.
Transfer coefficient
is low, requires
relatively large
space if heat load
is high.
0.5-0.7
Plate and
frame
Composed of
metal-formed thin
plates separated
by gaskets.
Compact, easy to
clean.
Viscous fluids,
corrosive fluids,
slurries, high heat
transfer.
Not well suited for
boiling or
condensing; limit
350-500
o
F by
gaskets. Used for
liquid-liquid only;
not gas-gas.
0.8-1.5
Spiral
Compact,
concentric plates;
no bypassing, high
turbulence.
Cross-flow,
condensing,
heating.
Process corrosion,
suspended
materials.
0.8-1.5
SHE## "ND TU%E HE"T E>CH"N&ER
&*
PRODUCTON OF CARBON DOXDE FROM FUEL OL
n process industries, shell and tube exchangers are used in great
numbers, far more than any other type of exchanger. More than 90% of
heat exchangers used in industry are of the shell and tube type. The shell
and tube heat exchangers are the "work horses of industrial process heat
transfer. They are the first choice because of well-established procedures
for design and manufacture from a wide variety of materials, many years of
satisfactory service, and availability of codes and standards for design and
fabrication. They are produced in the widest variety of sizes and styles.
There is virtually no limit on the operating temperature and pressure.
Classification of Shell and T,be Heat Exchan)ers
There are four basic considerations in choosing a mechanical
arrangement that provides for efficient heat transfer between the two
fluids while taking care of such practical matters as preventing leakage
from one into the other.
Consideration for differential thermal expansion of tubes and
shell.
Means of directing fluid through the tubes.
Means of controlling fluid flow through the shell.
Consideration for ease of maintenance and servicing.
Heat exchangers have been developed with different approaches to
these four fundamental design factors. Three principal types of heat
exchangers
1) Fixed tube-sheet exchangers
2) U-tube exchangers and
3) Floating head exchangerssatisfy these design requirements.
(+
PRODUCTON OF CARBON DOXDE FROM FUEL OL
t1 t2
T2 T1
?2 shell and t,be heat exchan)er
3@PH$SC"# PROPERTIES/
RICH CO#D "!INE #E"N HOT "!INE
t
1
=
55 C
0
T
1
= 120 C
0
t
2
= 102C
0
T
2
= ?
m = 4.25 kg/s M = 4.13 kg/s
1
= 0.0065 pa.s
2
= 0.004 pa.s
C
p
= 4016.6 J/kg.
o
C cp = 4037 J/kg.
o
C
1
= 979 kg/m
3
2
= 960 kg/m
3
k
1
= 0.5 k
2
= 0.5
!"#EAT LOAD
Q = mc
p
t
= (4.25)(4016.6)(102-55)
= 802315.85 J/s
Q = Mc
p
T
802315.85=(4.13)(4037)(120-T
2
)
T
2
=70
o
C (as above written)
8@ #!TD/
T
h
= T
1
-t
2
= 120-102 = 20
o
C
T
c
= T
2
-t
1
= 70-55 = 15
o
C
(1
PRODUCTON OF CARBON DOXDE FROM FUEL OL
LMTD = (T
h
- T
c
)/ln(T
h
/T
c
)
= 17.5C
o
2@ "SSU!ED '"#UE O- U
o
/
U
o
= 1500 W/m
2
.k
7@ HE"T TR"NS-ER "RE"/
A = Q /(U
o
.LMTD)
= 30.5 m
2
A@ CORRECTED "RE"/
R = (T
1
- T
2
)/(t
2
-t
1
)
= 1.025
S = (t
2
-t
1
)/( T
1
-t
1
)
= 0.645
F
t
= 0.88 (For 2-4 heat exchanger)
63T7 becomes 1 1%.$
o
/
A = Q/(U
o
.LMTD)
A = 34.5 m
2
4@ SE#ECTED P"R"!ETERS/
(2
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Length of Tube = 4.876 m
14 BWG D= 0.01483 m
OD = 0.01905 m
Pitch =P
t
= 0.02381 m
Baffle Spacing = Shell Diameter
9@ SHE## SIDE C"#CU#"TIONS/
9@3 %UND#E DI"!ETER/
For 4 Tube Passes:
K
1
= 0.175, n
1
=2.285
(Table 12.4 R&C VOL.6)
D
b
=OD (N
t
/k
1
)
1/n1
= 0.788 m
For Fixed Tube Heat Exchanger:
Dia = 0.016 m
9@ SHE## DI"!ETER/
D
s
= D
b
+ Dia
= 0.8 m
Baffle spacing(L
b
):
(L
b
=D
s
) =0.8 m
No of baffles used(N
b
):
(L/L
b
)= 6.25= 6
No of passes:
N
p
= 2
Baffles per pass:
(N
b
/N
p
)= 3
9@8 SHE## "RE"/
A
s
= ((P
t
OD)/P
t
)*D
s
*L
b
(3
PRODUCTON OF CARBON DOXDE FROM FUEL OL
= 0.13 m
2
Equivalent Diameter (D
e
):
=(1.10/OD)*(P
t
2
(0.917*OD
2
))
D
e
=0.01352 m
2
Vol. Flow Rate on Shell:
M/
2
= 0.0043 m
3
/s
9@2 SHE##'E#OCIT$ *,
s
+/
Vol. Flow Rate On Shell Side)/As
= 0.033 m/s
9@7 SHE## SIDE HE"T TR"NS-ER COE--ICIENT/
Reynolds Number
(
2
*D
e
* u
s
)/
2
= 1079.4
Prandtl Number
(c
p
*
2
)/k
2
= 0.32296
Heat Transfer Factor = (j
H
) = 0.009
(Fig: 12.29 R&C, VOL.6)
Nusselt Number
(Re*j
H
)*(P
r
)
1/3
= 143.0
h
o
= h
s
= (N
u
*k)/D
e
= 5290 W/m
2.
k
B@ TU%E SIDE C"#CU#"TIONS/
($
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Area of One Tube:
(DL) = 0.291667 m
No of Tubes (N
t
):
A/Area of One Tube = 116
No of Passes (N
p
) = 4.
Tubes per Pass:
N
t
/4 = 29
B@3 TU%E CROSS SECTION"# "RE"/
(/4)*(D)
2
= 0.0001726 m
2
Area Per Pass:
(Tubes Per 4 Pass)*(Tube Cross Sectional Area)
= 0.0055 m
2
Volumetric Flow Rate:
m/
1
= 0.0044 m
3
/s
B@ TU%E SIDE 'E#OCIT$/
(Vol. Flow Rate)/(Area Per Pass)
= 0.8 m/s
B@8 TU%E SIDE HE"T TR"NS-ER COE--ICIENT/
Reynolds Number
(
1
*D*u
t
)/
1
= 2593.2
Prandtl Number
(cp*
1
)/k
1
= 5.222
(%
PRODUCTON OF CARBON DOXDE FROM FUEL OL
L/D = 328.8
Heat Transfer Factor = j
H
= 0.007
Nusselt Number
(Re*j
H
)*(Pr)
1/3
= 313.2
h
i
= (Nu*k)/D
= 10559 W/m
2.
k
3C@ O'ER "## HE"T TR"NS-ER COE--ICINT/
We have a Relation:
1/U
o
= (d
o
/d
i
)(1/h
id
+ 1/h
i
)+(1/h
o
)+(1/h
od
)+(d
o
.ln(d
o
/d
i
)/2.K
w
))
=0.00069
U
o
= 1445 W/m
2
.k
h
id
= 0.0001 m
2
.k/W (Page: 661 P & D) (inside Dirt Coefficient)
h
od
= 0.0002 m
2
.k/ (Page: 663 P & D) (outside Dirt Coefficient)
K
w
= Thermal conductivity of wall = 45 Carbon Steel.
