Professional Documents
Culture Documents
Phenol
Uploaded by
issraaflowerCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Phenol
Uploaded by
issraaflowerCopyright:
Available Formats
Phenol also known as carbolic acid is an aromatic organic compound with the molecular formula C6H5OH.
H. It is a white crystalline solid that is volatile. The molecule consists of a phenyl group (-C6H5) bonded to a hydroxyl group (-OH). It is mildly acidic, but requires careful handling due to its propensity to cause burns. Phenol was first extracted from coal tar, but today is produced on a large scale (about 7 billion kg/year) from petroleum. It is an important industrial commodity as a precursor to many materials and useful compounds.[4] Its major uses involve its conversion to plastics or related materials. Phenol and its chemical derivatives are key for building polycarbonates, epoxies, Bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceutical drugs. Although similar to alcohols, phenols have unique distinguishing properties. Unlike in alcohols where the hydroxyl group is bound to a saturated carbon atom,[5] in phenols the hydroxyl group is attached to an unsaturated ring such as benzene or other arene ring.[6] Consequently, phenols have greater acidity than alcohols due to stabilization of the conjugate base through resonance in the aromatic ring. Properties:
Phenol is appreciably soluble in water, with about 8.42 g dissolving in 100 mL (0.88 M). Homogeneous mixtures of phenol and water at phenol to water mass ratios of ~2.6 and higher are also possible. The sodium salt of phenol, sodium phenoxide, is far more water soluble. Properties Molecular formula Molar mass Appearance Odor Density Melting point Boiling point Solubility in water C6H6O 94.11 g mol1 Transparent crystalline solid Sweet and tarry 1.07 g/cm3 40.5 C, 314 K, 105 F 181.7 C, 455 K, 359 F 8.3 g/100 mL (20 C)
Acidity (pKa)
9.95 (in water), 29.1 (in acetonitrile)[2]
max Dipole moment
270.75 nm[1] 1.7 D
Acidity: Phenol is weakly acidic and at high pH's gives the phenolate anion C6H5O (also called phenoxide):*7] PhOH PhO + H+ (K = 1010)
Compared to aliphatic alcohols, phenol is about 1 million times more acidic, although it is still considered a weak acid. It reacts completely with aqueous NaOH to lose H+, whereas most alcohols react only partially. Phenols are less acidic than carboxylic acids, and even carbonic acid. One explanation for the increased acidity over alcohols is resonance stabilization of the phenoxide anion by the aromatic ring. In this way, the negative charge on oxygen is delocalized on to the ortho and para carbon atoms.[8] In another explanation, increased acidity is the result of orbital overlap between the oxygen's lone pairs and the aromatic system.[9] In a third, the dominant effect is the induction from the sp2 hybridised carbons; the comparatively more powerful inductive withdrawal of electron density that is provided by the sp2 system compared to an sp3 system allows for great stabilization of the oxyanion. The pKa of the enol of acetone is 10.9, comparable to that for phenol.[10] The acidities of phenol and acetone enol diverge in the gas phase owing to the effects of solvation. About 1/3 of the increased acidity of phenol is attributable to inductive effects, with resonance accounting for the remaining difference. Reactions:
Neutral phenol substructure "shape". An image of a computed electrostatic surface of neutral phenol, showing neutral regions in green, electronegative areas in orange-red, and the electropositive phenolic proton in blue. Phenol is highly reactive toward electrophilic aromatic substitution as the oxygen atom's pi electrons donate electron density into the ring. By this general approach, many groups can be appended to the ring, via halogenation, acylation, sulfonation, and other processes. However, phenol's ring is so strongly activated second only to aniline - that bromination or chlorination of phenol leads to substitution on all carbons ortho and para to the hydroxy group, not only on one carbon.
