Professional Documents
Culture Documents
GCF Sampling
Uploaded by
Ferdinan PasaribuCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
GCF Sampling
Uploaded by
Ferdinan PasaribuCopyright:
Available Formats
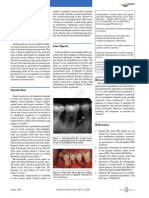
GCF sampling GCF was sampled 1 week after the clinical examination in order not to alter the nature
of the GCF. Two non-contiguous diseased sites (i.e. PD and CAL > 5 mm, BoP and no furcation involvement) and two noncontiguous healthy sites (i.e. PD and CAL < 4 mm without BoP and/or MB) were randomly chosen per patient for GCF sampling. After removal of the supragingival biofilm with sterile cotton pellets, the sites were isolated with cotton rolls and gently dried with an air syringe to eliminate the possibility of contamination with saliva. GCF was collected by inserting standard paper strips (Periopaper; Oraflow Inc., Smithtown, NY, USA) of approximately 2 mm into the sulcus/pocket for 20 s. Strips that were visually contaminated with blood were discarded. The GCF volume was measured in a calibrated device (Periotron 8000; Proflow Inc., Amityville, NY, USA) and the readings were converted to an actual volume (ll) by reference to a standard curve. Strips from the four selected sites were immediately placed into separate microcentrifuge tubes and stored at 80C for subsequent assays. Multiplex bead immunoassay The tubes were vortexed for 15 s and centrifuged for 5 min at 1500 g to elute. Samples were analysed using a multi-analyte method by means of a 14-multiplex fluorescent bead-based immunoassay for 14 cyto/chemokines [eotaxin, G-CSF, GM-CSF, interferon (IFN)-c, IL-1b, IL-2, IL-6, IL-7, IL-8, IL-10, IL-12, protein chemoattractant monocyte (MCP)-1, MIP-1a and tumour necrosis factor (TNF)-a] using commercially available kits (Milliplex Human Cytokine/Chemokine; EMD Millipore, Billerica, MA, USA) and a plate reader (Luminex 100TM; EMD Millipore), according to the manufacturer's recommendations. The amount of protein in each sample was extrapolated from standards using an appropriate software (Beadview; EMD Millipore). The
minimum detectable concentration for eotaxin, G-CSF, GM-CSF, IFNc, IL-1b, IL-2, IL-6, IL-7, IL-8, IL-10, IL-12, MCP-1, MIP-1a and TNF-a are 1.2, 0.5, 9.5, 0.1, 0.4, 0.3, 0.3, 1.8, 0.2, 0.3, 10.5, 0.9, 3.5 and 0.1 pg/ml, respectively. The results were reported as concentrations of cyto/chemokines per volume of GCF (pg/ll). Statistical analysis As no precise information regarding the clinical significance of the differences in the levels of the studied mediators between diabetic and nondiabetic groups exists, no formal sample size calculation could be performed for this study. The number of sites for GCF sampling was based on a previous study that evaluated the levels of the same cyto/chemokines in GCF (Tymkiw et al. 2011). Statistical analyses were performed using commercial software (SigmaPlot 11.0; Systat Software, San Jose, CA, USA). Data were first examined for normality by the Shapiro Wilk test and the data that did not achieve normality were analysed using non-parametric methods. The percentage of sites with visible PI, MB, BoP and SUP, mean PD and CAL and levels of HbA1c and FPG were computed for each subject and then averaged across diabetic and non-diabetic groups. GCF volume and the concentration of cyto/chemokine were evaluated in two subsets: healthy and diseased sites. Subsequently, all data were averaged in the diabetic and nondiabetic groups. The differences in age, clinical and glycemic parameters between groups were compared using the Unpaired Student's t-test. The MannWhitney U-test was used to compare the cyto/cytokine levels between diabetic and non-diabetic groups. The v2 test was used to detect differences in the frequencies of gender between groups. Eight models of multivariable logistic regression (MLR) analysis were undertaken to estimate the association between DM and GCF concentrations of relevant chemokines (eotaxin, IL-8 and MIP-1a), innate response-related cytokines
(GM-CSF, IL-6 and TNF-a), T helper-(Th) related cytokines (IFN-c, IL-10, IL-12) and T cell-growth factor cytokines (IL-2 and IL-7) in healthy and diseased sites. For each model, the dependent variable was the presence of DM and, an odds ratio (OR) with a 95% confidence limit was calculated. After using random effects models, the ORs were estimated as follows: OR > 1 nondiabetic subjects had higher levels of cyto/chemokine than the dependent group (i.e. diabetic subjects); OR < 1 non-diabetic subjects had lower levels of cyto/chemokine than the dependent group. The level of significance was set at 5%. Adjustments were made for multiple comparisons when the levels of the 14 cyto/ chemokines were evaluated. An overall p of 0.05 = 1 (1 k)14 was computed, where k was the desired individual p value. Therefore, for cyto/chemokine comparisons, p < 0.0035 was considered statistically significant at p < 0.05. Results Clinical The study was conducted between July 2010 and December 2011. Table 1 presents the mean clinical parameters and the demographic characteristics of the studied population. The only clinical parameter that differed significantly between diabetic and non-diabetic groups was the mean PD. Diabetic subjects showed a higher mean full-mouth PD than non-diabetic subjects (p < 0.05). As expected, the nondiabetic group presented lower levels of HbA1c and FPG than the diabetic group (p < 0.05) (Table 1). The duration of DM reported by the subjects was 6.4 0.9 years. Regarding DM regimens, 18 subjects reported that they were under diet control and using oral hypoglycemic agents, six reported to be under diet control and in use of insulin whereas two subjects reported to be under diet control only. There were no differences between groups for GCF volume, PD and CAL in healthy and diseased sampled sites (p > 0.05).
