Professional Documents
Culture Documents
27-30 Sudden Death of A Premature New-Born With Hypoplastic Left Heart Syndrome. Morfological Study of The Heart
Uploaded by
Loredana MorosanuOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
27-30 Sudden Death of A Premature New-Born With Hypoplastic Left Heart Syndrome. Morfological Study of The Heart
Uploaded by
Loredana MorosanuCopyright:
Available Formats
Rom J Leg Med [21] 27-30 [2013]
DOI: 10.4323/rjlm.2013.27
2013 Romanian Society of Legal Medicine
27
Sudden death of a premature new-born with Hypoplastic Left Heart Syndrome.
Morfological study of the heart
Florin Mihail Filipoiu
1
, Mihaela Balgradean
2,*
, Viorel Jinga
3
_________________________________________________________________________________________
Abstract: The Hypoplastic left heart syndrome (HLHS), also known as the Norwood Syndrome, is a complex clinical
entity, a part of the cardiac congenital maladies, with a birth frequency of 1/15000. It is a severe disease with a huge mortality
index after-birth. The lack of development of the left ventricle and the atresia with mitral or aortic stenosis mainly characterizes
the syndrome.
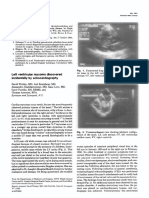
We report a case of a premature male fetus which was diagnosed with HLHS at 6 moths of age by cardiac ecoghraphic
examination. The new-born died at 3 hours after birth by cardio-respiratory arrest due to ventricular fbrillation.
We noticed that the specialized literature does not include photographic iconographies, owing to the fact that all articles
use only explaining schemes. This fact has determined us to achieve a suggestive dissection of the heart.
Key Words: Hypoplastic left heart syndrome, HLHS, dissection.
1) Associate Professor, Department of Morphological Sciences, Carol Davila University of Medicine and Pharmacy,
Bucharest, Romania
2) *Corresponding author: Associate Professor, Department of Pediatrics, Carol Davila University of Medicine and
Pharmacy, Tel: 0722.280.110, E-mail: mbalgradean@gmail.com, Bucharest, Romania
3) Associate Professor, Department of General Surgery, Carol Davila University of Medicine and Pharmacy, Bucharest,
Romania
T
he Norwood syndrome known as the
hypoplastic left heart syndrome (HLHS)
describes a group of cardiac malformations which
consist in various underdevelopment stages of the left
heart and of the aorta. Its consequences are related
with the incapacity of the heart to sustain the systemic
circulation and with a signifcant obstruction to blood
fow.
Lev [1] in 1952 was the frst to use the term
hypoplasia of the aortic tract complexes.
The frst to use the term of HLHS was Noonan
and Nadas [2] in 1958 describing an atresia or stenosis
of the left side of the heart, but in a general sense, the
area of discussions ranging from the aortic atresia to
an underdevelopment of the left ventricle.
In the 1980's the Norwood operation was
developed and resulted in a synonymous use with
HLHS, because it implies the inability of the left heart
to support systemic circulation [3].
The subject related to the HLHS was debated
by the Heart Surgery Nomenclature and by the
European Association for Cardiothoracic Surgery to
establish a common nomenclature [4].
MATERIAL AND METHODS
The investigated heart has been harvested
from a seven-month fetus, deceased in an obstetrics
service, three hours after a premature birth. The fetus
was previously diagnosed by fetal eco-cardiography
with HLHS.
After the demise of the child, the mother
28
F.M. Filipoiu et al. Sudden death of a premature new-born with Hypoplastic Left Heart Syndrome
gave her approval so that the corpse may be used in
scientifc purposes.
The heart has been dissected and we made
sagittal sections through both left and right part. The
dissection stages were photographed using a digital
camera.
RESULTS
The examined corpse presented generalized
adenopathy. The thymus was hypertrophied. Between
the thymus and the base of the heart a tumoral mass
can be observed, having the aspect of a lymphoma
(Figure 1).