Aqueous solution of phenol is weakly acidic and turns blue litmus slightly to red. Phenol is easily neutralized by sodium hydroxide forming sodium phenate or phenolate, but it being weaker than carbonic acid cannot be neutralized by sodium bicarbonate or sodium carbonate to liberate carbon dioxide C6H5OH + NaOH C6H5ONa + H2O When a mixture of phenol and benzoyl chloride when shaken in presence of dilute sodium hydroxide solution, phenyl benzoate is formed. This is an example of Schotten-Baumann reaction: C6H5OH + C6H5COCl C6H5OCOC6H5 + HCl Phenol is reduced to benzene when it is distilled with zinc dust or its vapour is passed over granules of zinc at 400 C:[15] C6H5OH + Zn C6H6 + ZnO When phenol is reacted with diazomethane in the presence of boron trifluoride (BF3), anisole is obtained as the main product and nitrogen gas is released: C6H5OH + CH2N2 C6H5OCH3 + N2 Uses: The major uses of phenol, consuming two thirds of its production, involve its conversion to precursors to plastics. Condensation with acetone gives bisphenol-A, a key precursor to polycarbonates and epoxide resins. Condensation of phenol, alkylphenols, or diphenols with formaldehyde gives phenolic resins, a famous example of which is Bakelite. Partial hydrogenation of phenol gives cyclohexanone, a precursor to nylon. Nonionic detergents are produced by alkylation of phenol to give the alkylphenols, e.g., nonylphenol, which are then subjected to ethoxylation.[4] Phenol is also a versatile precursor to a large collection of drugs, most notably aspirin but also many herbicides and pharmaceutical drugs. Phenol is also used as an oral anesthetic/analgesic in products such as Chloraseptic or other brand name and generic equivalents, commonly used to temporarily treat pharyngitis. Niche uses[edit] Phenol is so inexpensive that it attracts many small-scale uses. It once was widely used as an antiseptic, especially as carbolic soap, from the early 1900s through the 1970s. It is a component of industrial paint strippers used in the aviation industry for the removal of epoxy, polyurethane and other chemically resistant coatings.[18] Phenol derivatives are also used in the preparation of cosmetics including sunscreens,[19] hair colorings, and skin lightening preparations.[20]
Concentrated phenol liquids are commonly used in the surgical treatment of ingrown toenails to prevent a section of the toenail from growing back. This process is called phenolization. Production[edit]
Because of phenol's commercial importance, many methods have been developed for its production. The dominant current route, accounting for 95% of production (2003), involves the partial oxidation of cumene (isopropylbenzene) via the Hock rearrangement:[4] C6H5CH(CH3)2 + O2 C6H5OH + (CH3)2CO Compared to most other processes, the cumene-hydroperoxide process uses relatively mild synthesis conditions, and relatively inexpensive raw materials. However, to operate economically, there must be demand for both phenol, and the acetone by-product. An early commercial route, developed by Bayer and Monsanto in the early 1900s, begins with the reaction of a strong base with benzenesulfonate:[16] C6H5SO3H + 2 NaOH C6H5OH + Na2SO3 + H2O Other methods under consideration involve: hydrolysis of chlorobenzene, using base or steam (RaschigHooker process):[17] C6H5Cl + H2O C6H5OH + HCl direct oxidation of benzene with nitrous oxide, a potentially "green" process: C6H6 + N2O C6H5OH + N2 oxidation of toluene, as developed by Dow Chemical: C6H5CH3 + 2 O2 C6H5OH + CO2 + H2O In the Lummus Process, the oxidation of toluene to benzoic acid is conducted separately. Phenol is also a recoverable byproduct of coal pyrolysis
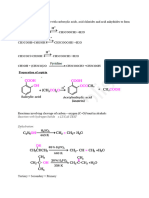
Phenol can be manufactured from Benzene using several ways Benzene hydrochlorination to form Benzyl chloride followed by hydrolysis of benzyl chloride to form phenol. Benzene chlorination to form benzyl chloride which is transformed to sodium benzoate and eventually to phenol using NaOH and HCl Benzene sulfonate process: In this process, benzene is convered to benzene sulfonate using sulphuric acid and eventually through neutralization, fusion and acidification, the benzene sulfonate is gradually transformed to phenol. In this lecture, we restrict our discussion to the manufacture of phenol from Benzene hydrochlorination route Benzene from chlorobenzene route Phenol using Hydro chlorination route Reactions First reactio Benzene + HCl + Oxygen Benzyl chloride + Water Catalyst: FeCl3 + CuCl2 Operating conditions: 240C and atmospheric pressure Second reaction Benzyl chloride + water Phenol + HCl Catalyst: SiO2 Here, HCl is regenerated and will be recycled. Operating conditions: 350C and atmospheric pressure In this process, Benzene is used to extract phenol from phenol +water mixture. This unit is termed as an extraction unit (liquid liquid extraction principle). Therefore, this unit takes up fresh benzene and phenol + water mixture and produces two streams namely water stream (bottom product) and benzene + phenol stream (top product). The water stream is fed to a scrubber unit (i.e., Unit B that will be described later).