3C@3 PRESSURE DROP ON SHE## SIDE/
Friction Factor = j
f
= 0.038
(Correspond to Re = 10794)
P
s
= (8*jf*(D
s
/D
e
)*(L/L
b
)*((
2
*u
s
2
)/2))
P
s
= 5825 N/m
2
As,
1 N/m
2
= 0.000145 psi
P
s
= 0.845 psi
(&
PRODUCTON OF CARBON DOXDE FROM FUEL OL
3C@ PRESSURE DROP ON TU%E SIDE/
Friction Factor = j
f
= 0.0039
(Correspond to Re = 25932)
P
t
= N
p
((8*jf*(L/D)+2.5)*(
1
*u
t
2
)/2)
= 33670 N/m
2
1 N/m
2
=0.000145 psi
so,
P
t
= 4.88 psi.
$PECIFICATION $#EET
#EAT E%C#ANGER
dentification8 tem heat exchanger
2o. 9e#uired 1 1
!unction #eat e&changer is the e'ui()ent used to e&change heat bet*een t*o +luids
having di++erent te)(eratures, through a +i&ed *all, *ithout )i&ing the
t*o +luids"
0peration8 /ontinuous
Type8 2-$ counter flow shell : tube heat exchanger
Heat 7uty 1 )+231%;.s
Tube <ide8
!luid handled 1 9ich /old 5mine
Tubes8 = in. 1 +.+1*+% m
1$ >'? thickness
((
PRODUCTON OF CARBON DOXDE FROM FUEL OL
!low rate 1 $.2% kg.s
Temp 1 %&
o
/ to 1+2
o
/
.7 8 +.+1$)3 m
+.+23)1 in. triangular pitch
2o of tubes 8 11&
@ressure drop 133&(+ 2.m
2
1$.)) psi
<hell <ide8
!luid handled 1 6ean Hot 5mine
!low rate 1 $.13 kg.s
Temp 1 12+
o
/ to ((
o
/
<hell 7iameter8 +.) m
2 passes
>affles spacing 1 6
b
17
s
@ressure drop 1))2% 2.m
2
1+.)$% psi
A
o
1 assumed 1 1%++ '.m
2o
/
A
o
calculated 1 1$$% '.m
2o
/
STRIPPER DESI&N
Steps/
Determining the preliminary diameter of the column
Select the type of column i.e. packed or plate.
Selection of packing type and its material
Calculating the size of packing
Calculating the actual diameter of column
Determining the Number of transfer units
Determine the Height of Transfer Unit
Determining the total height of the column
Determining the pressure drop.
()
PRODUCTON OF CARBON DOXDE FROM FUEL OL
!aterial %alance
Rich "mine
kmol/hr
CO
2
19.0
H
2
O 641.5
MEA 47.4
Total 707.9
Prod,ct
kmol/hr
CO
2
9.5
H
2
O 18.2
MEA 0.2
Total 27.9
#ean "mine
kmol/hr
CO
2
9.5
H
2
O 623.3
MEA 47.2
Total 680.0
172.3 kPa
Steam
(*
PRODUCTON OF CARBON DOXDE FROM FUEL OL
!aterial %alance/
Stripper Entering Leaving 1 Leaving 2
Component Rich Amine
Kg/hr
Lean Amine
Kg/hr
Product Gas
Kg/hr
CO
2
833 416.5 416.5
H
2
O 11563 11236 327
MEA 2891 2880.8 10.2
Total 15287 14533.3 753.7
Operatin) Conditions/
Bottom Temperature = 120
o
C
Column Operating Pressure = 173 kPa
Rich Loading = 0.4 mol CO
2
/mol MEA
Lean Loading =0.2 mol CO
2
/mol MEA
Top Temperature =105
o
C
Preliminary diameter calc,lation/
Vapor flow rate =753.7 kg/hr
=
$ . 1 3&++
( . (%3
m
3
/s
=0.149 m
3
/s
Assume a superficial velocity =0.61 m/s (2 ft/s)
)+
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Preliminary diameter =
&1 . +
1$* . +
=0.25m <2.5ft
So a packed column will be appropriate
Pac5ed Col,mn
Usually packed columns gives lower pressure drop than plate
columns.
f the liquid causes corrosion a packed column is cheaper than a
plate column.
Packed columns are more suitable for foaming liquids.
More contact time is available for the mass transfer.
Not suitable for very low liquid rates.
Economical with small diameter
Plate Col,mns
Easy to withdraw side streams
Can handle wider range of flow rates
f the liquid contains solids, cleaning is easier in plate columns
For larger diameters plate columns are economical and preferred.
Our solvent (20wt.% MEA) is :
Moderately Foaming
Very Corrosive at high temperature
Low pressure drop required
So our choice is a
Pac5ed Col,mn
This is also supported by present installations in gas treating industry.
-eed To the Stripper
Rich amine =708 kg-mol/hr
)1
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Composition:
CO
2
2.67 % by mol
H
2
O 90.65%
MEA 6.68%
Stripped Prod,ct Composition
Equilibrium pressure of CO
2
at top conditions is
=59 kPa
CO
2
in stripped product
y
1
=59/170
=34.1%
Similarly from VLE
As
y
i
= x
i
P
i
sat
/ P
total
For water at 105
o
C,
P
sat
=120kPa
y
2
= 65.3 %
And for Mono-ethanolamine
P
sat
=11 kPa
y
2
= 0.6 %
Therefore total Vapor Rate, V = 9.5/0.34
=27.8 kg-mol/hr
Therefore Top product composition is
COMPONENT KG-MOL MOL% KG
MEA 0.20 0.75% 12
H
2
O 17.36 64.15% 312
CO
2
9.50 35.11% 418
Total 27.06 100.00% 744
)2
PRODUCTON OF CARBON DOXDE FROM FUEL OL
#ean amine/ =680.9kg-mol/hr
Composition of the lean solvent
CO
2
1.39 % by mol
H
2
O 91.68%
MEA 6.93%
Pac5in) Selection
Stripping operation is at high temperature
Plastic may not work safely
Amine is corrosive
Metal packing is not viable
So the choice is ceramic ntalox saddles
Size 38 mm
DI"!ETER O- CO#U!N
Specify Design pressure drop
= 25 mmH
2
O/m packing height
Liquid flow rate is
L
w
= 15283 kg/hr
Vapor flow rate is
V
w
= 754 kg/hr
Calculate vapor density using avg. molecular wt.