The heart is generally hypertrophied, with
a dominant right hypertrophy. After removing the
pericardium the following aspects can be observed:
- on the sternocostal side the coronary vessels
are turgid and sinuous, with a developed collateral
circulation.
- the left auricle is very large in dimenssions. It
comes in contact and even covers the right auricle
(Figure 2).
On the base of the heart the coronary sinus
requires a special attention. It appears to have stopped
evolving, without reducing its diameter size up to the
usual. It still has the aspect of a horn of the venous
sinus, in which opens the great cardiac vein. (Figure 3).
Proximal to the junction with the great cardiac
vein, the oblique vein of left atrium (Marshall) can be
observed.
Between the ascending aorta and the pulmonary
artery we observe two embryological remains of the
branhial arteries. At their origins they are united in a
conjunctive mass and have no lumen. They have no
orifces of origin in the aorta or in the pulmonary artery.
The ductus arteriosus is permeable and well
represented, above the pulmonary artery origin.
(Figure 4).
On sagittal section we can observe a hypoplasic
left atrium with the endocardium being thickened and
undifferentiated. Unlike the atrium, the left auricle is
large, but atretic and almost without a lumen.
On the interatrial septum we can see that there
is no interatrial communication and the oval fossa
was closed by the overlapping of various successive
structures (Figure 5).
The mitral valve is atresic, the cuspids are
small, thickened, inserted in the ventricle on small
papillary muscles. There are no chordae tendinae.
The left ventricle is hypoplasic. Its endocardium
is thickened. The left ventricular myocardium is very
thick, has no regular structure and keeps a poorly
differentiated appearance (Figure 6).
The aortic orifce is atresic and stenosed. The
free edges of the semilunar valves are stuck together
Figure 1. Heart-lungs-thymus ensemble, antero-lateral view.
Figure 2. Heart-anterior view. Figure 3. Heart-pulmonary and diaphragmatic surfaces.
Romanian Journal of Legal Medicine Vol. XXI, No 1(2013)
29
in a common mass. The left coronary sinus and the non
coronary one are differentiated and have lumen.
The coronary right sinus is atresic and has no
lumen. There is a coronary right permeable artery. The
section includes the origin of the left coronary artery,
which presents a lumen and thick walls. We mention
that beyond the level of the coronary ostium the
ascending aortic lumen is present and well formed.
DISCUSSION
The left ventricular hypoplasia refers to the
absence of forming a normal-sized ventricular cavity
[4-7]. But the left ventricle exists and has very thick
walls, with a nearly embrionar aspect of the myocardium,
which stopped its evolution. This issue might be
due to the lack of blood fow through the ventricle.
The presence of the coronary, both sinuous and
turgid, suggests that the left ventricular myocardial
mass is not suffciently permeable for the coronary fow.
The coronary ostium is located above the
atresic area. The coronary perfusion is retrograde
through the arterial duct.
- The atresia of the left auricle prevents the
achievement of an effcient surgical communication
with the right auricle. Because in the Norwood type
operations the surgeons apply for an interauricular
anastomosis, our observation becomes important for
that moment when the surgical technique is to be
chosen [9].
- The presence of the generalized adenopathy and
thymus type of hypertrophy draws attention to the
associated pathologies that may be involved in the
pathogenesis or aggravation of the heart disease.
CONCLUSION
We consider that presenting and commenting
the dissection of a heart with the Norwood syndrome,
has several benefts:
- shows the clinician the seriousness of this
pathology.
- accommodates the cardiac surgeon with anatomic
details of the hypoplastic left heart syndrome.
- it represents a good reference for the effort of the
imagistic diagnosis.
- the grim prognosis may be better explained to
the patient's caregiver, using these images.
Figure 4. The base of the heart.
Figure 6. Sagittal cross-section through the left ventricle-left
view.
Figure 5. Sagittal cross-section through the left atrium. Left
view of the interatrial septum, the left atrial cavity and of the
mitral orifce.
