Professional Documents
Culture Documents
2 Gram Staining
Uploaded by
Michelle ErikaCopyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
2 Gram Staining
Uploaded by
Michelle ErikaCopyright:
Available Formats
Ateneo de Zamboanga University
COLLEGE OF NURSING
NURSING SKILLS OUTPUT Report No. 1
EAR DROPS INSTILLATION AND EAR IRRIGATION
I. DESCRIPTION
Gram staining is a quick procedure used to look for the presence of bacteria in
tissue samples and to characterise bacteria as Gram-positive or Gram-negative,
based on the chemical and physical properties of their cell walls. The Gram stain
should almost always be done as the first step in diagnosis of a bacteria infection.
The Gram stain is named after the Danish scientist Hans Christian Gram (1853
1938), who developed the technique in 1882 and published it in 1884 as a technique
to discriminate between two types of bacteria with similar clinical symptoms:
Streptococcus pneumoniae (also known as the pneumococcus) and Klebsiella
pneumoniae bacteria.
II. MATERIALS/EQUIPMENT NEEDED:
III. PROCEDURE:
1. Get a tissue sample for the Gram stain. For example, if a pneumonia is
suspected, get a sputum sample. If an urinary tract infection is suspected, get a
urine sample. If an intestinal infection is suspected, get a stool sample. If a brain
infection is suspected, get a cerebrospinal fluid (CSF) sample (centrifuge the fluid
and obtain the sediment for the Gram stain). If a skin infection is suspected, get
a skin biopsy sample. If an ear infection is suspected, get a middle ear fluid
sample (to increase yield, or the likelihood of finding the bacteria, centrifuge the
middle ear fluid sample to allow it to settle into three layers: supernatant (top),
buffy coat (middle), and red cells (bottom); get the buffy coat layer for the Gram
stain). Essentially any human tissue can be obtained for the Gram stain.
2. Add 1-2 drops of the tissue sample onto a glass slide. Spread it evenly on the
slide to form a thin smear, which can be done by sliding the edge of another
glass slide across the glass slide containing the tissue sample. Allow it to air dry.
3. Heat fix the smear, by quickly passing it two to three times through a flame, or
heat it on top of an electric slide warmer. Do not overheat, to avoid distortion.
Alternatively, the smear may be fixed by methanol instead, by adding 1-2 drops
of methanol onto the dried smear, draining off excess methanol, and allowing it
to air dry. Methanol has the advantage that it does not lyse red cells and it
minimises damage to host cells, giving a cleaner background.
Bunsen burner
alcohol-cleaned microscope slide
water
Crystal violet, Gram's iodine solution, acetone/ethanol (50:50 v:v),
0.1% basic fuchsin solution
4. Flood the smear with crystal violet. Wait thirty seconds. Crystal violet (CV)
dissociates in aqueous solutions into CV+ and chloride (Cl) ions. These ions
penetrate through the cell wall and cell membrane of both gram-positive and
gram-negative cells. The CV+ ion interacts with negatively charged components
of bacterial cells to stain the cells purple.
5. Gently rinse off the crystal violet with tap water. Do not rinse excessively,
which might remove the stain from Gram positive bacteria.
6. Flood the smear with iodine. Leave for at least three seconds. Iodine, in the form
of negatively charged ions, interacts with CV+ to form large complexes of crystal
violet and iodine (CVI complexes) within the inner and outer layers of the cell.
Iodine acts as a trapping agent to retain the purple crystal violet colour in the
cell.
7. Gently rinse off the iodine with tap water.
8. Decolorize by adding alcohol or acetone to the smear while holding the slide at
an angle to allow the decoloriser to drain. Stop when runoff becomes clear,
within seconds. The decolorisation step is critical and must be timed correctly. If
left on too long, the decolorising agent will remove the crystal violet stain from
both gram-positive and negative cells.
9. Gently rinse off excess decoloriser with tap water.
10. Flood the smear with safranin counterstain. Wait thirty seconds. Counterstain is
applied last to stain the decolorised gram-negative bacteria a pink or red
shadeBasic fuchsin, which stains anaerobic bacteria more intensely, may be
substituted for safranin, but it is less commonly used.
11. Gently rinse off excess safranin with tap water.
12. Drain slide and allow it to air dry. The Gram stain is done.
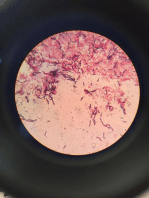
13. Examine the slide under the light microscope. Gram-positive bacteria appear
purple as stained by crystal violet, which is trapped within their thick cell walls.