M = 27 kg/kg-mol
p
v
=1.4 kg/m3
Liquid density is
p
L
= 970 kg/m3
And
)3
PRODUCTON OF CARBON DOXDE FROM FUEL OL
L*
w
/V*
w
= L
w
/ V
w
= 19.3
Now flow factor
F
LV
= L*
w
/V*
w
( p
v
/ p
L
)
Therefore
F
LV
= 0.79
So, From figure 11.44 Coulson Vol. 6
K4 = 0.38
As
K
4
=
( )
( )
v " v
" " p w
F (
1 . +
2
. B C D 1 . 13
Liquid viscosity
L
=3.4x10-4Pa.s
Packing factor for 50 mm Ceramic ntalox Saddles
F
p
=130 m
-1
Therefore
V
*
w
= 1.2 kg/m2s
As
V= 755 kg/hr =0.21 kg/s
So
A= V
w
/V
*
w
=0.21/1.2
=0.175 m
2
As
2
$
!
A
=
Therefore
D = (4A/)
1/2
)$
PRODUCTON OF CARBON DOXDE FROM FUEL OL
=0.47 m
Rounding off
=0.5m
So
Lw* = Lw/A
=21.6 kg/m
2
s
HEI&HT O- THE CO#U!N
N,mber of Transfer Units *NTU+
Equilibrium Curve is given
Operating Line can be found by material Balance
Slope of operating line is
L/V=25.4
Where L and V are molar flow rates
Top Composition of CO
2
is
(x, y) = (0.027, 0.34)
Now plot operating line on the equilibrium line graph.
Find x and x
e
at number of values of y
Y X X
E
e
x x
1
0.05 0.0152 0.0125 370
0.10 0.0172 0.0155 588
0.15 0.0192 0.0178 714
0.20 0.0211 0.0195 625
0.25 0.0232 0.0210 454
0.30 0.0251 0.0220 322
0.34 0.0267 0.0230 270
N
OL
=
e
x
x
x x
dx
2
1
By Graphical integration of R.H.S between the limits 0.0139 to 0.0267
)%
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Plotting x vs
e
x x
1
and finding area under the curve
N
OL
=6
Equilibrium Curve for Desorption
Reference: Gas Treating with chemical solvents, Astarita et. al
Hei)ht of Transfer Unit
Using ceramic intalox saddle of 38mm
H
OL
is given in literature i.e.
H
OL
=4.1 ft at given flow rate
H
OL
=1.25 m
Hei)ht of Pac5in)
Z=H
OL
N
OL
Z=1.25 x 6
=7.5 m
A distribution allowance of 1m will be made at top and bottom
)&
PRODUCTON OF CARBON DOXDE FROM FUEL OL
So height of the column =7.5+2
=9.5m
-loodin) 'elocity/
Percentage Flooding =(K
4
/K
4
at flooding)
1/2
*100%
=0.56%
So flooding velocity is=1.78 kg/m
2
s
SPECI-IC"TION SHEET
Operatin) press,re 3485Pa
Operatin) Temperat,re *bottom+ 3C
o
C
Hei)ht of transfer ,nit 3@7m
N,mber of transfer ,nits A
Hei)ht of pac5ed section 4@7m
Total hei)ht of the col,mn B@7 m
Inside diameter C@7m
Press,re drop 5Pa
Col,mn Internals
Type of pac5in) Intalox saddles
!aterial of pac5in) Ceramic
SiDe of pac5in) 89mm
Pac5in) S,pport CPS Plate
#i.,id distrib,tor :eir Tro,)h
#i.,id redistrib,tor -,ll redistrib,tor
)(
PRODUCTON OF CARBON DOXDE FROM FUEL OL
T = 46 C T = 30 C
CO
2
= 417 Kg CO
2
= 417 Kg
Steam = 328 Kg Steam = 9 Kg
T = 105C T = 48C
P = 170 kN/m2 P = 170 kN/m2
Enterin) !oles
Steam = 18.2Kg mole
CO
2
= 9.5Kg mole
Dew point = 378 K
M
m
= 26.9Kg mole
"t inlet:
V.P of Steam = 111.7 KPa
nert Press = 170-111.7
= 58.3KPa
"t O,tlet
Partial p water = 12 KPa
nert Pressure = 158 KPa
Steam condensed = 17.5 Kg mole
Heat d,ty/
Partial Condenser
))
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Q
1
= heat of condensation +heat from UN condensed steam + heat from
CO
2
= 522311KJ/h
Similarly,
Q
2
= 9224541 KJ/h
Total Q = Q1
E
Q
2
Q = 1444762 KJ/h
!ass of Coolin) 0ater
Mw = 1444762 / 4.187 * (319-303)
= 5.04 Kg / sec
Exchan)er #ayo,t
1-2, shell & tube heat exchanger
1OD, 1 sq. pitch,
A = a
t
N
t
Lt
L
t
= 16 ft.
a
t
= 0.1963
A = 0.2618 16 Nt
N
t
= 111
N
t
= 166 (Nearest count)
So
A = 695.3 ft
2
U
D
= 57.91 Btu/hr. ft
2
.
o
F
)*
PRODUCTON OF CARBON DOXDE FROM FUEL OL
SPECI-IC"TIONS
Shell Side T,be Side
21 , C = 0.25 1 OD, 1 sqr. Pitch
B = 5 in 16. BWG
T,be side heat transfer coefficient
a
t
= N
t
* a
t
'
/ n
a
t
= 0.0120 m
2
G
W
= 700 Kg / sec m
2
At which
hi = 6.36 kW / m
2
K
hi
O =
hi * D / OD
= 5.25 kW / m
2
K
Shell side heat transfer coefficient
!ean Properties/
Cp
m
= 1.58 kJ / Kg K
k = 0.025 kW / mK
= 0 .015 mNs / m
2
a
s
=
t
1$$@
> / .7
a
S = 0.0411
m
2
&
s
F m1a
s
G
S
= 9.7 Kg / m
2
sec
De = 0.024 m
Re
s
= De Gs/
Re
S
= 24,000
J
H
= 300
h
o
= J
H
(K/De)(C/K)
1/3
h
o
= 107 W/ m
2
K
*+
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Clean o(erall heat transfer coefficient
(CP / k)
.A4
= 1.01
( / pD) = 0.62
k
G
= h
o
*(CP / k)
.A4
/cPsf*M
m
*( / pk
d
)
= 0.102/Psf
Point/ 3
T
g
= 278 K
p
g ==
120 kPa
p
S ==
38 kPa
T
w =
319K
A T = 59 K
Estimate T
c
= 352K
p
S
= 50 kPa
p
g =
120
kPa
Psf = 80 kPa
Now
h
g
(T
S
-T
c
) + k
G
(p
g
-p
S
) = hi
O
*(Tc-T
cm
)
229.7 = 230
AT = 230
U = 230/ (378-319)
= 3.89kW/ m
2
*K
Desi)n o(erall heat transfer coefficient
Point T
$
T
C
U -T .U -T/
a0
1 A
1 3() 3%3 23+ -- -- -
2 33* 3+1 1*& 213 1$% &.)
