Professional Documents
Culture Documents
Chem c2 Exer1
Uploaded by
jalrizal7Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Chem c2 Exer1
Uploaded by
jalrizal7Copyright:
Available Formats
1. Name the alcohol with the molecular formula as shown below. 2.
Name the alcohol with the molecular formula:
3. Name the alcohol with the molecular formula:
4. Name the alcohol with the molecular formula:
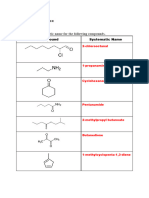
5. What is the general formula of the alcohol family? 6. Name the functional group of the alcohol family. . When ethanol is !issol"e! in water# the solution !oes not affect litmus paper. $owe"er# when so!ium hy!ro%i!e is !issol"e! in water# the solution turns re! litmus paper into blue. With rele"ant e&uations# e%plain this obser"ation. '. What is the role that the functional group plays in the chemical properties of the alcohol family? (. )i"e the *+,-. name of the organic compoun! gi"en below
12. 11.
12.
13.
14.
15.
a/ Write the structural formula for 20methylpropan010ol. b/ 1tate the similarity an! !ifference between the organic compoun!s abo"e with butan020ol Write the chemical e&uation for the complete combustion of propan020ol Write the chemical e&uation for each of the following reactions an! name the organic pro!uct obtaine!. a/ ,ropan010ol 3 mi%ture of potassium !ichromate45*/ an! !ilute sulphuric aci! b/ 6utan020ol 3 concentrate! sulphuric aci! at 1'27. c/ 6utan010ol "apour passe! o"er hot porous pot chips Which of the following molecular formulae represents the first three members of the alcohol family? -. .2$58$# .3$ 8$# .4$(8$ 6. .$48$# .2$68$# .3$'8$ .. .$38$# .2$58$# .3$ 8$ 9. .$8$# .2$48$# .3$68$ When ethanol "apour is channele! through hot porous clay pieces# a certain gas is pro!uce!. Which of the following is not the characteristic of the gas pro!uce!? -. .hanges the litmus solution colour to re! 6. 9ecolourises bromine water .. 9ecolourises aci!ifie! potassium manganate4*5/ solution 9. ,ro!uces carbon !io%i!e gas an! water when burnt 9iscuss the or!er of change in the boiling points of members of alcohol homologous series as the number of carbon atoms per molecule increases. :thanol reacts with so!ium accor!ing to the following e&uation 2.2$58$ 4a&/ 3 2Na 4s/ ; 2.2$58Na 4a&/ 3 $2 4g/ 1o!ium etho%i!e an! hy!rogen gas are forme!. ,re!ict the reaction of butan010ol with so!ium.
1(. 6utane can be con"erte! to butanol at a temperature of 3227. an! in the presence of a catalyst which is -. ,otassium manganite45**/ 6. .opper4**/ o%i!e .. Nic<el 9. ,hosphoric aci! 22. :thanol can be prepare! in laboratory from the reaction between -. :thene an! hy!rogen 6. :thene an! steam .. :thane an! hy!rogen 9. :thane an! steam 21. When ethanol "apour is channele! through hot porous clay pieces# a certain gas is pro!uce!. Which of the following is not the characteristic of the gas pro!uce!? -. .hanges the litmus solution colour to re! 6. 9ecolourises bromine water .. 9ecolourises aci!ifie! potassium manganite45**/ solution 9. ,ro!uces carbon !io%i!e an! water when burnt 22. Which of the following reactions will pro!uce carbon !io%i!e? *. =ermentation **. .ombustion of alcohol ***. >he reaction between carbo%ylic aci! with so!ium carbonate *5. >he reaction between alcohol an! carbo%ylic aci! -. * an! *** only 6. ** an! *5 only .. *# ** an! *** only 9. *# **# *** an! *5 23. Which of the following statements !escribes the characteristic of ethanol? -. :thanol has the ?8$ group which ma<es it al<aline 6. :thanol has the ?8$ group which forms a bon! with water when it !issol"es in water .. :thanol can react with water when it !issol"es in water 9. :thanol can be o%i!ise! into ethane 24. What is the general formula of carbo%ylic aci!? 25. 