Professional Documents
Culture Documents
E26 Questions
Uploaded by
polypeptide0 ratings0% found this document useful (0 votes)
18 views5 pageschemistry lab
Original Title
e26 Questions
Copyright
© © All Rights Reserved
Available Formats
DOCX, PDF, TXT or read online from Scribd
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this Documentchemistry lab
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
0 ratings0% found this document useful (0 votes)
18 views5 pagesE26 Questions
Uploaded by
polypeptidechemistry lab
Copyright:
© All Rights Reserved
Available Formats
Download as DOCX, PDF, TXT or read online from Scribd
You are on page 1of 5
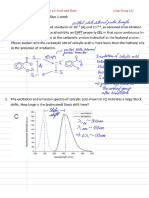
E26 QUESTIONS & DISCUSSION
1. According to the UV-Vis spectrum X, both HCrO
4
-
and CrO(O
2
)
2
absorb at 360nm.
Therefore, when the absorbance at this wavelength would include the absorbance of both
species. How would this affect the reaction rate determination? How can this be fixed?
Since the absorbance at 360nm would include both that of the reactant (Cr
7+
) and the product
(Cr
5+
), it would probably overestimate the reaction rate by making the disappearance rate
(decrease in absorbance) of HCrO
4
-
seem larger than it really is by including the appearance rate
(increase in absorbance) of CrO(O
2
)
2
. This problem can be fixed by using the other absorption
peak of Cr(V) at 580nm, a wavelength at which only Cr(V) absorbs:
A
380
= A[Cr(VII)] + A[Cr(V)]
A
580
= A[Cr(V)]
A
380
> A
580
A[Cr(VII)]=A
380
A
580
By subtracting the absorbance at 580nm from the absorbance at 380nm, the absorbance of Cr(VII)
can be determined.
2. When using the stopped-flow method to determine the rate constant and the spectrum
shows a first-order decay/growth, which method is more accurate? Why?
Usually, both methods (reaction rate determination using a first-order decay or first-order growth)
should be equally valid and accurate since (1/a[A])d[A]/dt = (1/b[B])d[B]/dt for a reaction
aAbB. In this experiment, however, one of the absorbances (A
380
) used in this experiment
includes both that of the reactant and the product, and H
2
O
2
is involved in the forward reaction.
H
2
O
2
is thermodynamically unstable and decomposes to H
2
O and O
2
, with the rate of
decomposition increasing with increasing temperature, pH, and concentration. In addition, many
transition metals also catalyze the decomposition of H
2
O
2.
Therefore, due to the instability of
H
2
O
2
and the fact that A
380
= A[Cr(VII)] + A[Cr(V)], the first-order decay should be used here.
SOURCE: N.N. Greenwood, A. Earnshaw (1997). Chemistry of the Elements (2nd ed.). Oxford UK:
Butterworth-Heinemann. A great description of properties & chemistry of H2O2.
3. In this experiment, which reactant has a larger impact on the reaction half life, H
+
or
H
2
O
2
? How does this affect the determination of the reaction mechanism?
HCrO
4
-
+ 2 H
2
O
2
+ H
+
CrO(O
2
)
2
H
2
O + 2 H
2
O
R = k[HCrO
4
-
]
m
[H
2
O
2
]
n
[H
+
]
o
=>
vial 15 vial 19 vial 20
H
2
O
2
600 L 500 L 600 L
1M HNO
3
300 L 400 L 400 L
290.7 331.4 375.7
The absorption spectrum for the product is used since only CrO(O
2
)
2
absorbs at this
wavelength, and no H
2
O
2
is involved in the backward reaction. Even though the reaction
orders m,n,o are still unknown, the reaction rate will always be proportional to k
obs
, the
reaction rate constant, regardless of the order of the reaction. The rate constants were
determined by fitting the experimentally obtained spectra were fitted with the exponential
function above to obtain k
obs
. Since the concentration of H
2
O
2
is the same in both vials 15 and
20, their ratio can be used to determine the reaction order of H
+
. Similarly, the concentration
of H+ is the same in vials 19 and 20 can their ratio can therefore be used to determine the
reaction order of H
2
O
2..