30
F.M. Filipoiu et al. Sudden death of a premature new-born with Hypoplastic Left Heart Syndrome
References
1. Lev M. Pathologic anatomy and interrelationship of hypoplasia of the aortic tract complexes. Lab Invest 1952;1:6170.
2. Noonan JA, Nadas AS. The hypoplastic left heart syndrome. An analysis of 101 cases. Pediatr Clin North Am 1958;5: 102956
3. Norwood WI, Lang P, Castan eda AL, Campbell DN. Experience with operations for hypoplastic left heart syndrome. J Cardio Thorac
Surg 1981;82:5119.
4. Page DA, Levine MM. Left ventricular growth in a patient with critical coarctation of the aorta and hypoplastic left ventricle. Pediatr
Cardiol 1995;16:176 8.
5. Maurice Beghetti, Genve et Renzo Ghisla, St. Gall, Hypoplasie Coeur gauche Diagnostic, traitement et prognosis, PAEDRIATICA, Vol
16 No. 5 2005
6. Christo I. Tchervenkov Marshall L. Jacobs, and Stephen A. Tahta, Congenital Heart Surgery Nomenclature and Database Project:
Hypoplastic Left Heart Syndrome Ann Thoracic Surge in 2000, 69: S170-9 The Annals of Thoracic Surgery
7. Doty DB, Knott HW. Hypoplastic left heart syndrome. J Thorac Cardiovasc Surg 1977;74:62430.
8. Starnes VA, Griffn ML, Pitlick PT, et al. Current approach to hypoplastic left heart syndrome. Palliation, transplantation or both? J Thorac
Cardiovasc Surg 1992;104:18995.
9. Ghanayem NS, Hoffmann GM, Mussatto KA, et al. Home surveillance program prevents interstage mortality after the Norwood procedure.
J Thorac Cardiovasc Surg 2003; 126: 13671377
You might also like
- Hypoplastic Right Heart Syndrome, Absent Pulmonary Valve, and Non-Compacted Left Ventricle in An AdultDocument4 pagesHypoplastic Right Heart Syndrome, Absent Pulmonary Valve, and Non-Compacted Left Ventricle in An AdultReinaldi octaNo ratings yet
- Tow Bin 2015Document13 pagesTow Bin 2015Raúl Pérez GilNo ratings yet
- Particularities of Scimitar Syndrome in Adult PatientsDocument5 pagesParticularities of Scimitar Syndrome in Adult PatientsIJAR JOURNALNo ratings yet
- Cor Triatriatum: Edited by - HAPPY MALIK (3 Year Student)Document26 pagesCor Triatriatum: Edited by - HAPPY MALIK (3 Year Student)Olga GoryachevaNo ratings yet
- Congenital Heart DiseasesDocument10 pagesCongenital Heart DiseasesRohit NaiduNo ratings yet
- Echocardiography and More Recently With CT and MRI. However, The Chest FilmDocument10 pagesEchocardiography and More Recently With CT and MRI. However, The Chest FilmNoor Al Zahraa AliNo ratings yet
- Beating with Precision: The Science of Cardiology: Understand the Intricacies of the Human HeartFrom EverandBeating with Precision: The Science of Cardiology: Understand the Intricacies of the Human HeartNo ratings yet
- Topic 10 CVSDocument10 pagesTopic 10 CVSSneha GaneshNo ratings yet
- CHEST X-RAY Presentation CVSDocument45 pagesCHEST X-RAY Presentation CVSNorsafrini Rita Ahmad100% (1)
- SMJ 59 279Document5 pagesSMJ 59 279Faradiba MaricarNo ratings yet
- Ebstein's Anomaly - Review of A Multifaceted Congenital Cardiac ConditionDocument14 pagesEbstein's Anomaly - Review of A Multifaceted Congenital Cardiac ConditionSep NeufNo ratings yet
- E7.5 E8.0 E8.5 E12.5Document11 pagesE7.5 E8.0 E8.5 E12.5خـޢޢـطـޢޢـ آحـޢޢـمـޢޢـرNo ratings yet