9raw the structural formula for ethanoic aci! an! propanoic aci!. 26. Which of the following compoun!s belong to the same homologous series as ethanoic aci!? -. .2$5.$28$ 6. .$3.88.$3 .. .2$5.88$ 9. $.88.2$5 2 . )i"e two physical properties of ethanoic aci!. 2'. Name the pro!ucts of reaction between ethanoic aci! an! nagnesium. 2(. Name the reaction between ethanoic aci! an! so!ium hy!ro%i!e.Write an e&uation for reaction. 32. Write the e&uation for the reaction between calcium carbonate with ethanoic aci!. 31. Name the first member of the carbo%ylic aci! family. 9raw the structural formula of the aci! molecule. With the help of rele"ant e&uations# !escribe how the aci! in reacts with a/ 1o!ium b/ @agnesium carbonate c/ .opper4**/ hy!ro%i!e 32. .ompoun!s , an! A ha"e the same number of carbon atoms. When 1 mole of compoun! , is burne! completely# 3 moles of carbon !io%i!e are pro!uce!. .ompoun! , is neutral whereas compoun! A is aci!ic.
16. :thanol can be pro!uce! by the fermentation of glucose using yeast in the laboratory. Write the e&uation for this process. 1 . .omplete the following table Process 8%i!ation Alcohol :thanol ,ropanol Product ,ropene
1'. Name the alcohol use! to manufacture butane. Write an e&uation for the reaction.
a/ Name the compoun!s , an! A. b/ .ompoun! , is con"erte! into compoun! A by process i. name the reaction in process i. c/ Write an e&uation for process i. !/ What is the name of compoun! B? e/ Name the process ii. f/ )i"e an e&uation to represent process ii. 33. )i"e the general formula of the ester family. 34. - carbo%ylic aci! reacts with an alcohol in the presence of concentrate! sulphuric aci! to pro!uce a salt an! water. *s this statement correct? :%plain your answer. 35. Name an! !raw the structural formula of the ester pro!uce! from a reaction between a/ @ethanol 3 propanoic aci!
b/ :thanol 3 butanoic aci! c/ ,ropan010ol 3 ethanoic aci! 36. -n esterification is carrie! out to prepare an ester from the first member of the carbo%ylic aci! family. >he ester has a total of three carbon atoms. a/ What is the functional group of the esters? b/ Name the alcohol nee!e! to prepare the ester c/ Write an e&uation for the reaction !/ 6riefly !escribe how the reaction can be carrie! out 3 . -n ester B is prepare! from an alcohol , an! a carbo%ylic aci! A. the molar mass , an! A are gi"en below Substan Molar mass (g mol-1) ce , 46 A 4 a/ )i"en that the relati"e atomic mass of $: 1# .: 12 an! 8: 16. 9etermine the molecular formula of alcohol , an! carbo%ylic aci! A b/ $ow an! un!er what con!itions will alcohol , an! carbo%ylic aci! A react to pro!uce an ester B? c/ Write an e&uation for the reaction in b. 3'. Name the elements that ma<e up fats 3(. Name the sources of each the following fats Source Common name 1tearic aci! Cauric aci! ,almitic aci! 42. Name glycerol using the *+,-. system. 9raw the structural formula for glycerol 41. 1tate the two uses of fat in human 42. 8ur bo!ies nee! fats an! oils. >hey are essential in helping us maintain healthy an! strong bo!ies. 9iscuss the roles fats an! oils play in helping us to maintain a healthy bo!y. 43. 1tate one importance of fats to our health 44. 1tate one a!"erse effect of e%cessi"e inta<e of fats to our bloo! "essels 45. 6ase! on the !iagram below
e/ Name one cleaning agent in our househol! that is ma!e from palm oil 4(. - natural polymer has a structure shown in figure below.