Then onwards, the organic mixture is fed to a distillation column that produces purer benzene as the top product. The bottom product is phenol with other impurities. The bottom phenol rich product is sent to the phenol fractionator to obtain waste product as top product and pure phenol as bottom product. The purer benzene then enters the hydrochlorination reactor in which a mixture of HCl and O2 is fed at 220C. Under these conditions, Benzene will be also in vapour state. Therefore, the reactor is a gas solid reactor. The conversions are pretty low and not more than 20% of the benzene is converted to benzyl chloride. Eventually, the products are sent to two fractionators that separate unreacted benzene, crude benzyl chloride and poly benzyl chlorides as various products. The unreacted benzene is sent back to the hydrochlorination reactor as a recycle stream. The crude benzyl chloride then enters an absorber unit A where phenol is used to purify the benzyl chloride from other organic compounds (such as benzene and polybenzyl chlorides). The purified benzyl chloride stream then enters the hydrolysis reactor in which water is passed along with benzyl chloride over the silica catalyst. The reactor itself is a furnace with catalyst loaded in the tubes and hot fuel gases are circulated in the shell to obtain the desired higher temperature. Under these conditions, both reactants are in vapour state (with the benzyl chloride boiling point of 179C) and therefore, the reaction is also a gas solid reaction. After hydrolysis reaction, the product vapors are sent to a partial condenser that separates the HCl from the organic phase. The HCl is recycled to the hydrochlorination reactor.
The phenol rich product stream is sent as a solvent for the scrubber (unit A) that purifies crude benzyl chloride to purer benzyl chloride. The bottom product from the scrubber (i.e., unit A) enters another scrubber (unit B) that receives water from the extractor. The unit B enables washing of the phenol to remove any water soluble impurities. The water from the unit B enters the hydrolysis reactor.
You might also like
- Hydroxy CompoundsDocument9 pagesHydroxy Compoundschong56No ratings yet
- Importance of LubricationDocument14 pagesImportance of LubricationBijendra PrajapatiNo ratings yet
- Organic Chemistry NotesDocument24 pagesOrganic Chemistry NotesSweatNo ratings yet
- BTX SeparationDocument21 pagesBTX Separationhhhhosh100% (2)
- Index: Sr. No. Topics Page NoDocument69 pagesIndex: Sr. No. Topics Page NoRoshan Rohit100% (3)
- Cape Chemistry Unit Ii Module I Alcohols and Phenol and Alkenes Worksheet and Revision GuideDocument10 pagesCape Chemistry Unit Ii Module I Alcohols and Phenol and Alkenes Worksheet and Revision GuideAshli GrantNo ratings yet
- PHENOLDocument50 pagesPHENOLencik dugaNo ratings yet
- Pce2 Unit-II AromaticsDocument87 pagesPce2 Unit-II AromaticsMeghana SNo ratings yet
- Chemistry - of - PETROCHEMICAL NEWDocument116 pagesChemistry - of - PETROCHEMICAL NEWvivaline AchiengNo ratings yet
- Chemistry List of ExperimentDocument3 pagesChemistry List of ExperimentKaiswan GanNo ratings yet