3 321 2** 1*$ 1*% 2%& 2+.3
*1
PRODUCTON OF CARBON DOXDE FROM FUEL OL
U
D
= Q / A * U AT
= 0.200kW / m
2
K
Desi)n Coefficient
Available surface area on
out side of tubes
= 0.060 m2/ m
= 33.9 m
2
Uc = 0.159 kW / m
2
K
Rd = (0.200 0.159) / (0.200 * 0.159)
= 1.2 m
2
K / kW
Press,re Drop
Shell side press,re drop
Separate flo0 model
(AP/L)
TP
= 4
L
2
(AP/L)
L
4
L
2
= E + 3.24 * F * H / (F r
.045
* Wg
.035
)
E = 0.488 F r = 0.4058
F = 2.97 Wg = 0.7528
H = 0.66
AP = 4 f
L
[L/ di ] * m
t
2
( 1 - x
2
) [ 1/ 2p
L
]
AP = 1.493 psi
GP
TP
F @ psi
T,be Side Press,re Drop
AP = f * G
t
2
* L * n/ (5.22 * 10
10
)
f =. 00026 from graph
AP
t
= 6 psi
AP
r
= (4 n / S) (V
2
/ 2g)
= 0.065 psi
Total pressure drop = 6.065 psi
*2
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Specification Sheet
dentification: Partial condenser
No. Required = 1
Function: Condense vapors by removing the latent heat of vaporization
Operation: Continuous
Type: 1-2 Horizontal Condenser
Shell side condensation
Heat Duty = 1444762 KJ/h
Tube Side:
Fluid handled cold water
Flow rate = 5.04 Kg / sec
Pressure = 170 kPa
Temperature = 30
o
C to 45
o
C
Tubes: 1 in. dia. 16 BWG
166 tubes each 16 ft long
2 passes
1 in square pitch
Shell Side:
Fluid handledCO
2
+ Steam
Flow rate 745 kg/hr
Pressure 170 kPa
Temperature 105
o
C to 48
o
C
Shell: 0.54 m. dia. 1 passes
Baffles spacing 0.127 m.
Utilities: Cold water
Ud assumed = 195 W/m
2
K Ud calculated = 200 W/m
2
K
Uc calculated = 159 W/m
2
K Allowed dirt factor = Rd = 1.2
PU!P SE#ECTION
-"CTORS "--ECTIN& CHOICE O- " PU!P
*3
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Many different factors can influence the final choice of a pump for a
particular operation. The following list indicates the major factors that
govern pump selection.
.
1) The amount of fluid that must be pumped. This factor determines the
size of pump (or pumps) necessary.
2) The properties of the fluid. The density and the viscosity; of the fluid
influence the power requirement for a given set of operating
conditions, corrosive properties of the fluid determine the acceptable
materials of construction. f solid particles are suspended in the fluid,
this factor dictates the amount of clearance necessary and may
eliminate the possibility of using certain types of pumps.
3) The increase in pressure of the fluid due to the work input of the
pumps. The head change across the pump is influenced by the inlet
and downstream reservoir pressures, the change in vertical height of
the delivery line, and frictional effects. This factor is a major item in
determining the power requirements.
4) Type of flow distribution. f nonpulsating flow is required, certain
types of pumps, such as simplex reciprocating pumps, may be
unsatisfactory. Similarly, if operation is intermittent, a self-priming
pump may be desirable, and corrosion difficulties may be increased.
5) Type of power supply. Rotary positive-displacement pumps and
centrifugal pumps are readily adaptable for use with electric-motor or
internal-combustion-engine drives; reciprocating pumps can be used
with steam or gas drives.
6) Cost and mechanical efficiency of the pump.
INSTRU!ENT"TION "ND CONTRO#
PROCESS CONTRO#
*$
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Measurement is a fundamental requisite to process
control. Either the control can be affected automatically, semi-automatically
or manually. The quality of control obtainable also bears a relationship to
the accuracy, re-producibility and reliability of the measurement methods,
which are employed. Therefore, selection of the most effective means of
measurements is an important first step in the design and formulation of
any process control system.
ObHecti(e of process control/
The basic objective of process control is;
Suppressing the influence of external disturbance
Ensuring the stability of chemical process
Optimize the performance of chemical process
Controllers
A typical controller is a process element that produces an
actuating signal to keep the out put at desired position
TE!PER"TURE !E"SURE!ENT "ND CONTRO#
Temperature measurement is used to control the temperature of
outlet and inlet streams in heat exchangers, reactors, etc.
Most temperature measurements in the industry are made by means of
thermo-couples to facilitate bringing the measurements to centralized
location. For local measurements at the equipment bi-metallic or filled
system thermometers are used to a lesser extent. Usually, for high
measurement accuracy, resistance thermometers are used.
All these meters are installed with thermo-wells when used locally. This
provides protection against atmosphere and other physical elements.
*%
PRODUCTON OF CARBON DOXDE FROM FUEL OL
PRESSURE !E"SURE!ENT "ND CONTRO#
Like temperature pressure is a valuable indication of material
state and composition. n fact, these two measurements considered
together are the primary evaluating devices of industrial materials.
Pumps, compressor and other process equipment associated with
pressure changes in the process material are furnished with pressure
measuring devices. Thus pressure measurement becomes an indication
of energy increase or decrease.
Most pressure measurement in industry are elastic element devices, either
directly connected for local use or transmission type to centralized location.
Most extensively used industrial pressure element is the Bourderi Tube or
a Diaphragm or Bellows gauges.
-#O: !E"SURE!ENT "ND CONTRO#
Flow-indicator-controllers are used to control the amount of liquid.
Also all manually set streams require some flow indication or some easy
means for occasional sample measurement. For accounting purposes,
feed and product stream are metered. n addition utilities to individual and
grouped equipment are also metered.
Most flow measures in the industry are/ by Variable Head devices. To a
lesser extent Variable Area is used, as are the many available types as
special metering situations arise.
Control schemes of re)eneration to0er
ObHecti(es
*&
PRODUCTON OF CARBON DOXDE FROM FUEL OL
n regeneration tower control any of following may be the goals
to achieve
1. pressure in the condenser
2. composition of vapors from Reboiler
3. Pressure in the Reboiler .
!anip,lated (ariables
Any one or any combination of following may be the manipulated variables
1. Steam flow rate to Reboiler.
2. Reflux rate.
3. Overhead product withdrawn rate.
5. Water flow rate to condenser.
#oads or dist,rbances
Following are typical disturbances
1. Flow rate of feed
2. Composition of feed.
3. Temperature of feed.
4. Steam rate to Reboiler
5. nlet temperature of water for condenser.
Control scheme/
Overhead product rate is fixed and any change in feed rate must be
absorbed by changing bottom product rate. The change in vapor pressure
of Reboiler is controlled by manipulating the steam rate to Reboiler,hence
controlling the internal reflux to the bottom of Regenerator.
H"IOP STUD$
HaDop
*(
PRODUCTON OF CARBON DOXDE FROM FUEL OL
A HAZOP survey is one of the most common and widely accepted
methods of systematic qualitative hazard analysis. t is used for both new
or existing facilities and can be applied to a whole plant, a production unit,
or a piece of equipment t uses as its database the usual sort of plant and
process information and relies on the judgment of engineering and safety
experts in the areas with which they are most familiar. The end result is,
therefore reliable in terms of engineering and operational
expectations, but it is not quantitative and may not consider the
consequences of complex sequences of human errors.
The objectives of a HAZOP study can be summarized as follows:
1) To identify (areas of the design that may possess a significant
hazard potential.
2) To identify and study features of the design that influence the
probability of a hazardous incident occurring.
3) To familiarize the study team with the design information
available.
4) To ensure that a systematic study is made of the areas of
significant hazard potential.