What is a polymer? What class of polymer is shown abo"e? Name the monomer for this polymer. .arbohy!rate is another e%ample of a natural polymer. i. Name two e%amples of carbohy!rates. ii. What is the monomer of carbohy!rates? 52. ,art of the structure of a natural rubber chain is shown below.
a/ b/ c/ !/
a/ )i"e the molecular formula of the fat molecule shown abo"e b/ $ow can this molecule be prepare! in the laboratory? c/ :%plain the meaning of saturate! fat an! unsaturate! fat. !/ $ow can an unsaturate! fat be con"erte! into a saturate! fat? 46. >he following e&uation shows the formation of fat an! oil from a fatty aci! an! glycerol. =atty aci! 3 )lycerol ; =at an! oil a/ Name the functional group foun! in fatty aci!s b/ Name the functional group foun! in glycerol c/ When a fatty aci! an! glycerol are combine!# fat is forme!. What can one !e!uce about the chemical nature of fat? !/ )i"e one e%ample of a saturate! fatty aci! e/ Name the fat or oil pro!uce! when the saturate! fatty aci! reacts with glycerol 4 . =ats an! oils are classifie! as saturate! fats an! unsaturate! fats. 1aturate! fats are ma!e from saturate! fatty aci!s whereas unsaturate! fats are ma!e from unsaturate! fatty aci!s. a/ )i"e one effect of eating a !iet high in saturate! fats b/ )i"e one health benefit of consuming palm oil c/ >he structure of an unsaturate! fatty aci! is shown below
a/ Name the monomer of this polymer b/ What is the type of reaction that Doins the monomers together to form natural rubber? c/ 6riefly !escribe how coagulation of late% ta<en place when an aci! is a!!e!? 51. =igure below shows a section of "ulcaniEe! rubber a/ $ow is "ulcaniEation of rubber carrie! out in the school laboratory? b/ *!entify the atoms that lin< the rubber chains together c/ What is the structure in the rubber chain allows "ulcaniEation to occur? !/ 1tate three properties in which "ulcaniEe! rubber is better than un"ulcanise! rubber 52. Which of the following are the properties of "ulcaniEe! rubber? i. 5ulcaniEe! rubber is har!er than un"ulcanise! ii. 5ulcaniEe! rubber is blac< in colour iii. 5ulcaniEe! rubber has sulphur lin<ages between the polymer chains i". 5ulcaniEe! rubber is more elastic than un"ulcanise! rubber -. i# ii an! iii 6. i# ii an! i" .. i# iii an! i" 9. ii# iii an! i" 53. Which of the following chemicals can pre"ent the coagulation of late%? -. 1ulphur !ichlori!e 6. $y!rochloric aci! .. 1o!ium hy!ro%i!e 9. ,ropanol 54. =reshly0tape! rubber late% will coagulate if it is left for a !ay without any chemical a!!e!. >his is !ue to the presence of F in the late%. Which of the following is F? -. 6acteria 6. 1o!ium hy!ro%i!e .. -mmonia 9. 1ulphuric aci! 55. >he !iagram shows the con"ersion of late% to "ulcanise! rubber
a/ Name the reaction i b/ Name the chemical that is a!!e! to late% in reaction i c/ 9raw the structural formula of the monomer of natural rubber an! name it !/ 9raw the repeating unit for natural rubber e/ $ow is reaction ii carrie! out in the in!ustry 56. Gust li<e al<anes# ester compoun!s also form a homologous series. 9iagram below shows the part structure of an ester.