- Phenol From Cuemen and TolueneDocument9 pagesPhenol From Cuemen and TolueneAnonymous RJkpep7D0rNo ratings yet
- Production of PhenolDocument22 pagesProduction of PhenolShubhranshu Kathuria71% (7)
- Lab #1: Polymers Testing: How To Identify Different PlasticsDocument6 pagesLab #1: Polymers Testing: How To Identify Different Plasticsazeezsadiq100% (4)
- Petrochem 10 - SEM 1 12-13Document40 pagesPetrochem 10 - SEM 1 12-13Saifuddin AzizNo ratings yet
- International Reference Guide To Hazardous AreasDocument13 pagesInternational Reference Guide To Hazardous Areasmalileo100% (1)
- Production of PhenolDocument65 pagesProduction of Phenolchaitanyavura67% (3)
- Phenol ProductionDocument9 pagesPhenol ProductionPlant Design100% (1)
- AmuphenolDocument5 pagesAmuphenolanu malikNo ratings yet
- FinalDocument29 pagesFinalabhishek_nitwNo ratings yet
- Topic 2 PhenolsDocument41 pagesTopic 2 PhenolsMark K DavidNo ratings yet
- PhenolDocument20 pagesPhenolUmar TahirNo ratings yet
- Phenols (1) - 24840322 - 2023 - 11 - 16 - 16 - 47Document22 pagesPhenols (1) - 24840322 - 2023 - 11 - 16 - 16 - 47ap376808No ratings yet
- 08 Chapter 4Document42 pages08 Chapter 4Alda Sadilillah RNo ratings yet
- Nomenclature of PhenolsDocument3 pagesNomenclature of PhenolsRockyNo ratings yet
- CT-308 Phenol Production-2023Document22 pagesCT-308 Phenol Production-2023Divyansh NagarNo ratings yet
- Topic 4: Major Bulk Organic Major Bulk Organic Chemicals From BenzeneDocument23 pagesTopic 4: Major Bulk Organic Major Bulk Organic Chemicals From BenzeneYong LiNo ratings yet
- PROCESS - 3 - Chemical and Process Design HandbookDocument4 pagesPROCESS - 3 - Chemical and Process Design HandbookEdrian A. MañalongNo ratings yet
- Although This Process Is No Longer in Common UseDocument15 pagesAlthough This Process Is No Longer in Common Usedia_aldy100% (1)
- Types of Phenol Manufacturing ProcessDocument4 pagesTypes of Phenol Manufacturing ProcessIsma AzraNo ratings yet
- KimiDocument29 pagesKimiDejan KrajaNo ratings yet
- Production of CumeneDocument5 pagesProduction of CumeneMohit YaduwanshiNo ratings yet
- Phenols: Ahmet Kaan Dikici 03130021005Document23 pagesPhenols: Ahmet Kaan Dikici 03130021005Ahmet Kaan DikiciNo ratings yet
- Evolution of A Process The Manufacture of Diphenyl OxideDocument2 pagesEvolution of A Process The Manufacture of Diphenyl OxideAshwini SwamiNo ratings yet
- Proceeding Bromometric Phenol Assay Without Starch IndicatorDocument6 pagesProceeding Bromometric Phenol Assay Without Starch IndicatorAsiyahNo ratings yet
- Alkohol (Repaired)Document38 pagesAlkohol (Repaired)LiviaAsriNo ratings yet
- MCQ Alcohols Phenols and EtherDocument4 pagesMCQ Alcohols Phenols and EtherVARUN SNo ratings yet
- KIT 458 - Report (Group G) Task 6Document25 pagesKIT 458 - Report (Group G) Task 6Lim LeepingNo ratings yet
- Phenol 203Document47 pagesPhenol 203ajibolaakorede20No ratings yet
- First Review Report On Production of Phenol: Done by GuideDocument27 pagesFirst Review Report On Production of Phenol: Done by GuideRuban RkNo ratings yet