5) To identify pertinent design information not currently available to
the team.
6) To provide a mechanism for feedback to the client of the study
team's detailed comments.
A HAZOP study is conducted in the following steps:
1) Specify the purpose, objective, and scope of the study. The
purpose may he the analysis of a yet to be built plant or a review
*)
PRODUCTON OF CARBON DOXDE FROM FUEL OL
of the risk of un existing unit. Given the purpose and the
circumstances of the study, the objectives listed above can he
made more specific. The scope of the study is the boundaries of
the physical unit, and also the range of events and variables
considered. For example, at one time HAZOP's were mainly
focused on fire and explosion endpoints, while now the scope
usually includes toxic release, offensive odor, and environmental
end-points. The initial establishment of purpose, objectives, and
scope is very important and should be precisely set down so that
it will be clear, now and in the future, what was and was not
included in the study. These decisions need to be made by an
appropriate level of responsible management.
2) Select the HAZOP study team. The team leader should be skilled
in HAZOP and in interpersonal techniques to facilitate successful
group interaction. As many other experts should be included in
the team to cover all aspects of design, operation, process
chemistry, and safety. The team leader should instruct the team
in the HAZOP procedure and should emphasize that the end
objective of a HAZOP survey is hazard identification; solutions to
problems are a separate effort.
3) Collect data. Theodore16 has listed the following materials that
are usually needed:
Process description
Process flow sheets
**
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Data on the chemical, physical and toxicological properties of
all raw materials,, intermediates, and products.
Piping and instrument diagrams (P&Ds)
Equipment, piping, and instrument specifications
Process control logic diagrams
Layout drawings
Operating procedures
Maintenance procedures
Emergency response procedures
Safety and training manuals
1++
PRODUCTON OF CARBON DOXDE FROM FUEL OL
1+1
PRODUCTON OF CARBON DOXDE FROM FUEL OL
H"IOP &,ide :ords and !eanin)s
&,ide :ords !eanin)
No
Less
More
Part of
As well as
Reverse
Other than
Negation of design intent
Quantitative decrease
Quantitative increase
Qualitative decrease
Qualitative ncrease
Logical opposite of the intent
Complete substitution
4) Conduct the study. Using the information collected, the unit is
divided into study "nodes" and the sequence diagrammed in
Figure , is followed for each node. Nodes are points in the
process where process parameters (pressure, temperature,
composition, etc.) have known and intended values. These
values change between nodes as a result of the operation of
various pieces of equipment' such as distillation columns, heat
exchanges, or pumps. Various forms and work sheets have been
developed to help organize the node process parameters and
control logic information.
When the nodes are identified and the parameters are identified, each
node is studied by applying the specialized guide words to each
parameter. These guide words and their meanings are key elements of the
HAZOP procedure. They are listed in Table(10.1).
1+2
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Repeated cycling through this process, which considers how and why each
parameter might vary from the intended and the consequence, is the
substance of the HAZOP study.
5) Write the report. As much detail about events and their
consequence as is uncovered by the study should be recorded.
Obviously, if the HAZOP identifies a not improbable sequence of
events that would result in a disaster, appropriate follow-up action
is needed. Thus, although risk reduction action is not a part of the
HAZOP, the HAZOP may trigger the need for such action.
The HAZOP studies are time consuming and expensive. Just getting the P
& D's up to date on an older plant may be a major engineering effort. Still,
for processes with significant risk, they are cost effective when balanced
against the potential loss of life, property, business, and even the future of
the enterprise that may result from a major release.
3@H"IOP STUD$ O- CO
/
PRODUCT N"!E/ CARBON DOXDE
CHE!IC"# N"!E/ Carbon Dioxide
3@3 H"I"RD IDENTI-IC"TION/
EMERGENCY OVERVEW
Oxygen levels below 19.5% may cause asphyxia. Carbon dioxide
exposure can cause nausea and
respiratory problems. High concentrations may cause vasodilation leading
to circulatory collapse.
Contact with liquid product may cause frostbite in exposed tissues.
1+3
PRODUCTON OF CARBON DOXDE FROM FUEL OL
ROUTE O- ENTR$/
Skin Contact Yes
Skin Absorption No
Eye Contact Yes
nhalation Yes
ngestion Yes
HE"#TH E--ECTS/
Exposure Limits Yes
rritant No
Sensitization No
Teratogen No
Reproductive Hazard No
Mutagen No
Synergistic Effects None reported
E$E E--ECTS/
Contact with evaporating liquid may cause frostbite.
SJIN E--ECTS/
Contact with liquefied product may cause frostbite upon evaporation.
Frostbite effects are a change in color of
the skin to gray or white, possibly followed by blistering. Skin may become
inflamed and painful.
IN&ESTION E--ECTS/
ngestion is unlikely. Contact with liquid may cause frostbite.
INH"#"TION E--ECTS/
Carbon dioxide is the most powerful cerebral vasodilator known. nhaling
large concentrations causes rapid circulatory insufficiency leading to coma
and death. Asphyxiation is likely to occur before the effects of carbon
dioxide overexposure. Chronic, harmful effects are not known from
repeated inhalation of low concentrations.
1+$
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Low concentrations of carbon dioxide cause increased respiration and
headache.
Effects of oxygen deficiency may include: rapid breathing, diminished
mental alertness, impaired muscular coordination, faulty judgement,
depression of all sensations, emotional instability, and fatigue. As
asphyxiation progresses, nausea, vomiting, prostration, and loss of
consciousness may result, eventually leading to convulsions, coma, and
death.
Oxygen deficiency during pregnancy has produced developmental
abnormalities in humans and experimental animals.
3@ -IRST "ID !E"SURES/
E$ES/
Never introduce oil or ointment into the eyes without medical advice! n
case of freezing or cryogenic "burns"
by rapidly evaporating liquid. DO NOT WASH THE EYES WTH HOT OR
EVEN TEPD WATER! Remove
victim from the source of contamination. Open eyelids wide to allow liquid
to evaporate. f pain is present,
refer the victim to an ophthalmologist for further treatment and follow up. f
the victim cannot tolerate light,
protect eyes with a light bandage or handkerchief.
SJIN/
Remove contaminated clothing and flush affected area with cold water and
soap. DO NOT USE HOT WATER.
A physician should see the patient promptly if frostbite has occurred.
IN&ESTION/
A physician should see the patient promptly if frostbite has occurred.
INH"#"TION/
1+%
PRODUCTON OF CARBON DOXDE FROM FUEL OL
prompt medical attention is mandatory in all cases of overexposure to
carbon dioxide. Rescue personnel should be equipped with self-contained
breathing apparatus. Conscious persons should be assisted to an
uncontaminated area and inhale fresh air. Quick removal from the
contaminated area is most important. Unconscious persons should be
moved to an uncontaminated area, given mouth-to-mouth resuscitation
and supplemental oxygen. Further treatment should be symptomatic and
supportive.
H"ND#IN& "ND STOR"&E/
Non-Hazardous.
Dry carbon dioxide can be handled in most common structural materials.