You might also like
- GINGIVECTOMYDocument6 pagesGINGIVECTOMYFerdinan Pasaribu100% (1)
- Sling and Tag Suturing Technique For Coronally Advanced FlapDocument7 pagesSling and Tag Suturing Technique For Coronally Advanced FlapFerdinan Pasaribu0% (1)
- Shalu Bathla - Chronic PeriodontitisDocument6 pagesShalu Bathla - Chronic PeriodontitisFerdinan PasaribuNo ratings yet
- Abscesses of The Periodontium Article-PDF-bansi M Bhusari Rizwan M Sanadi Jayant R Ambulgeka-558Document4 pagesAbscesses of The Periodontium Article-PDF-bansi M Bhusari Rizwan M Sanadi Jayant R Ambulgeka-558Ferdinan PasaribuNo ratings yet
- Ektopic Enamel PearlDocument2 pagesEktopic Enamel PearlFerdinan PasaribuNo ratings yet
- Vestibuloplasty New TechniqueDocument4 pagesVestibuloplasty New TechniqueFerdinan Pasaribu0% (1)
- Non-Carious Cervical Lesions Associated With Gingival Recessions: A Decision-Making ProcessDocument12 pagesNon-Carious Cervical Lesions Associated With Gingival Recessions: A Decision-Making ProcessFerdinan PasaribuNo ratings yet
- Immediate Implant Placement PDFDocument11 pagesImmediate Implant Placement PDFFerdinan PasaribuNo ratings yet
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (74)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (345)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- RLE Module 3F Course Module and Procedure Guide 2Document7 pagesRLE Module 3F Course Module and Procedure Guide 2KaiNo ratings yet
- Climbing Training Log - TemplateDocument19 pagesClimbing Training Log - TemplateKam Iqar ZeNo ratings yet
- Top 10 Ranking Universities in West Africa 2022Document1 pageTop 10 Ranking Universities in West Africa 2022Bright OtchereNo ratings yet
- The Art and Practice of Diagnosis in Chinese Medicine. NIGEL CHINGDocument794 pagesThe Art and Practice of Diagnosis in Chinese Medicine. NIGEL CHINGИгорь Клепнев100% (13)
- Case Group 1Document10 pagesCase Group 1JASMEEN RAVALNo ratings yet
- Enrolled Nurses FADocument11 pagesEnrolled Nurses FAjoayou23No ratings yet
- Jurnal Asli PBL 3 Blok 9Document6 pagesJurnal Asli PBL 3 Blok 9Annisa FadilasariNo ratings yet
- Periodontal AbscessDocument27 pagesPeriodontal AbscessAhmed Tawfig GamalNo ratings yet
- Diastasis Recti Ebook DownloadDocument19 pagesDiastasis Recti Ebook DownloadMertcan DamatNo ratings yet
- Invenia ABUS USA Brochure Feb2016Document14 pagesInvenia ABUS USA Brochure Feb2016Asim AliNo ratings yet
- LPV Fault Detection of Glucose-Insulin System: Cite This PaperDocument6 pagesLPV Fault Detection of Glucose-Insulin System: Cite This PaperfatihaNo ratings yet
- Reflection PaperDocument7 pagesReflection Paperapi-623973327No ratings yet
- Experiment 4: Roadway Lighting Evaluation And: DesignDocument12 pagesExperiment 4: Roadway Lighting Evaluation And: DesignEdogawa ConanNo ratings yet
- Dokumen - Tips Biology Investigatory Project 561e79b91f5a0Document17 pagesDokumen - Tips Biology Investigatory Project 561e79b91f5a0Upendra LalNo ratings yet
- Ufgs 01 57 19.01 20Document63 pagesUfgs 01 57 19.01 20jackcan501No ratings yet
- Physical Examination FormDocument4 pagesPhysical Examination FormDawit KumsaNo ratings yet
- My Evaluation in LnuDocument4 pagesMy Evaluation in LnuReyjan ApolonioNo ratings yet
- Action Plan GPPDocument3 pagesAction Plan GPPMa Rk AntonioNo ratings yet
- Project ProposalDocument3 pagesProject ProposalSkrrtt SkrrttNo ratings yet
- WEEK 2 ILP Grade 4Document5 pagesWEEK 2 ILP Grade 4jean arriolaNo ratings yet
- Objectives of The Nairobi SummitDocument12 pagesObjectives of The Nairobi SummitJoachim ChijideNo ratings yet
- Arihant DRX Ascend Case Study PDFDocument2 pagesArihant DRX Ascend Case Study PDFsaurav.martNo ratings yet
- The Barrier To Implementation of Evidence-Based Practice Among Novice TherapistsDocument11 pagesThe Barrier To Implementation of Evidence-Based Practice Among Novice TherapistsInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- Free End SaddleDocument5 pagesFree End SaddleAdry AnnaNo ratings yet
- Esmeron 10 MG/ML Solution For Injection Rocuronium Bromide Prescribing InformationDocument3 pagesEsmeron 10 MG/ML Solution For Injection Rocuronium Bromide Prescribing InformationVeeta Ruchi LieNo ratings yet
- Selective, Differential, & Enriched Media Lab ChartDocument2 pagesSelective, Differential, & Enriched Media Lab ChartFrankiesgirl6yr100% (6)
- Et CareDocument15 pagesEt CarePaulo GarciaNo ratings yet
- Jonathan Glover-Alien LandscapesDocument448 pagesJonathan Glover-Alien LandscapesIrina Soare100% (1)
- Pork TocinoDocument1 pagePork TocinoMaria Ivz ElborNo ratings yet
- Agri SBA (Broiler)Document20 pagesAgri SBA (Broiler)Shanti KissoondyalNo ratings yet