O C H O
i. What is obser"e! when this fatty aci! is sha<en with bromine water? ii. 2.12 mole of this fatty aci! is reacte! with e%cess bromine. .alculate the number of moles of bromine reacte! 4'. ,alm oil has many uses. *t is wi!ely use! in the foo! in!ustry as well as for in!ustrial processes a/ )i"e one reason why palm oil is consi!ere! as a better source of coo<ing oil compare! to other plant oils b/ Name the "itamin which is rich in palm oil that can help in !elaying ageing an! fight against arteriosclerosis c/ Name a substance which imparts the re!!ish colour to palm oil an! has anti0cancer properties !/ Name one palm oil pro!uct which is use! in ma<ing ca<es an! biscuits
a/ What is a homologous series? b/ .opy an! complete the structure in !iagram abo"e to show an ester obtaine! from an esterification reaction between propan010ol an! methanoic aci!. c/ Write an e&uation for the esterification reaction in b. !/ )i"e the reaction con!itions for the esterification reaction
You might also like
- Chem and Phy RevisionDocument89 pagesChem and Phy RevisionBwalya kelvinNo ratings yet
- Carbon and Its CompoundsDocument10 pagesCarbon and Its CompoundsSankar MuthuswamyNo ratings yet
- CBSE Class 10 Chemistry Worksheet - Carbon and Its CompoundDocument4 pagesCBSE Class 10 Chemistry Worksheet - Carbon and Its Compoundomm2500100% (1)
- International Indian School Chemistry Worksheet on Carbon CompoundsDocument4 pagesInternational Indian School Chemistry Worksheet on Carbon CompoundsRaghav GuptaNo ratings yet
- Chemistry 10Document14 pagesChemistry 10BehruzNo ratings yet
- Chemistry Class: O-Level Time: 50 Min Marks: 45Document7 pagesChemistry Class: O-Level Time: 50 Min Marks: 45Zainab ShigriNo ratings yet
- Carbon and It's Compounds Assignment + WorksheetDocument34 pagesCarbon and It's Compounds Assignment + WorksheetDishant KumarNo ratings yet
- 02 Alcohol and Carboxylic Acid QPDocument7 pages02 Alcohol and Carboxylic Acid QPcharlesma123No ratings yet
- Macromolecules: Large Molecules Formed by Covalent BondsDocument8 pagesMacromolecules: Large Molecules Formed by Covalent Bondsafoo1234No ratings yet
- Carbon and Its CompoundsDocument3 pagesCarbon and Its CompoundsPooja Debnath100% (1)
- Class X Term Ii Fa Science PDFDocument15 pagesClass X Term Ii Fa Science PDFgodwinmodernschoolNo ratings yet
- Why Is Carbon So Important in Spite of It Being Present in A Very Small QuantityDocument2 pagesWhy Is Carbon So Important in Spite of It Being Present in A Very Small QuantitySuman DasNo ratings yet
- Cbse Test Paper-05: Science & Technology (Class-10) Chapter 4. Carbon and Its CompoundsDocument1 pageCbse Test Paper-05: Science & Technology (Class-10) Chapter 4. Carbon and Its CompoundsbannaduraiNo ratings yet
- DocumentDocument89 pagesDocumentRajeev Sharma100% (1)
- CHAPTER - 4-Carbon and Its CompoundDocument2 pagesCHAPTER - 4-Carbon and Its CompoundHimanshu JainNo ratings yet
- Chemistry Form 5 Module Organic CompoundDocument23 pagesChemistry Form 5 Module Organic CompoundMudzaffar Shah100% (3)
- Carbon and Its CompoundsDocument15 pagesCarbon and Its CompoundsSahil baggaNo ratings yet
- Carbon compounds guideDocument14 pagesCarbon compounds guideharryNo ratings yet