- Cape Chemistry Unit 2: Module 1: The Chemistry of Carbon CompoundsDocument16 pagesCape Chemistry Unit 2: Module 1: The Chemistry of Carbon CompoundsMalik MuhammadNo ratings yet
- PhenolDocument2 pagesPhenolBiswajit MohantyNo ratings yet
- Hydroxylation of Benzene To Phenol Via Hydrogen Peroxide in Hydrophilic Triethylammonium Acetate Ionic LiquidDocument5 pagesHydroxylation of Benzene To Phenol Via Hydrogen Peroxide in Hydrophilic Triethylammonium Acetate Ionic LiquidshivanshNo ratings yet
- About Phenol:: 2. Hydrolysis ofDocument3 pagesAbout Phenol:: 2. Hydrolysis ofNazmul NayeemNo ratings yet
- Chemical Properties of EthanolDocument7 pagesChemical Properties of EthanolKinkwan King100% (3)
- Phenol SDocument9 pagesPhenol SAnonymous 8rsxG4No ratings yet
- Rest Part of AlcoholsDocument6 pagesRest Part of Alcoholswww.seemasainirox123No ratings yet
- Aniline Project 1234Document6 pagesAniline Project 1234kareem100% (1)
- Chemistry Notes For Class 12 Chapter 11 Alcohols, Phenols and EthersDocument25 pagesChemistry Notes For Class 12 Chapter 11 Alcohols, Phenols and EthersMedicah Simon Peligrino100% (5)
- Phenol: Wullida Hayuning BidariDocument12 pagesPhenol: Wullida Hayuning BidariwullidaNo ratings yet
- Uses of The CompoundsDocument10 pagesUses of The CompoundsXin PurpleNo ratings yet
- Organic Chemistry Lecture (M6)Document6 pagesOrganic Chemistry Lecture (M6)cesia freniereNo ratings yet
- Unit II A PhenolsDocument16 pagesUnit II A Phenols36 : Tushar RajguruNo ratings yet
- PhenolDocument16 pagesPhenolAmanNo ratings yet
- Dev GDocument22 pagesDev GDeeksha GangwarNo ratings yet
- Classification and Nomenclature of Alcohols, Phenols and EthersDocument16 pagesClassification and Nomenclature of Alcohols, Phenols and EthersTr Mazhar PunjabiNo ratings yet
- PH Eno LS: Group No #5Document16 pagesPH Eno LS: Group No #5السید طیب علی البخاری القادری السہروردیNo ratings yet
- Petrochemical Industry: Eng/Bassem FathallaDocument45 pagesPetrochemical Industry: Eng/Bassem FathallaGalal Eldien GalalNo ratings yet
- CHM301Document24 pagesCHM301encik dugaNo ratings yet
- Hydroxyl Compounds: Alcohol & PhenolDocument59 pagesHydroxyl Compounds: Alcohol & PhenolUMMU MARDHIAH ABDUL HALIMNo ratings yet
- NCERT Solutions For Chapter 11 Alcohols Phenols and EtherDocument16 pagesNCERT Solutions For Chapter 11 Alcohols Phenols and EtherPrithvi AryaNo ratings yet
- CHAPTER 7 Alcohols RESTOREDDocument51 pagesCHAPTER 7 Alcohols RESTOREDsukaina fatimaNo ratings yet
- Chapter 5 - PhenolsDocument29 pagesChapter 5 - Phenolsdr.3nonaNo ratings yet
- Alcohols and PhenolsDocument47 pagesAlcohols and PhenolsniyazrahimNo ratings yet
- Shanto Mariam University of Creative Technology: Submitted ToDocument14 pagesShanto Mariam University of Creative Technology: Submitted ToMd Raim razzakNo ratings yet
- Anzenbacher-CV January 12 Venezuela-ShortversionDocument4 pagesAnzenbacher-CV January 12 Venezuela-ShortversionCELQUSBNo ratings yet
- NHDT FoulingDocument6 pagesNHDT FoulingJulio RamirezNo ratings yet
- Drinking Water Contamination and Treatment TechniquesDocument25 pagesDrinking Water Contamination and Treatment Techniquesaditya mohantyNo ratings yet
