Professional Documents
Culture Documents
Periodic Table Plus
Uploaded by
bl9nkverseCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Periodic Table Plus
Uploaded by
bl9nkverseCopyright:
Available Formats
Activity series, periodic table and solubility rules
Li
K
Ba
Ca
Na
Mg
Al
Mn
Zn
Cr
Fe
Co
Ni
Sn
Pb
H2
Cu
Ag
Hg
Pt
Au
1
1A
1 1
2
2A
1.008
3
4
Li
Be
14
4A
6
15
5A
7
16
6A
8
17
7A
9
4.003
10
Ne
He
3
3B
21
4
4B
22
5
5B
23
6
6B
24
7
7B
25
8
8B
26
9
8B
27
10
8B
28
11
1B
29
12
2B
30
Ca
Sc
Ti
V Cr Cr
Mn
Fe
Co
Ni
Cu
Zn
Ga
Ge
As
Se
Br
Kr
Sr
Zr
Nb
Tc
Ru
Rh
Pd
Ag
Cd
In
Sn
Sb
Te
Xe
Mg
22.99 24.31
19
20
13
3A
5
10.81 12.01 14.01 16.00 19.00 20.18
13
14
15
16
17
18
6.941 9.012
11
12
Na
18
8A
2
Al
Si
Cl
Ar
26.98 28.09 30.97 32.07 35.45 39.95
31
32
33
34
35
36
39.10 40.08 44.96 47.88 50.94 52.009854.94 55.85 58.93 58.69 63.55 65.39 69.72 72.59 74.92 78.96 79.90 83.80
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
54
53
Rb
Mo
85.47 87.62 88.91 91.22 92.91 95.94 ((98)) 101.1 102.9 106.4 107.9 112.4 114.8 118.7 121.8 127.6 126.9 131.3
55
56
72
73
74
75
76
77
79
80
81
82
83
84
85
86
78
57
Cs
Ba
La
Hf
Ta
Re
Os
132.9 137.3 138.9 178.5 180.9 183.8 186.2 190.2
89
88
104 105 106 107 108
87
Fr Ra Ac Unq
q Unp
p Unh Uns Uno
(223) (226) (227) (257) (260) (263) (262) (265)
Ir
Pt
Au
Hg
Tl
Pb
Bi
Po
At
Rn
192.2 195.1 197.0 200.6 204.4 207.2 209.0 (210) (210) (222)
109
Une
(266)
58
59
60
61
62
63
64
65
66
67
68
69
70
71
Ce
Pr
Nd
Pm
Sm
Eu
Gd
Tb
Dy
Ho
Er
Tm
Yb
Lu
140.1 140.9 144.2 (147) 150.4 152.0 157.3 158.9 162.5 164.9 167.3 168.9 173.0 175.0
92
93
94
95
96
97
98
99
100 101 102 103
90
91
Th
Pa
Np
Pu
Am
Cm
Bk
Cf
Es
Fm
Md
No
Lw
232.0 (231) 238.0 (237) (242) (243) (247) (247) (249) (254) (253) (256) (254) (257)
Soluble Compounds
1 alkali
1.
lk li metall salts,
l except potassium
i
perchlorate
hl
2.
2 ammonium
i
salts
l 3.
3 all
ll nitrates,
i
chlorates,
hl
perchlorates,
hl
andd acetates except silver
il
acetate andd
+
2+
2+
2+
potassium perchlorate 4. all chlorides, bromides, and iodides except those of Ag , Pb , Hg , and Hg2 . PbCl2 is slightly soluble. HgCl2 is soluble.
5.sulfates except those of Pb2+, Sr2+, Ba2+ and Hg22+ . sulfates of Ca2+ and Ag+ are slightly soluble Insoluble Compounds 1. metal oxides except those
of the alkali metals, Ca2+, Sr2+, and Ba2+ 2. hydroxides except those of the alkali metals, Ba2+, and Sr2+. calcium hydroxide is slightly soluble
3. carbonates, phosphates, sulfides, and sulfites except those of the alkali metals and the ammonium ion.