- Carbon and It's Compounds Theory and Worksheet Class 10Document15 pagesCarbon and It's Compounds Theory and Worksheet Class 10subham kumarNo ratings yet
- ICSE Sample Question Paper On Organic Chemistry Full Marks: 80 Time Alloted: An Hour and A HalfDocument5 pagesICSE Sample Question Paper On Organic Chemistry Full Marks: 80 Time Alloted: An Hour and A HalfSatya KamNo ratings yet
- Ujian 2 Form 5Document9 pagesUjian 2 Form 5Nazreen NashruddinNo ratings yet
- Combined Past Paper Questions On Organic ChemistryDocument155 pagesCombined Past Paper Questions On Organic ChemistryRamesh Iyer100% (2)
- CBSE Class 10 Chemistry Worksheet - Carbon and Its CompoundsDocument5 pagesCBSE Class 10 Chemistry Worksheet - Carbon and Its CompoundsSreejit0% (1)
- Carbon and Its Compounds HTTPDocument3 pagesCarbon and Its Compounds HTTPsanju sharmaNo ratings yet
- Revision ChemDocument32 pagesRevision ChemNada AlbuainainNo ratings yet
- ChemistryDocument6 pagesChemistrySafalsha BabuNo ratings yet
- Chapter Sk027 OverviewDocument12 pagesChapter Sk027 OverviewNor AfidahNo ratings yet
- Molecular Formula WorksheetTITLE Organic Compounds Nomenclature Worksheet TITLE Organic Reactions & Identification WorksheetTITLE Properties of Alcohols & Carboxylic AcidsDocument8 pagesMolecular Formula WorksheetTITLE Organic Compounds Nomenclature Worksheet TITLE Organic Reactions & Identification WorksheetTITLE Properties of Alcohols & Carboxylic Acidsbhumika motiyaniNo ratings yet
- NSS Chemistry Part 11 Chemistry of Carbon CompoundsDocument47 pagesNSS Chemistry Part 11 Chemistry of Carbon CompoundsFelix YueNo ratings yet
- Section II Q No. 2. Attempt Any Eight Parts Out of TwelveDocument4 pagesSection II Q No. 2. Attempt Any Eight Parts Out of TwelveUsama IjazNo ratings yet
- Carbon and Its CompoundsDocument27 pagesCarbon and Its CompoundstechvipreshNo ratings yet
- Previous Year Questions Carbon & Its CompoundsDocument41 pagesPrevious Year Questions Carbon & Its CompoundsRehan MotiwalaNo ratings yet
- Organic WsDocument38 pagesOrganic Wsranim ismailNo ratings yet
- CBSE Class 10 Science - Carbon and Its CompoundsDocument9 pagesCBSE Class 10 Science - Carbon and Its CompoundsAbhishek kumarNo ratings yet
- CQ Petroleum ProductsDocument15 pagesCQ Petroleum Productsapi-3826629No ratings yet
- HB Chapter 3 Test2013Document5 pagesHB Chapter 3 Test2013Julia MontoneNo ratings yet
- CH4 Hots QuestionsDocument1 pageCH4 Hots Questionsprabhat7969tiagoNo ratings yet
- Carbon and It S Compounds ANSWER KEYDocument6 pagesCarbon and It S Compounds ANSWER KEYSmrithi ChandrashekarNo ratings yet
- Class X Chapter4Document12 pagesClass X Chapter4kannan2030No ratings yet
- 12th-class-guess-papers-2024-chemistry-short (2)Document7 pages12th-class-guess-papers-2024-chemistry-short (2)tahajalil1074No ratings yet
- Assignment C CDocument3 pagesAssignment C CSumathi SrinivasNo ratings yet
- QB - Aldehydes, Ketones and Carboxylic AcidsDocument5 pagesQB - Aldehydes, Ketones and Carboxylic AcidsAkshith ReddyNo ratings yet
- IT Chem F5 Topical Test 2 (E)Document7 pagesIT Chem F5 Topical Test 2 (E)Norzawati NoordinNo ratings yet
- Carbon and Its CompoundsDocument2 pagesCarbon and Its Compoundsdeepan kumar100% (1)