- Current Management of Ebstein's Anomaly in The AdultDocument9 pagesCurrent Management of Ebstein's Anomaly in The AdultRJMNo ratings yet
- Left Ventricular Myxoma Discovered Incidentally by EchocardiographyDocument2 pagesLeft Ventricular Myxoma Discovered Incidentally by EchocardiographyAnonymous o2a75FBeRWNo ratings yet
- Hypoplastic Left Heart Syndrome Diagnosis and ManagementDocument10 pagesHypoplastic Left Heart Syndrome Diagnosis and ManagementMore InterestingNo ratings yet
- Situs Inversus Imaging - Practice Essentials, Radiography, Computed TomographyDocument9 pagesSitus Inversus Imaging - Practice Essentials, Radiography, Computed TomographyFadil DendiNo ratings yet
- Congenital Heart Disease EtiologyDocument8 pagesCongenital Heart Disease EtiologykudzaimuregidubeNo ratings yet
- Congenital Heart DiseaseDocument93 pagesCongenital Heart DiseaseManjunatha HR100% (1)
- 4 Surgical Anatomy of The HeartDocument79 pages4 Surgical Anatomy of The HeartKacper DaraszkiewiczNo ratings yet
- CVSassignement 1 AnatomyDocument12 pagesCVSassignement 1 AnatomyRista SimamNo ratings yet
- Review of Literature A. Overview of Cardiovascular System: Mitral Valve InsufficiencyDocument10 pagesReview of Literature A. Overview of Cardiovascular System: Mitral Valve InsufficiencyCha AlegriaNo ratings yet
- What Causes Increased Pulmonary Blood FlowDocument11 pagesWhat Causes Increased Pulmonary Blood FlowJc Pauline PabustanNo ratings yet
- Silent Myocardial IschaemiaDocument7 pagesSilent Myocardial IschaemiaSmitha ShettyNo ratings yet
- Cardiovascular DysfunctionDocument15 pagesCardiovascular DysfunctionJhasseryne Orias SanchezNo ratings yet
- Atrial Septal Defects: Presented by Dr. Maysa Abdul Haq Directed by Dr. Ali Halabi Jordan Hospital 11-9-2005Document36 pagesAtrial Septal Defects: Presented by Dr. Maysa Abdul Haq Directed by Dr. Ali Halabi Jordan Hospital 11-9-2005Joe JosephNo ratings yet
- Echo Heart4advancedDocument21 pagesEcho Heart4advanceddgina8800No ratings yet
- Transposition Septal Defect: of The and PulmonaryDocument6 pagesTransposition Septal Defect: of The and Pulmonarydrafabiola.cardiopediagmail.comNo ratings yet
- Pi Is 1878540914000462Document6 pagesPi Is 1878540914000462aandakuNo ratings yet
- Europace 2013 Brugada Europace Eut082Document46 pagesEuropace 2013 Brugada Europace Eut082Griselle PortillaNo ratings yet
- Anatomical and Pathophysiological Classification of Congenital Heart DiseaseDocument16 pagesAnatomical and Pathophysiological Classification of Congenital Heart DiseasePietro PensoNo ratings yet
- 14 08 11.CV Anatomy Histology - Gundogan.Document6 pages14 08 11.CV Anatomy Histology - Gundogan.allisonNo ratings yet
- Cardio BasicsDocument8 pagesCardio Basicsanandn22No ratings yet
- Diagnosis of Double-Chambered Left Ventricle by Contrast Echocardiography: A Case ReportDocument6 pagesDiagnosis of Double-Chambered Left Ventricle by Contrast Echocardiography: A Case ReportMasithaNo ratings yet
- Anomalous Origin of The Coronary Artery From The Pulmonary Artery in Children and Adults - A Pictorial Review of Cardiac Imaging FindingsDocument10 pagesAnomalous Origin of The Coronary Artery From The Pulmonary Artery in Children and Adults - A Pictorial Review of Cardiac Imaging FindingsChici FarlinaNo ratings yet