Moist carbon dioxide is generally corrosive by its formation of carbonic
acid. For applications with moist Carbon Dioxide, 316, 309 and 310
stainless steels may be used as well as Hastelloy A, B, & C, and Monel
. Ferrous Nickel alloys are slightly susceptible to corrosion. At normal
temperatures carbon dioxide is compatible with most plastics and
elastomers. Use only in well-ventilated areas. Carbon dioxide vapor is
heavier than air and will accumulate in low areas. Valve protection caps
must remain in place unless container is secured with valve outlet piped to
use point. Do not drag, slide or roll containers. Use a suitable hand truck
for container movement. Do not heat containers by any means to increase
the discharge rate of product from the cylinder. Use a check valve or trap
in the discharge line to prevent hazardous back flow into the system.
Provide proper pressure relief valve.
Protect containers from physical damage. Store in cool, dry, well-ventilated
area away from heavily trafficked areas and emergency exits. Do not allow
the temperature where containers are stored to exceed 125oF (52 C).
Containers should be stored upright and firmly secured to prevent falling or
being knocked over. Full and empty cylinders should be segregated. Use a
1+&
PRODUCTON OF CARBON DOXDE FROM FUEL OL
"first in-first out" inventory system to prevent full containers being stored for
excessive periods of time.
Never carry a compressed gas cylinder or a container of a gas in cryogenic
liquid form in an enclosed space such as a car trunk, van or station wagon.
A leak can result in a fire, explosion, asphyxiation or a toxic exposure.
EN&INEERIN& CONTRO#S/
Use local exhaust to prevent accumulation of high concentrations so as to
reduce the oxygen level in the air to less than 19.5% and the carbon
dioxide concentration below the exposure limit.
E$E1-"CE PROTECTION/
Safety goggles or glasses as appropriate for the job. A face shield is
recommended for handling cryogenic liquid.
SJIN PROTECTION/
Protective gloves of any material appropriate for the job. nsulated gloves
are recommended for cryogenic
liquids.
RESPIR"TOR$ PROTECTION/
Positive pressure air line with full-face mask and escape bottle or self-
contained breathing apparatusshould be
available for emergency use.
H"IOP STUD$ O- !E"/
CHE!IC"# N"!E/ MEA, monoethanolamine
PH$SIC"# K CHE!IC"# CH"R"CTERISTICS
Vapor Density (air=1): Not determined.
Water Solubility: Complete.
Melting/Freezing Point: 10 deg. F
Appearance: liquid. Slightly more viscous than water.
1+(
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Specific Grav. (water=1): 1.025
Odor:
Ammoniacal odor in concentrate;
fresh, slightly ammoniacal and vegetable soap odor in dilution.
PH$SIC"# H"I"RD D"T"
Flammable Limits: Not determined.
Fire Fighting Media: Water spray, CO
2
, dry chemical; -- Treat primary
cause of fire.
Special Fire Fighting Procedures: None.
Fire/Explosion Hazards: No unusual hazards known.
RE"CTI'IT$ D"T"
Stability: Stable.
Hazardous Polymerization: Will not occur.
Conditions to Avoid: Long exposure to materials containing copper,
aluminum and strong oxidizing agents may cause discoloration.
ncompatible Materials: Strong oxidizing or reducing agents.
Hazardous Decomposition Products: f heated to decomposition, CO, CO
2
,
NOx and ammonia may be produced.
@ S$!PTO!S O- O'ERE>POSURE/
Symptoms of ngestion: ngestion of significant quantity of concentrate may
cause nausea, vomiting, and abdominal pain, incoordination, diarrhea and
general weakness. May cause red blood cell hemolysis and possible liver
and kidney injury.
Symptoms of nhalation: f misted in concentrated form can cause irritation
of mucous membrane, nose, eye and throat.
Symptoms of Skin Contact: May cause dermatitis or irritation in some
individuals upon prolonged contact. Localized skin defatting can be
expected from any detergent on long contact.
1+)
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Symptoms of Eye Contact: Causes painful stinging or burning of eyes and
lids, watering of eye, conjunctivitis, and in concentrated undiluted form
many cause opaqueness of cornea, possibly leading to loss of sight.
E!ER&ENC$ -IRST "ID PROCEDURES/
For ngestion: DO NOT attempt to induce vomiting. Have the individual
drink one or more full glasses or water. NEVER give anything to an
unconscious person. Call a physician or your local Poison Control Center.
Treatment should be directed at the control of symptoms and the clinical
condition of the patient. There is no specific antidote.
For Skin: As for all foreign materials, wash off concentrate or diluted use
solution with water. Remove clothing that has been saturated by
concentrate.
For Eyes: PROMPTLY flush with large amounts of water occasionally
lifting the lower and upper lids. Call a physician if irritation persists.
OCCUP"TION"# CONTRO# PROCEDURES/
Ventilation: Use with adequate ventilation. Working solution should not
present any hazard. f misted or an aerosol is generated, local or
mechanical exhaust recommended to maintain vapor concentration below
TLV. This level, however, should not be reached under normal working
conditions.
Respiratory Protection: Not required under normal working/use conditions.
Eye Protection: Not normally required. Use if in specific applications
working solution will get into eyes.
Skin Protection: Use gloves if hands will be continuously in solution, not
normally necessary in general uses.
Personal Hygiene: As in handling any detergent, wash thoroughly after
using.
1+*
PRODUCTON OF CARBON DOXDE FROM FUEL OL
COST ESTI!"TION
An acceptable plant design must present a process that is capable of
operating under conditions which will yield a profit. Since,
Net profit = total income-all expenses
t is essential that chemical engineer be aware of the many different types
of cost involved in manufacturing processes. Capital must be allocated for
direct plant expenses; such as those for raw materials, labor, and
equipment. Besides direct expenses, many other indirect expenses are
incurred, and these must be included if a complete analysis of the total
cost is to be obtained. Some examples of these indirect expenses are
administrative salaries, product distribution costs and cost for interplant
communication.
ESTIMATIN ! E"UI#MENT $ST
E.,ipment Nomenclat,re Cost *L+
Absorber A-1 56890
Condenser C-1 4342
Heat Exchanger H.X 23765
Reboiloer K-1 11652
Stripper St-1 45873
Pump P-1 3254
Blower B-1 1303
Direct contact cooler QT 2897
ESTIMATIN! TTA% $A#ITA% IN&ESTMENT
Purchased equipment cost = 186875
11+
PRODUCTON OF CARBON DOXDE FROM FUEL OL
Purchased equipment installation = 0.47 186875= 87831
nstrumentation & Process Control = 0.12 186875= 22425
Piping (installed) = 0.66 186875= 123337
Building (ncluding Services) = 0.18 186875=33637
Yard improvements = 0.1 186875= 18687
Service facilities (installed) = 0.7 186875=130812
Land = 0.06 186875 = 11212
Total direct plant cost = 428941
Indirect $ost
Engg. & Supervision = 0.33 186875= 61700
Construction expenses = 0.41 186875 = 76620
Total ndirect Cost = 138320
Total Direct & ndirect Cost = 567260
Contractor's fee = 0.05 567260= 28363
Contingency = 0.1 567260 = 56726
Fixed Capital nvestment = Total direct + indirect cost + contigency +
Contractor's fee
= 652350
Total Capital nvestment = F.C. + W.C.