You might also like
- The Periodic Table NotesDocument23 pagesThe Periodic Table Notesapi-239426184No ratings yet
- Tabla Periódica de Elementos: MolybdenumDocument1 pageTabla Periódica de Elementos: MolybdenumMichael A. Alonso RodriguezNo ratings yet
- Atomic Structure 1Document1 pageAtomic Structure 1Iqbal SinghNo ratings yet
- Periodic Table Melt PointDocument1 pagePeriodic Table Melt PointFidree AzizNo ratings yet
- Application of IC-MS and IC-ICP-MS in Environmental ResearchFrom EverandApplication of IC-MS and IC-ICP-MS in Environmental ResearchRajmund MichalskiNo ratings yet
- Tabla Periodica de Los Elementos H Li Be B C N O F Na MG Al Si P S CLDocument2 pagesTabla Periodica de Los Elementos H Li Be B C N O F Na MG Al Si P S CLDanielaCastilloColoradoNo ratings yet
- Electronegativity ChartDocument1 pageElectronegativity ChartEhsan KhanNo ratings yet
- The Terrible and Wonderful Reasons Why I Run Long DistancesFrom EverandThe Terrible and Wonderful Reasons Why I Run Long DistancesRating: 4 out of 5 stars4/5 (97)
- Bishop Periodic TableDocument1 pageBishop Periodic TableAashay PatilNo ratings yet
- Bishop Periodic Table PDFDocument1 pageBishop Periodic Table PDFzelNo ratings yet
- Periodic TableDocument1 pagePeriodic TableCassidyNo ratings yet
- Name: Student ID Number: Section Number:: Version A KeyDocument2 pagesName: Student ID Number: Section Number:: Version A KeyAileen LiangNo ratings yet
- Key Information: Explore About The Chemical Elements Through This Periodic TableDocument1 pageKey Information: Explore About The Chemical Elements Through This Periodic TableNilanjana MishraNo ratings yet
- Periodic Table All ColorDocument1 pagePeriodic Table All ColorcingggggggggggNo ratings yet
- Final Exam Cover SheetDocument1 pageFinal Exam Cover SheetpaulaNo ratings yet
- Periodic Table of The ElementsDocument1 pagePeriodic Table of The ElementsRobert MarcoliniNo ratings yet
- Periodic Table From EocDocument1 pagePeriodic Table From Eocapi-87739323No ratings yet
- UntitledDocument3 pagesUntitledJoshi SanikaNo ratings yet
- P Table Update 2016 - Oths Aca Chem For WebsiteDocument2 pagesP Table Update 2016 - Oths Aca Chem For Websiteapi-254514513No ratings yet
- 4 Periodic Table 3Document1 page4 Periodic Table 3abhijeet_sangwanNo ratings yet
- Selig 4022 Polar DataDocument8 pagesSelig 4022 Polar DataAdrian DincaNo ratings yet
- Periodictable BWDocument1 pagePeriodictable BWShubham SinghNo ratings yet
- Table of Electron NegDocument2 pagesTable of Electron NegTarun GuptaNo ratings yet
- 1A Ground State Electron Configurations 8ADocument2 pages1A Ground State Electron Configurations 8ABhrugu PandyaNo ratings yet
- Periodic Table of Chemical ElementsDocument1 pagePeriodic Table of Chemical Elementsm afiq fahmiNo ratings yet
- Periodic Table of The ElementsDocument4 pagesPeriodic Table of The Elementsalpatil2No ratings yet
- T 2 Periodic Table GroupsDocument1 pageT 2 Periodic Table GroupsfanthiNo ratings yet
- Periodic TableDocument1 pagePeriodic Tableapi-21226401No ratings yet
- Periodic TableDocument1 pagePeriodic Tableapi-233187566No ratings yet
- Peroidic TableDocument1 pagePeroidic TableAnonymous XcVJCTG0No ratings yet
- Group 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Period 1 2 3 4 5 6Document2 pagesGroup 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 Period 1 2 3 4 5 6Oyedotun TundeNo ratings yet
- Atomic Properties of The Elements TableDocument1 pageAtomic Properties of The Elements TableMaahiNo ratings yet
- IUPAC - Periodic TableDocument1 pageIUPAC - Periodic TableNaren VmdNo ratings yet
- MG 1 N 2 C 0 o 6 H 0 Li 0 B 0 Mass of Sample 56.2Document4 pagesMG 1 N 2 C 0 o 6 H 0 Li 0 B 0 Mass of Sample 56.2gclebretNo ratings yet
- Jadual BerkalaDocument18 pagesJadual BerkalaMohd NizamNo ratings yet
- IUPAC Periodic Table-28Nov16Document1 pageIUPAC Periodic Table-28Nov16mmmaaallleeeNo ratings yet
- Periodic TableDocument1 pagePeriodic Tablenouran94No ratings yet
- Lampiran B C + GrafikDocument2 pagesLampiran B C + GrafikrikaariskaaaNo ratings yet
- Molecular Masses and Percent Composition CalculatorDocument4 pagesMolecular Masses and Percent Composition CalculatorsristisekharNo ratings yet
- Molecular Masses and Percent Composition CalculatorDocument4 pagesMolecular Masses and Percent Composition CalculatorsristisekharNo ratings yet