- Unit-4 Environmental Hazards PDFDocument103 pagesUnit-4 Environmental Hazards PDFpavanNo ratings yet
- SABIC NORYL Resin Injection Molding Processing GuideDocument20 pagesSABIC NORYL Resin Injection Molding Processing GuideKrishnan AnanthanarayananNo ratings yet
- Roadmap For Reactions of Alkanes, Alkenes, Alkynes, Alcohols & EthersDocument5 pagesRoadmap For Reactions of Alkanes, Alkenes, Alkynes, Alcohols & EthersUday Prakash SahuNo ratings yet
- ADT, Litho Scanner & XL-RockDocument3 pagesADT, Litho Scanner & XL-RockFasih HaiderNo ratings yet
- E. Coli Is Your Friend: Kathrin EngelDocument37 pagesE. Coli Is Your Friend: Kathrin EngelcambodianpisethNo ratings yet
- Nowadays, Pesticide Are Not Only Expensive But Also Not Guaranteed To Be Safe. With TheDocument12 pagesNowadays, Pesticide Are Not Only Expensive But Also Not Guaranteed To Be Safe. With TheFery AnnNo ratings yet
- Chalcone Synthesis 4-ClorochalconeDocument8 pagesChalcone Synthesis 4-ClorochalconeAngie RozoNo ratings yet
- Optimalisasi Variasi Volume Resin Komposit Serat Limbah Karung Glangsing Utsman Syah Amrullah, Riyanto Heri NugrohoDocument7 pagesOptimalisasi Variasi Volume Resin Komposit Serat Limbah Karung Glangsing Utsman Syah Amrullah, Riyanto Heri Nugrohohari juharaNo ratings yet
- Characterization of Landfilled Materials Screening of The Enhanced Landfill Mining PotentialDocument24 pagesCharacterization of Landfilled Materials Screening of The Enhanced Landfill Mining PotentialTri diahNo ratings yet
- 1504 Metals From MWIBA R. BungeDocument68 pages1504 Metals From MWIBA R. BungemhdsolehNo ratings yet
- Hespiridin PDFDocument4 pagesHespiridin PDFanon_753148412No ratings yet
- Zhihong Qiao 2010Document16 pagesZhihong Qiao 2010nlddoan Nguyễn Thị Lâm ĐoànNo ratings yet
- Iso 12966 2 2017Document11 pagesIso 12966 2 2017Carlitos JsjsNo ratings yet
- Data Obat Terjual Jan-Des 2018Document62 pagesData Obat Terjual Jan-Des 2018HanNo ratings yet
- Ecosystems Have Living and Nonliving ComponentsDocument5 pagesEcosystems Have Living and Nonliving ComponentsPaul Jeremy MendozaNo ratings yet
- A Plastic OceanDocument2 pagesA Plastic OceanSilvia LunguNo ratings yet
- X-Flow Aquaflex 64: Ultrafiltration MembraneDocument2 pagesX-Flow Aquaflex 64: Ultrafiltration Membranewahyu raharjoNo ratings yet
- Biology The Unity and Diversity of Life 14Th Edition Starr Test Bank Full Chapter PDFDocument38 pagesBiology The Unity and Diversity of Life 14Th Edition Starr Test Bank Full Chapter PDFBeckySmithnxro100% (11)
- DNA Damage and RepairDocument23 pagesDNA Damage and RepairbadrhashmiNo ratings yet
- Isolation of DNA From Cryostat Sections of Bone Using Nucleon BACC 1 (From Life Science News, Issue 1)Document1 pageIsolation of DNA From Cryostat Sections of Bone Using Nucleon BACC 1 (From Life Science News, Issue 1)AsmaNo ratings yet
- United States Patent (191: Brunke Et A1. (11) Patent Number: (45) Date of PatentDocument13 pagesUnited States Patent (191: Brunke Et A1. (11) Patent Number: (45) Date of PatentAnonymous vRpzQ2BLNo ratings yet