- Aortic Atresia With Normally Developed Left Ventricle in A Young AdultDocument3 pagesAortic Atresia With Normally Developed Left Ventricle in A Young AdultUmmul HidayahNo ratings yet
- Fishbein 2019Document8 pagesFishbein 2019hawk.man8No ratings yet
- PCU 200 Handbook 2018-19 PDFDocument177 pagesPCU 200 Handbook 2018-19 PDFVica CapatinaNo ratings yet
- 41 HLHS Ma AaDocument14 pages41 HLHS Ma AaVictor PazNo ratings yet
- Ovid - Cardiac Nursing7Document82 pagesOvid - Cardiac Nursing7Rapani LoebisNo ratings yet
- CHAPTER 35 Cardiac DisordersDocument93 pagesCHAPTER 35 Cardiac DisordersIfy OhansonNo ratings yet
- Fetal Cardiac Dextroposition in The Absence of An Intrathoracic MassDocument8 pagesFetal Cardiac Dextroposition in The Absence of An Intrathoracic MassCatalinNo ratings yet
- CardiomyopathyDocument17 pagesCardiomyopathyRashed ShatnawiNo ratings yet
- Research Paper On Heart MurmurDocument4 pagesResearch Paper On Heart Murmurnodahydomut2100% (1)
- Hypertensive Heart DiseaseDocument18 pagesHypertensive Heart DiseaseKaye ViolaNo ratings yet
- Acyanotic Congenital Heart DiseaseDocument7 pagesAcyanotic Congenital Heart DiseaseSam Raj100% (1)
- Anatomopathological Session: A 20 Year-Old Man With Heart Failure Due To Restrictive CardiomyopathyDocument8 pagesAnatomopathological Session: A 20 Year-Old Man With Heart Failure Due To Restrictive CardiomyopathyGuilherme AméricoNo ratings yet
- Hypertensive Heart DiseaseDocument18 pagesHypertensive Heart DiseaseAmanda Edwards100% (1)
- Mayo Applied Cardiac AnatomyDocument34 pagesMayo Applied Cardiac AnatomyMuhammad Naveed SaeedNo ratings yet
- Lokanadham Et AlDocument5 pagesLokanadham Et AleditorijmrhsNo ratings yet
- Cardio Vascular DisordersDocument62 pagesCardio Vascular DisordersUday Kumar100% (1)
- 1 Mitral Stenosis: InstructionDocument8 pages1 Mitral Stenosis: Instructionrashid.scribdNo ratings yet
- Congenital Heart Disease Is One or More Problems With The Heart's Structure ThatDocument37 pagesCongenital Heart Disease Is One or More Problems With The Heart's Structure Thatneelimawanker chinnariNo ratings yet
- Classic Imaging Signs of Congenital Cardiovascular Abnormalities 1Document45 pagesClassic Imaging Signs of Congenital Cardiovascular Abnormalities 1adhik_deepakNo ratings yet
- Rheumatic Heart DiseaseDocument15 pagesRheumatic Heart DiseasePdianghunNo ratings yet
- Nursing Care Plan For Acyanotic Heart DiseaseDocument55 pagesNursing Care Plan For Acyanotic Heart DiseaseDeepikaxena John79% (14)
- Alfonso Lopez CardiovascularDocument15 pagesAlfonso Lopez Cardiovascularjaniceli0207No ratings yet
- Arco DerechoDocument8 pagesArco DerechoCorazon MabelNo ratings yet
- DR Abdulkareem Al Othman Valvular HDDocument10 pagesDR Abdulkareem Al Othman Valvular HDDarawan MirzaNo ratings yet
- Minggu 2 LP ASDDocument15 pagesMinggu 2 LP ASDMuhammad PanduNo ratings yet
- Jaga 16 Maret 2019Document8 pagesJaga 16 Maret 2019RONY PERMANANo ratings yet
- Autopsy of Heart External ExaminationDocument20 pagesAutopsy of Heart External ExaminationchinnnababuNo ratings yet
- Pengangkutan: TransportationDocument22 pagesPengangkutan: TransportationsuhanaNo ratings yet
- Chapter 10-Rhythmical Excitation of The HeartDocument13 pagesChapter 10-Rhythmical Excitation of The Heartmuna s100% (1)