Thus,
Even though the reaction order for H
+
is slightly larger than that of H
2
O
2
, they are both
approximately equal to 1, which means that both have about the same impact on the reaction
half-time. This makes sense since slightly dilute acidic solutions are needed to ensure that
H
2
O
2
does not decompose and thus will be able to oxidize HCrO
4
-
to CrO(O
2
)
2
. In terms of
the reaction mechanism, this means that a reasonable reaction mechanism would have to
include both the participation of H+ and H
2
O
2
.
4. Please derive the relationship between [A] and time for n=0,1,2, where n= reaction
order.
You might also like
- NMR Yield CalculationDocument4 pagesNMR Yield CalculationpolypeptideNo ratings yet
- Units of Measurement: Introduction To ChemistryDocument4 pagesUnits of Measurement: Introduction To ChemistrypolypeptideNo ratings yet
- Glass Care Safe Handling PDFDocument16 pagesGlass Care Safe Handling PDFAdinda SaraswatiNo ratings yet
- Glass Care Safe Handling PDFDocument16 pagesGlass Care Safe Handling PDFAdinda SaraswatiNo ratings yet
- Homework 3Document13 pagesHomework 3polypeptideNo ratings yet
- Chemical Equilibrium (Final Review)Document20 pagesChemical Equilibrium (Final Review)polypeptideNo ratings yet
- Chem 14A Discussion Section: Coordination ChemistryDocument3 pagesChem 14A Discussion Section: Coordination ChemistrypolypeptideNo ratings yet
- Homework 3Document13 pagesHomework 3polypeptideNo ratings yet
- Mechanism of Microbial InfectionsDocument122 pagesMechanism of Microbial InfectionspolypeptideNo ratings yet
- Viral Diseases - Mechanisms of Microbial InfectionsDocument105 pagesViral Diseases - Mechanisms of Microbial InfectionspolypeptideNo ratings yet
- Lecture of Zoonoses at NTU 2015Document159 pagesLecture of Zoonoses at NTU 2015polypeptideNo ratings yet
- Midterm RecitationDocument12 pagesMidterm RecitationpolypeptideNo ratings yet
- Animal ConditioningDocument27 pagesAnimal ConditioningpolypeptideNo ratings yet
- Aic I HW7Document2 pagesAic I HW7polypeptideNo ratings yet
- Functional Groups in Organic ChemistryDocument1 pageFunctional Groups in Organic ChemistrygznmemberNo ratings yet
- Chemistry GRE SampleDocument0 pagesChemistry GRE Sampleyoostan100% (2)
- Pka TablesDocument6 pagesPka TablesAlessandro MoreniNo ratings yet
- General Chemistry C Homework 6 Due On 12/11 Name: ID: DepartmentDocument2 pagesGeneral Chemistry C Homework 6 Due On 12/11 Name: ID: DepartmentpolypeptideNo ratings yet
- Chapter 28 LCDocument5 pagesChapter 28 LCpolypeptideNo ratings yet
- Chapter24 Materials ChemDocument25 pagesChapter24 Materials ChempolypeptideNo ratings yet
- FLUXIONALITYDocument7 pagesFLUXIONALITYpolypeptideNo ratings yet
- Chapter 7 Intro To Optical InstrumentsDocument10 pagesChapter 7 Intro To Optical InstrumentspolypeptideNo ratings yet
- Chapter1s StatMechDocument22 pagesChapter1s StatMechpolypeptideNo ratings yet
- C H ActivationDocument55 pagesC H Activationpolypeptide100% (1)
- Est-Ce QueDocument6 pagesEst-Ce QuepolypeptideNo ratings yet
- Protective GroupDocument26 pagesProtective Groupchhan4No ratings yet
- Alkyne Metathesis & Enyne MetathesisDocument3 pagesAlkyne Metathesis & Enyne MetathesispolypeptideNo ratings yet
- Literature Report 2Document12 pagesLiterature Report 2polypeptideNo ratings yet