Now W.C= 0.15 !.C."#
T.C. = 0.15 (652350+ W.C)
W.C = 115120
T.C. = 652350 + 115120
= 767500 US$
111
You might also like
- PCMS RBI Technical ManualDocument69 pagesPCMS RBI Technical ManualFebri Ramdani Nugraha100% (1)
- Encyclopedia of Chemistry From Wileypdf PDFDocument1,846 pagesEncyclopedia of Chemistry From Wileypdf PDFMira SucheaNo ratings yet
- DBE For Paint Stripping ApplicationsDocument5 pagesDBE For Paint Stripping ApplicationsGhufran SaeedNo ratings yet
- Mechanism of Buffering SystemDocument8 pagesMechanism of Buffering SystemRezaul Karim TutulNo ratings yet
- Carbon Storage and SequestrationDocument2 pagesCarbon Storage and SequestrationRadhaAnanthalekshmi100% (1)
- Cansolve TechnologiesDocument10 pagesCansolve TechnologiesBongibethu Msekeli HlabanoNo ratings yet
- Operating Manual GsuDocument89 pagesOperating Manual GsuVijendra Kumar GuptaNo ratings yet
- CO2 SMR White Paper - Shell Catalysts & TechnologiesDocument12 pagesCO2 SMR White Paper - Shell Catalysts & Technologiespierre-françois Le BouilleNo ratings yet
- The Cansolv System ProcessDocument33 pagesThe Cansolv System ProcessAsociación Juventud HuayránNo ratings yet
- Metalworking Brochure HuntsmanDocument58 pagesMetalworking Brochure Huntsman12bisiNo ratings yet
- Infographic Green HydrogenDocument1 pageInfographic Green HydrogenMASOUDNo ratings yet
- H2S Scavenger - OilfieldWikiDocument2 pagesH2S Scavenger - OilfieldWikiizzybjNo ratings yet
- Treat LPGs With AminesDocument12 pagesTreat LPGs With Amineskaaskopdawie5755No ratings yet
- CO2 RemovalDocument32 pagesCO2 Removalaehque04100% (2)
- 07 Hydrogen From SMRDocument6 pages07 Hydrogen From SMRmaheshNo ratings yet
- Chapter6a Hydrotreating 170106114901 PDFDocument26 pagesChapter6a Hydrotreating 170106114901 PDFMI MNo ratings yet
- Literature Review On Carbon Dioxide Capture by AbsorptionDocument21 pagesLiterature Review On Carbon Dioxide Capture by AbsorptionTU_MTECH_ENV11No ratings yet
- Atmospheric CO2 To MethanolDocument16 pagesAtmospheric CO2 To MethanolMUTHU KESHAV KNo ratings yet
- Ibp1502 12Document9 pagesIbp1502 12Marcelo Varejão CasarinNo ratings yet
- Gasification: The Waste-To-Energy SolutionDocument14 pagesGasification: The Waste-To-Energy Solutionmamboking1No ratings yet
- 2017-04 CO2 Capture in Nat Gas ProductionDocument86 pages2017-04 CO2 Capture in Nat Gas ProductionDiegoNo ratings yet
- Fossil Power Generation With Carbon Capture and Storage (CCS) : Policy Development For Technology DeploymentDocument24 pagesFossil Power Generation With Carbon Capture and Storage (CCS) : Policy Development For Technology Deploymentjubatus.libroNo ratings yet
- Aspect of Degradation of Monoethanolamine PDFDocument379 pagesAspect of Degradation of Monoethanolamine PDFFaisal MoeNo ratings yet
- Assignment 2 Process and Dynamic System Modelling (PPSD)Document15 pagesAssignment 2 Process and Dynamic System Modelling (PPSD)Husaini ZaidanNo ratings yet
- CCS For LNG LiquefactionDocument17 pagesCCS For LNG Liquefactionbkonly4uNo ratings yet
- Lspom Biogas Plant FlowchartDocument1 pageLspom Biogas Plant Flowchartkiller120No ratings yet
- Lurgi and Ammonia Casale Have Disclosed Their Jointly Developed Ammonia Process TechDocument2 pagesLurgi and Ammonia Casale Have Disclosed Their Jointly Developed Ammonia Process TechSisca AmeliaNo ratings yet
- Zarei 2016Document34 pagesZarei 2016IffatNo ratings yet
- Case Story DK - Green Methanol - Web 1Document4 pagesCase Story DK - Green Methanol - Web 1Pao M. MorenoNo ratings yet
- Selexol Vs RectisolDocument3 pagesSelexol Vs RectisolRaguNo ratings yet
- CCS Directive Evaluation Interim Report PDFDocument89 pagesCCS Directive Evaluation Interim Report PDFmardiradNo ratings yet
- Petrochemical 1Document68 pagesPetrochemical 1AnilKumarNo ratings yet
- Fossil Hydrocarbons: Chemistry and TechnologyFrom EverandFossil Hydrocarbons: Chemistry and TechnologyRating: 3 out of 5 stars3/5 (1)
- Carbon Capture and Sequestration-A Review: IOP Conference Series: Earth and Environmental ScienceDocument11 pagesCarbon Capture and Sequestration-A Review: IOP Conference Series: Earth and Environmental ScienceVika XieNo ratings yet
- Crude and ProductsDocument65 pagesCrude and ProductsAmit KrNo ratings yet
- Bringing It Together: Sophie Babusiaux Leandro Labanca Marie-Amélie Lambert Remi MoniotDocument41 pagesBringing It Together: Sophie Babusiaux Leandro Labanca Marie-Amélie Lambert Remi MoniotvietnampetrochemicalNo ratings yet
- Process Systems and Materials for CO2 Capture: Modelling, Design, Control and IntegrationFrom EverandProcess Systems and Materials for CO2 Capture: Modelling, Design, Control and IntegrationAthanasios I. PapadopoulosNo ratings yet
- Ethanolamines From Ethylene Oxide and AmmoniaDocument1 pageEthanolamines From Ethylene Oxide and AmmoniaBramJanssen76100% (1)
- 12-Modelling For Capture of Carbon Dioxide Using Aqueous Ammonia From Flue Gases of A Brick KilnDocument8 pages12-Modelling For Capture of Carbon Dioxide Using Aqueous Ammonia From Flue Gases of A Brick KilnWARP-World Academy of Research and PublicationNo ratings yet
- 2019jan - IECM Amine-Based CO2 Capture PDFDocument63 pages2019jan - IECM Amine-Based CO2 Capture PDFFunky labsNo ratings yet
- CO2 Absorption Simulation PaperDocument11 pagesCO2 Absorption Simulation PaperUmer AzharNo ratings yet
- Part 1: Design, Modeling and Simulation of Post-Combustion CO Capture Systems Using Reactive SolventsDocument24 pagesPart 1: Design, Modeling and Simulation of Post-Combustion CO Capture Systems Using Reactive SolventsBenjamin F ZavalaNo ratings yet
- Green Hydrogen Characterisation Initiatives Definitions, StandardsDocument13 pagesGreen Hydrogen Characterisation Initiatives Definitions, Standardsjorge bustosNo ratings yet
- Combined ProjectDocument83 pagesCombined ProjectArpit Patel100% (1)