- Organic Chem ExDocument15 pagesOrganic Chem Exwan zhouNo ratings yet
- Tutorial 5.1Document3 pagesTutorial 5.1Ijal JaminNo ratings yet
- Chapter 19: Carboxylic Acids and The Acidity of The O-H BondDocument22 pagesChapter 19: Carboxylic Acids and The Acidity of The O-H BondeliNo ratings yet
- Untitled Document-4Document9 pagesUntitled Document-4contacts.sawanNo ratings yet
- Crude Oil Fractions Distillation ProcessDocument11 pagesCrude Oil Fractions Distillation Processcharlesma123No ratings yet
- Chapter 19 The Chemistry of Metabolism: Essential Organic Chemistry (Bruice)Document11 pagesChapter 19 The Chemistry of Metabolism: Essential Organic Chemistry (Bruice)tyron9520No ratings yet
- Organic Chemistry: Carboxylic Acids and EstersDocument20 pagesOrganic Chemistry: Carboxylic Acids and EstersAbhi RamNo ratings yet
- Chemistry work sheet for grade 12th on polymersDocument4 pagesChemistry work sheet for grade 12th on polymersousmanwizdan6No ratings yet
- Alcohol and Carboxylic Acid 4Document6 pagesAlcohol and Carboxylic Acid 4Geraldine LatupeirissaNo ratings yet
- Organic Chemistry Practice TestDocument4 pagesOrganic Chemistry Practice TestGirishmaNo ratings yet
- Organic Chemistry: Tutor: Abhiram Date: 29/11/2016 Cambridge IGCSEDocument20 pagesOrganic Chemistry: Tutor: Abhiram Date: 29/11/2016 Cambridge IGCSEAbhi RamNo ratings yet
- Class 12 Chemistry Chapter 14 Biomolecules MCQs SolvedDocument19 pagesClass 12 Chemistry Chapter 14 Biomolecules MCQs SolvedTayseer SaudiaNo ratings yet
- Practice Makes Perfect in Chemistry: Organic ChemistryFrom EverandPractice Makes Perfect in Chemistry: Organic ChemistryRating: 3 out of 5 stars3/5 (1)
- Set K2Document2 pagesSet K2jalrizal7No ratings yet
- New Doc Apr 3, 2020Document6 pagesNew Doc Apr 3, 2020jalrizal7No ratings yet
- Chem c2 Exer1Document3 pagesChem c2 Exer1jalrizal7No ratings yet
- Qualitative AnalysisDocument4 pagesQualitative Analysisjalrizal7No ratings yet
- Nature of MatterDocument1 pageNature of Matterjalrizal7No ratings yet
- Understanding Pressure and its ApplicationsDocument19 pagesUnderstanding Pressure and its Applicationsjalrizal7No ratings yet
- F 5 C 2Document14 pagesF 5 C 2jalrizal7No ratings yet
- Calculating Solutions to Systems of EquationsDocument17 pagesCalculating Solutions to Systems of Equationsjalrizal7No ratings yet
- c4c8 Exer1Document2 pagesc4c8 Exer1jalrizal7No ratings yet
- F 4 C 4Document3 pagesF 4 C 4jalrizal7No ratings yet
- C 5 C 5Document2 pagesC 5 C 5jalrizal7No ratings yet
- Balancing Chemical EquationsDocument4 pagesBalancing Chemical Equationsjalrizal7No ratings yet
- c4c8 Exer1Document2 pagesc4c8 Exer1jalrizal7No ratings yet
- FZ Kps 20131Document83 pagesFZ Kps 20131jalrizal7No ratings yet
- NovelDocument4 pagesNoveljalrizal7No ratings yet
- f5c4 ExerDocument3 pagesf5c4 Exerjalrizal7No ratings yet
- KPMT - Bi - 2013Document3 pagesKPMT - Bi - 2013jalrizal7No ratings yet
- AD Circular MeasureDocument4 pagesAD Circular Measurejalrizal7No ratings yet
- 4 Simultaneous Equations MajuDocument3 pages4 Simultaneous Equations Majujalrizal7No ratings yet
- Chai Sing4Document4 pagesChai Sing4jalrizal7No ratings yet