- Test Taking StrategiesDocument193 pagesTest Taking StrategiesAmy Kennedy100% (1)
- The Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeFrom EverandThe Subtle Art of Not Giving a F*ck: A Counterintuitive Approach to Living a Good LifeRating: 4 out of 5 stars4/5 (5794)
- The Little Book of Hygge: Danish Secrets to Happy LivingFrom EverandThe Little Book of Hygge: Danish Secrets to Happy LivingRating: 3.5 out of 5 stars3.5/5 (400)
- Shoe Dog: A Memoir by the Creator of NikeFrom EverandShoe Dog: A Memoir by the Creator of NikeRating: 4.5 out of 5 stars4.5/5 (537)
- Hidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceFrom EverandHidden Figures: The American Dream and the Untold Story of the Black Women Mathematicians Who Helped Win the Space RaceRating: 4 out of 5 stars4/5 (895)
- The Yellow House: A Memoir (2019 National Book Award Winner)From EverandThe Yellow House: A Memoir (2019 National Book Award Winner)Rating: 4 out of 5 stars4/5 (98)
- The Emperor of All Maladies: A Biography of CancerFrom EverandThe Emperor of All Maladies: A Biography of CancerRating: 4.5 out of 5 stars4.5/5 (271)
- A Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryFrom EverandA Heartbreaking Work Of Staggering Genius: A Memoir Based on a True StoryRating: 3.5 out of 5 stars3.5/5 (231)
- Never Split the Difference: Negotiating As If Your Life Depended On ItFrom EverandNever Split the Difference: Negotiating As If Your Life Depended On ItRating: 4.5 out of 5 stars4.5/5 (838)
- Grit: The Power of Passion and PerseveranceFrom EverandGrit: The Power of Passion and PerseveranceRating: 4 out of 5 stars4/5 (588)
- On Fire: The (Burning) Case for a Green New DealFrom EverandOn Fire: The (Burning) Case for a Green New DealRating: 4 out of 5 stars4/5 (73)
- Elon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureFrom EverandElon Musk: Tesla, SpaceX, and the Quest for a Fantastic FutureRating: 4.5 out of 5 stars4.5/5 (474)
- Devil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaFrom EverandDevil in the Grove: Thurgood Marshall, the Groveland Boys, and the Dawn of a New AmericaRating: 4.5 out of 5 stars4.5/5 (266)
- The Unwinding: An Inner History of the New AmericaFrom EverandThe Unwinding: An Inner History of the New AmericaRating: 4 out of 5 stars4/5 (45)
- Team of Rivals: The Political Genius of Abraham LincolnFrom EverandTeam of Rivals: The Political Genius of Abraham LincolnRating: 4.5 out of 5 stars4.5/5 (234)
- The World Is Flat 3.0: A Brief History of the Twenty-first CenturyFrom EverandThe World Is Flat 3.0: A Brief History of the Twenty-first CenturyRating: 3.5 out of 5 stars3.5/5 (2259)
- The Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreFrom EverandThe Gifts of Imperfection: Let Go of Who You Think You're Supposed to Be and Embrace Who You AreRating: 4 out of 5 stars4/5 (1090)
- The Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersFrom EverandThe Hard Thing About Hard Things: Building a Business When There Are No Easy AnswersRating: 4.5 out of 5 stars4.5/5 (344)
- The Sympathizer: A Novel (Pulitzer Prize for Fiction)From EverandThe Sympathizer: A Novel (Pulitzer Prize for Fiction)Rating: 4.5 out of 5 stars4.5/5 (121)
- Her Body and Other Parties: StoriesFrom EverandHer Body and Other Parties: StoriesRating: 4 out of 5 stars4/5 (821)