- Integration of Gasification With Thermal Residue Conversion in RefineriesDocument15 pagesIntegration of Gasification With Thermal Residue Conversion in Refineriesrameshkarthik810No ratings yet
- Claus ProcessDocument6 pagesClaus ProcessRafi AlgawiNo ratings yet
- Desulfurization of Natural Gas LiquidsDocument21 pagesDesulfurization of Natural Gas LiquidsZoran ČeralinacNo ratings yet
- 3.2 - Ballaguet & Barrère-Tricca - Sulphur CycleDocument24 pages3.2 - Ballaguet & Barrère-Tricca - Sulphur CyclesantiagoNo ratings yet
- CO2 FloodingDocument15 pagesCO2 FloodingUzumaki28No ratings yet
- SCCS CO2 EOR JIP Tax Study Non Technical Report 2013Document23 pagesSCCS CO2 EOR JIP Tax Study Non Technical Report 2013Farzaneh SedighiNo ratings yet
- Review IChemE PartA Full Paper V9 28may2010 PDFDocument22 pagesReview IChemE PartA Full Paper V9 28may2010 PDFaziziNo ratings yet
- Advances in Carbon CaptureDocument3 pagesAdvances in Carbon Capturetassili17No ratings yet
- Integrated Gasification Combined Cycle: Acid Gas RemovalDocument3 pagesIntegrated Gasification Combined Cycle: Acid Gas RemovaljuhriloverNo ratings yet
- A New Dawn For CO2 EORDocument5 pagesA New Dawn For CO2 EORmnoriegalNo ratings yet
- Cryogenic TurboexpandersDocument9 pagesCryogenic TurboexpandersDwinaRahmayaniNo ratings yet
- Desulfurization v1.2 (Final)Document35 pagesDesulfurization v1.2 (Final)Talha JamilNo ratings yet
- Biodiesel 36244Document110 pagesBiodiesel 36244api-3802837100% (1)
- Paper On Regenerator Duty of CO2 Absorption System Using DETA and AmineDocument7 pagesPaper On Regenerator Duty of CO2 Absorption System Using DETA and AmineHuraira TahirNo ratings yet
- Rectisol Wash Units: Acid Gas Removal For Polygeneration Concepts Downstream GasificationDocument34 pagesRectisol Wash Units: Acid Gas Removal For Polygeneration Concepts Downstream Gasificationpippo2378793No ratings yet
- Steam Reforming Carbon FormationDocument26 pagesSteam Reforming Carbon FormationBilalNo ratings yet
- Enhanced Oil RecoveryDocument2 pagesEnhanced Oil RecoveryEl Sayed Zakaria100% (1)
- Crude Oil Refinery-Short VersionDocument14 pagesCrude Oil Refinery-Short Versionligia hancuNo ratings yet
- Improve Amine Unit Efficiency by Optimazing Operating Conditiong PDFDocument4 pagesImprove Amine Unit Efficiency by Optimazing Operating Conditiong PDFRobert MontoyaNo ratings yet
- Incineration of Municipal Waste: Specialized Seminars on Incinerator Emissions of Heavy Metals and Particulates, Copenhagen, 18–19 September 1985 and Emission of Trace Organics from Municipal Solid Waste Incinerators, Copenhagen, 20–22 January 1987From EverandIncineration of Municipal Waste: Specialized Seminars on Incinerator Emissions of Heavy Metals and Particulates, Copenhagen, 18–19 September 1985 and Emission of Trace Organics from Municipal Solid Waste Incinerators, Copenhagen, 20–22 January 1987Robert B. DeanNo ratings yet
- Lect. 2Document5 pagesLect. 2xa53dasNo ratings yet
- Industrial GasesDocument56 pagesIndustrial GasesnadyahginiceNo ratings yet
- Hazop Study Worksheet: Inlet Valve Malfunction Fully Open Inlet Valve Malfunction CloseDocument12 pagesHazop Study Worksheet: Inlet Valve Malfunction Fully Open Inlet Valve Malfunction CloseGhufran SaeedNo ratings yet
- Lesson 3Document11 pagesLesson 3Ghufran SaeedNo ratings yet
- Biolime Brochure Nov 2010Document16 pagesBiolime Brochure Nov 2010Ghufran SaeedNo ratings yet
- Material Safety Data Sheet: SECTION I. Chemical Product and Company IdentificationDocument6 pagesMaterial Safety Data Sheet: SECTION I. Chemical Product and Company IdentificationGhufran SaeedNo ratings yet
- Unite: Sartori Et A1.Document13 pagesUnite: Sartori Et A1.haddig8No ratings yet
- Carbon Dioxide Adborption Into Promoted Potassium Carbonate SolutionsDocument13 pagesCarbon Dioxide Adborption Into Promoted Potassium Carbonate SolutionsLucia RojasNo ratings yet
- Patente Proceso aMDEA de BASFDocument9 pagesPatente Proceso aMDEA de BASFizurietaeduardoNo ratings yet
- Fosfa InternationalDocument29 pagesFosfa InternationaljesusNo ratings yet
- Diffusivity of MEA in WaterDocument7 pagesDiffusivity of MEA in WaterHeng-Ji ChanNo ratings yet
- 2016 OLI SimSci Partnership - Rasika - Nimkar - FINAL RevDocument26 pages2016 OLI SimSci Partnership - Rasika - Nimkar - FINAL RevShayne229No ratings yet
- TeaDocument7 pagesTeajohnsopranaNo ratings yet
- Us 5362842Document7 pagesUs 5362842giovanniNo ratings yet
- KGSP PDFDocument12 pagesKGSP PDFEmiZNo ratings yet
- FOSFA List of Banned Immediate Previous Cargoes With Flowchart Nov 2022Document3 pagesFOSFA List of Banned Immediate Previous Cargoes With Flowchart Nov 2022jesusNo ratings yet
- Unit-5, POC-1, SEM-2Document53 pagesUnit-5, POC-1, SEM-2Avinash rajNo ratings yet
- Mass Transfer Study Using An Electrochemical MethodDocument7 pagesMass Transfer Study Using An Electrochemical MethodinstrutechNo ratings yet
- Izopropanolaminlerle Meral Sıvıları FormuluDocument4 pagesIzopropanolaminlerle Meral Sıvıları FormuluahmetNo ratings yet
- European Patent Application: Method and Plant For The Production of Ethanol AminesDocument17 pagesEuropean Patent Application: Method and Plant For The Production of Ethanol AmineswanNo ratings yet
- Chapter 8 - Manufacture of C2 CompoundDocument39 pagesChapter 8 - Manufacture of C2 CompoundMicrosoft GamingNo ratings yet
- Oxidative Degradation of MeaDocument9 pagesOxidative Degradation of Meacosmicbabe_2000No ratings yet
- Sabic® Diethanolamine Dea 99: DescriptionDocument2 pagesSabic® Diethanolamine Dea 99: DescriptionHarisNo ratings yet
- Monoethanolamine Methods 2520of 2520productionDocument2 pagesMonoethanolamine Methods 2520of 2520productionSanjay KumarNo ratings yet
- Absorption of Carbon Dioxide in Aqueous Ammonia: Energy ProcediaDocument8 pagesAbsorption of Carbon Dioxide in Aqueous Ammonia: Energy ProcediaElnovistaNo ratings yet
- Diacid Corrosion Water Based FluidsDocument18 pagesDiacid Corrosion Water Based FluidsNgũ Viên Gia CácNo ratings yet