- Periodic Table of Chemical ElementsDocument1 pagePeriodic Table of Chemical ElementsOusseynou DiagneNo ratings yet
- The Periodic TableDocument7 pagesThe Periodic TableJake Giuseppe PriceNo ratings yet
- REGRESIONDocument8 pagesREGRESIONBryan SuttaNo ratings yet
- Periodic Table of The Elements: For Chemistry Practice Problems and Video Lessons VisitDocument1 pagePeriodic Table of The Elements: For Chemistry Practice Problems and Video Lessons VisitlmlNo ratings yet
- Electronic ConfigurationDocument3 pagesElectronic ConfigurationSushobhan SanyalNo ratings yet
- Hidro StatistikaDocument3 pagesHidro StatistikaKuzmi21No ratings yet
- Cal FormulaesDocument3 pagesCal Formulaesrazamehdi3No ratings yet
- 1Document2 pages1Nina Ixora SelviajavanicaNo ratings yet
- Handbook: A-38 LightingDocument1 pageHandbook: A-38 LightingreacharunkNo ratings yet
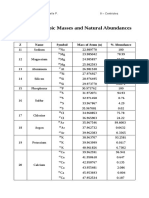
- Table of Isotopic Masses and Natural Abundances: Z Name Symbol Mass of Atom (U) % AbundanceDocument1 pageTable of Isotopic Masses and Natural Abundances: Z Name Symbol Mass of Atom (U) % AbundanceAaliyah Gabrielle SambaleNo ratings yet
- Kunci Jawaban PPKN B Indo KLS 7 8 SMT 2Document2 pagesKunci Jawaban PPKN B Indo KLS 7 8 SMT 2Adi WmNo ratings yet
- Taller 3 - Daniel Augusto Bautista MoraDocument7 pagesTaller 3 - Daniel Augusto Bautista MorabasuramiamNo ratings yet
- Chemistry Reference SheetDocument2 pagesChemistry Reference SheetTimothy TopolskiNo ratings yet
- Calculos Metabolicos: Estado MetabolicoDocument15 pagesCalculos Metabolicos: Estado MetabolicoMaggie PonceNo ratings yet
- Nonideal BehaviorDocument9 pagesNonideal BehaviorSusiilawatiiNo ratings yet
- Cheby TableDocument2 pagesCheby Tablebl9nkverseNo ratings yet
- WritingABudget One Pager OURProjectAward WITH EXAMPLESDocument3 pagesWritingABudget One Pager OURProjectAward WITH EXAMPLESbl9nkverseNo ratings yet
- Butterworth TableDocument1 pageButterworth Tablebl9nkverseNo ratings yet
- Feb 23 ClassDocument2 pagesFeb 23 Classbl9nkverseNo ratings yet
- Chapter 3 - Organizing: Dr. C. M. ChangDocument86 pagesChapter 3 - Organizing: Dr. C. M. Changbawardia100% (2)
- Quadratics PDFDocument5 pagesQuadratics PDFBəndiyeva ElnarəNo ratings yet
- GAO Cost Estimating GuideDocument476 pagesGAO Cost Estimating Guidepirotte100% (2)
- Undated Weekly Classic SundayDocument2 pagesUndated Weekly Classic Sundaybl9nkverseNo ratings yet
- BPH ChartDocument3 pagesBPH Chartbl9nkverseNo ratings yet
- Amplitude ModulationDocument14 pagesAmplitude ModulationsonalibhagwatkarNo ratings yet
- Tims Advanced Modules and Tims Special Applications Modules User ManualDocument113 pagesTims Advanced Modules and Tims Special Applications Modules User ManualSubhashree DashNo ratings yet
- LM 348Document17 pagesLM 348bl9nkverseNo ratings yet
- A1-03 - DSBSC Generation PDFDocument14 pagesA1-03 - DSBSC Generation PDFkick singhNo ratings yet
- 1 (A) - Spectral AnalysisDocument10 pages1 (A) - Spectral Analysisbl9nkverseNo ratings yet
- Timeextender ClassicDocument2 pagesTimeextender Classicbl9nkverseNo ratings yet
- Learning Objectives For The Pharmacology of Parkinson Disease Drugs.Document5 pagesLearning Objectives For The Pharmacology of Parkinson Disease Drugs.bl9nkverseNo ratings yet
- Watertracker Classic Sunday v1 PDFDocument1 pageWatertracker Classic Sunday v1 PDFbl9nkverseNo ratings yet
- GlaucomaDocument2 pagesGlaucomabl9nkverseNo ratings yet
- CYP450 Chart 5.2016Document8 pagesCYP450 Chart 5.2016bl9nkverseNo ratings yet
- Case 2 Chief ComplaintDocument2 pagesCase 2 Chief Complaintbl9nkverseNo ratings yet
- Short Acting Beta AgonistsDocument3 pagesShort Acting Beta Agonistsbl9nkverseNo ratings yet
- Drugs That Decrease Aqueous Production I. Beta-BlockersDocument2 pagesDrugs That Decrease Aqueous Production I. Beta-Blockersbl9nkverseNo ratings yet
- Antibiotics Handout ReferenceDocument3 pagesAntibiotics Handout Referencebl9nkverseNo ratings yet
- Violin I KijeDocument10 pagesViolin I Kijebl9nkverseNo ratings yet
- Functional GroupsDocument1 pageFunctional Groupsbl9nkverseNo ratings yet
- Case 1Document3 pagesCase 1bl9nkverseNo ratings yet
- Build 2 Verification MatrixDocument4 pagesBuild 2 Verification Matrixbl9nkverseNo ratings yet
- EMIS7320-01s 2017Document46 pagesEMIS7320-01s 2017bl9nkverseNo ratings yet
- Periodic TableDocument1 pagePeriodic Tablebl9nkverseNo ratings yet