Gram-negative bacteria appear pink as stained by the safranin counter-stain, as
their thin cell walls allow the crystals violet to wash out during decolorisation.
Bacteria are further classified by their shape under the microscope, most
commonly as cocci (spherical) or rods (cylindrical).
IV. DIAGRAM/ILLUSTRATION:
V. NURSING RESPONSIBILITIES:
Before Procedure:
During Procedure:
After Procedure:
REFERENCE:
http://www.uphs.upenn.edu/bugdrug/antibiotic_manual/Gram2.htm
http://www.wikihow.com/Gram-Stain
DATE CLINICAL INSTRUCTOR
MICHELLE ERIKA F. MEJIA
BSN III- B
You might also like
- Microbiology: a QuickStudy Laminated 6-Page Reference GuideFrom EverandMicrobiology: a QuickStudy Laminated 6-Page Reference GuideNo ratings yet
- Sputum Culture TestDocument5 pagesSputum Culture TestpraveenASPNo ratings yet
- Stool Examination (4)Document29 pagesStool Examination (4)محمد رحيم حسن محمودNo ratings yet
- Staining Techniques in Microbiology.Document9 pagesStaining Techniques in Microbiology.rajendraprasadreddyNo ratings yet
- Gastric AnalysisDocument3 pagesGastric AnalysisMichelle Erika0% (1)
- Monoclonal Antibody and Immunosensor Technology: <b>The production and application of rodent and human monoclonal antibodies</b>From EverandMonoclonal Antibody and Immunosensor Technology: <b>The production and application of rodent and human monoclonal antibodies</b>No ratings yet
- Psychiatric Nursing Documentation MethodsDocument25 pagesPsychiatric Nursing Documentation MethodsMichelle ErikaNo ratings yet
- Gram Staining ProtocolDocument19 pagesGram Staining ProtocolmourighoshNo ratings yet
- 3 Mic125Document8 pages3 Mic125nadiazkiNo ratings yet
- Gram Stain Final DraftDocument6 pagesGram Stain Final Draftcxs5278No ratings yet
- Blood Culture Test: Detecting Bacteria in the BloodDocument3 pagesBlood Culture Test: Detecting Bacteria in the BloodEllah MaeNo ratings yet
- Lab Safety Rules and Gram StainingDocument4 pagesLab Safety Rules and Gram StainingAsad MahmoodNo ratings yet
- Lab ReportDocument4 pagesLab ReportDiane AlecksaNo ratings yet
- LAB 2: Staining and Streaking: Series of Stains and Chemical Reagents To Increase Contrast and Reveal Information AboutDocument10 pagesLAB 2: Staining and Streaking: Series of Stains and Chemical Reagents To Increase Contrast and Reveal Information AboutrabkaNo ratings yet
- Richmond CompleteDocument3 pagesRichmond CompleteKojo PappoeNo ratings yet
- Staining MethodsDocument8 pagesStaining MethodsMd Arshad100% (1)
- Simple and Differential Staining of Bacteria: Figure1 Principles Behind Gram StainingDocument4 pagesSimple and Differential Staining of Bacteria: Figure1 Principles Behind Gram StainingNurulJazirohNo ratings yet
- Laboratory Activity Revil Angelica MDocument16 pagesLaboratory Activity Revil Angelica MAngelica Malacay RevilNo ratings yet
- StainingDocument5 pagesStainingyuppie_raj2175No ratings yet
- Germ Tube TestDocument4 pagesGerm Tube TestJericho SingcoNo ratings yet
- Bachelor's degree in Microbiology covers bacterial growthDocument10 pagesBachelor's degree in Microbiology covers bacterial growthOumia HarbitNo ratings yet
- Staining, Bacteria, and Use of The MicroscopeDocument9 pagesStaining, Bacteria, and Use of The MicroscopeHusna AliasNo ratings yet
- Staining Bacteria and MicroscopeDocument9 pagesStaining Bacteria and MicroscopeNur Athirah KhairinaNo ratings yet
- Lab 5 Microbiology sbl1023Document9 pagesLab 5 Microbiology sbl1023api-385038701No ratings yet
- Acid Fast Stain: PROCEDURE (Ziehl-Neelsen Method)Document3 pagesAcid Fast Stain: PROCEDURE (Ziehl-Neelsen Method)62991No ratings yet
- SBL 1023 Lab 9 Exp Gram Staining AsepticDocument10 pagesSBL 1023 Lab 9 Exp Gram Staining Asepticapi-384057570No ratings yet