- Masiag National High SchoolDocument6 pagesMasiag National High SchoolJESSAN DE PEDRONo ratings yet
- CardioDocument40 pagesCardioOctavio González InversonNo ratings yet
- Anatomy and Physiology of The Heart Made Easy Part 1Document22 pagesAnatomy and Physiology of The Heart Made Easy Part 1adedayodavid860No ratings yet
- Anatomy & PhysiologyDocument38 pagesAnatomy & PhysiologyParsa EbrahimpourNo ratings yet
- Important SEQs Heart Physiology by Dr. RoomiDocument3 pagesImportant SEQs Heart Physiology by Dr. RoomiMudassar Roomi100% (2)
- Echo Petunjuk PenggunaanDocument20 pagesEcho Petunjuk PenggunaanabdulkadirmunsyNo ratings yet
- Buret TerbaruDocument33 pagesBuret TerbaruEka Rahayu UtamiNo ratings yet
- HeartDocument28 pagesHeartZahidKhanNo ratings yet
- K Sembulingam Essentials of Medical Physiology 6th 105 PDFDocument11 pagesK Sembulingam Essentials of Medical Physiology 6th 105 PDFBlerta Deari100% (2)
- Bme Unit-1Document6 pagesBme Unit-1Sowmya ChowdaryNo ratings yet
- Heart Guided Notes External Heart AnatomyDocument6 pagesHeart Guided Notes External Heart AnatomyElliot SynderNo ratings yet
- Congenital Heart Diseases: Non Cyanotic PlethoraDocument43 pagesCongenital Heart Diseases: Non Cyanotic PlethoraFauzi SatriaNo ratings yet
- The Cardiovascular System: (Chapter 15)Document59 pagesThe Cardiovascular System: (Chapter 15)Hasanuddin NuruNo ratings yet
- Anatomy of Heart: Mr. Binu Babu MBA, M.Sc. (N) Asst. Professor Mrs. Jincy Ealias M.Sc. (N) LecturerDocument71 pagesAnatomy of Heart: Mr. Binu Babu MBA, M.Sc. (N) Asst. Professor Mrs. Jincy Ealias M.Sc. (N) Lecturerjyoti kunduNo ratings yet
- LOGIQ E11LOGIQ E20 R3 English Advanced Reference Manual (Domestic) - UM - 5919872-1EN - 1Document203 pagesLOGIQ E11LOGIQ E20 R3 English Advanced Reference Manual (Domestic) - UM - 5919872-1EN - 1Hani Al-NassNo ratings yet
- Mitral StenosisDocument25 pagesMitral StenosisnindaNo ratings yet
- Interior of The Right AtriumDocument2 pagesInterior of The Right AtriumAanavi MalikNo ratings yet
- Brachiocephalic TrunkDocument3 pagesBrachiocephalic TrunkstephNo ratings yet
- Cardiovascular System: The Heart: Student Learning OutcomesDocument10 pagesCardiovascular System: The Heart: Student Learning Outcomeslily1liang-1No ratings yet
- CVS. Questions and AnswersDocument6 pagesCVS. Questions and AnswersOmar HNo ratings yet
- Regurgitation The Tricuspid Valve: Current Perspective and Evolving Management of TricuspidDocument9 pagesRegurgitation The Tricuspid Valve: Current Perspective and Evolving Management of TricuspidAngga Maulana IbrahimNo ratings yet
- PericardiumDocument2 pagesPericardiumUDIT MANCHANDANo ratings yet
- The Cardiovascular SystemDocument20 pagesThe Cardiovascular Systembuzz QNo ratings yet
- The Cardiovascular System: Lecture Presentation by Patty Bostwick-Taylor Florence-Darlington Technical CollegeDocument124 pagesThe Cardiovascular System: Lecture Presentation by Patty Bostwick-Taylor Florence-Darlington Technical Collegelourd nabNo ratings yet
- Echo A. A.made PermadiDocument1 pageEcho A. A.made PermadiIndrayaniNo ratings yet
- Development of The Cardiovascular System: Mais Al-Kawaz Rim El ChakiDocument12 pagesDevelopment of The Cardiovascular System: Mais Al-Kawaz Rim El ChakiMais Al-KawazNo ratings yet