- PHYS 237 Syllabus 2014Document2 pagesPHYS 237 Syllabus 2014spylogo3No ratings yet
- Aldehydes PresentationDocument40 pagesAldehydes PresentationEric ArcillasNo ratings yet
- 10 Root LocusDocument13 pages10 Root LocusAhmed Tanveer Ashraff100% (1)
- 591 Homework v1 - 3Document45 pages591 Homework v1 - 3Michael FralaideNo ratings yet
- Basic Laplace Fourier TransformsDocument3 pagesBasic Laplace Fourier TransformsWayne LeeNo ratings yet
- Explosive Welding 1 WeldingDocument4 pagesExplosive Welding 1 WeldingRabindra DashNo ratings yet
- Classical Mechanics 2Document109 pagesClassical Mechanics 2pticicaaaNo ratings yet
- m1 SuvatDocument7 pagesm1 SuvatdrkhansacademyNo ratings yet
- Module 6: Electromagnetism Topic 1.1: Charged Particles in Electric/Magnetic FieldsDocument13 pagesModule 6: Electromagnetism Topic 1.1: Charged Particles in Electric/Magnetic FieldsReacher ElliotNo ratings yet
- TugasDocument7 pagesTugastemizzhNo ratings yet
- Schneider Zelio Control RM4TR32-356472Document8 pagesSchneider Zelio Control RM4TR32-356472TERASAT SANo ratings yet
- Sebellino StaticsDocument3 pagesSebellino StaticsJoby Jobzz SebellinoNo ratings yet
- Compressor BasicsDocument65 pagesCompressor BasicsGiang T Le100% (1)
- FSMQ Force Diagrams PDFDocument6 pagesFSMQ Force Diagrams PDFgrace_lo_1No ratings yet
- Esolutions Manual - Powered by CogneroDocument26 pagesEsolutions Manual - Powered by CogneroZar ArhNo ratings yet
- Shimpo ER SeriesDocument10 pagesShimpo ER SeriesElectromateNo ratings yet
- Ai TS 1 (XI) - SET ADocument14 pagesAi TS 1 (XI) - SET Akanishk namdev100% (1)
- Induction Motor Tradeoff For VSD Driven Pumps and Fans PDFDocument5 pagesInduction Motor Tradeoff For VSD Driven Pumps and Fans PDFkankokwahNo ratings yet
- Lesson 1 Coulombs LawDocument16 pagesLesson 1 Coulombs LawPeach BubbleNo ratings yet
- Matrix Assisted Laser DesorptionDocument9 pagesMatrix Assisted Laser DesorptionYASHIKA raniNo ratings yet
- Aspirin Loading and Release From MCM-41 Functionalized With Aminopropyl Groups Via Co-Condensation or Postsynthesis Modi Fication MethodsDocument9 pagesAspirin Loading and Release From MCM-41 Functionalized With Aminopropyl Groups Via Co-Condensation or Postsynthesis Modi Fication MethodsBasofi HabibiNo ratings yet
- M1A 01 Algebra Foundation 06 Dimensional AnalysisDocument25 pagesM1A 01 Algebra Foundation 06 Dimensional AnalysisMuhlis AliNo ratings yet
- Haulotte H 41 PXDocument2 pagesHaulotte H 41 PXAthox QhurienNo ratings yet
- Structural Elements in FLAC 3DDocument186 pagesStructural Elements in FLAC 3DSergio Andres ZerreitugNo ratings yet
- Jest 2023 Question With Solution 1Document31 pagesJest 2023 Question With Solution 1Muskan MukhijaNo ratings yet
- Design of Raft FoundationDocument4 pagesDesign of Raft FoundationDayo Idowu100% (2)
- Motion With Uniform AccelerationDocument3 pagesMotion With Uniform AccelerationShidevNo ratings yet
- Chapter 5: Laws of Motion Class: XI Subject: Physics: Topic: Dynamics of Uniform Circular Motion, Banking of RoadDocument5 pagesChapter 5: Laws of Motion Class: XI Subject: Physics: Topic: Dynamics of Uniform Circular Motion, Banking of RoadKirtan KumarNo ratings yet
- Self Assessment Questions OneDocument3 pagesSelf Assessment Questions OneTendus StephanNo ratings yet