- GLENDADocument3 pagesGLENDAKojo PappoeNo ratings yet
- Gram Positive and Gram Negative Lab Practice - Victor H.Document3 pagesGram Positive and Gram Negative Lab Practice - Victor H.Victor HernandezNo ratings yet
- Gram Stain Procedure ExplainedDocument6 pagesGram Stain Procedure ExplainedJFcp VazquezNo ratings yet
- SBL 1023 Exp 5Document9 pagesSBL 1023 Exp 5api-383623349No ratings yet
- Gram Staining of Genital DischargeDocument11 pagesGram Staining of Genital DischargeDenden AllatifNo ratings yet
- Bacterial Colonial MorphologyDocument5 pagesBacterial Colonial MorphologyMegasonNo ratings yet
- Gram StainDocument26 pagesGram StainFahad FarrukhNo ratings yet
- Simple, Differential Staining and MotilityDocument8 pagesSimple, Differential Staining and MotilitySai SridharNo ratings yet
- Miscellaneous Protozoa1Document5 pagesMiscellaneous Protozoa1Haki TozakiNo ratings yet
- MIKAL (Salmonella) 1Document13 pagesMIKAL (Salmonella) 1Kausalyaa Gajapathi RaoNo ratings yet
- JASMIN, Kisha Jane P.Document2 pagesJASMIN, Kisha Jane P.Kisha Jane JasminNo ratings yet
- Lab 5Document8 pagesLab 5api-383615212100% (1)
- Experiment 4 MicrobiologyDocument5 pagesExperiment 4 MicrobiologyFrancis TagnongNo ratings yet
- Microbiology ExperimentDocument9 pagesMicrobiology Experiment门门No ratings yet
- 368 FullDocument8 pages368 FullWindy MentariiNo ratings yet
- Bio LabbDocument3 pagesBio LabbAnyss Hasni Al-banjariNo ratings yet
- Microbiology Lab Test 2 Review: Acid Fast Stain, KOH, India Ink, LPCB, Gram StainDocument5 pagesMicrobiology Lab Test 2 Review: Acid Fast Stain, KOH, India Ink, LPCB, Gram StainRoyNo ratings yet
- Sop Gram StainDocument6 pagesSop Gram Staindavid mchembeNo ratings yet
- BIOL 240 Lab Report 1Document11 pagesBIOL 240 Lab Report 1Ben CharlesNo ratings yet
- Acid Fast StainingDocument17 pagesAcid Fast StaininglemuelNo ratings yet
- Spherical Rod: 2 Lab Microbiology 3 Grade Dentistry MSC. Elaf Mohammed The Morphology and Fine Structure of BacteriaDocument3 pagesSpherical Rod: 2 Lab Microbiology 3 Grade Dentistry MSC. Elaf Mohammed The Morphology and Fine Structure of BacteriaMohammed Yousif AbdualjabbarNo ratings yet
- RELIABLE DIAGNOSIS OF PARASITIC INFECTIONDocument31 pagesRELIABLE DIAGNOSIS OF PARASITIC INFECTIONNida RidzuanNo ratings yet
- Gram's Stain: The Key To MicrobiologyDocument7 pagesGram's Stain: The Key To Microbiologyalvaro tito loaizaNo ratings yet
- BC6055 - BM7122 Practical Booklet 2021-22Document10 pagesBC6055 - BM7122 Practical Booklet 2021-22MasoomaIjazNo ratings yet
- Cell Staining: The Simple StainDocument4 pagesCell Staining: The Simple StainAitlas KhanNo ratings yet
- TCBSDocument13 pagesTCBSMohammad Aklis AzmiNo ratings yet
- Methods and Materials For Aqua Magt LabDocument3 pagesMethods and Materials For Aqua Magt LabJon Irish AquinoNo ratings yet
- Lab Report On Endospore Staining: Escherichia Coli Mwajuma Katembo Texas A&M University-CommerceDocument5 pagesLab Report On Endospore Staining: Escherichia Coli Mwajuma Katembo Texas A&M University-CommerceMuwa KatemboNo ratings yet
- (2012) Performing Vaginal Lavage, Crystal Violet Staining, and Vaginal Cytological Evaluation - McLeanDocument6 pages(2012) Performing Vaginal Lavage, Crystal Violet Staining, and Vaginal Cytological Evaluation - McLeanleidyannegoncalvesNo ratings yet
- Antibiotic Senstivity of Various Pathogenic Bacteria in Case of Uti and Throat InfectionsDocument25 pagesAntibiotic Senstivity of Various Pathogenic Bacteria in Case of Uti and Throat InfectionsMishel AroraNo ratings yet
- The Science of MicrobiologyDocument30 pagesThe Science of MicrobiologyKristel AnneNo ratings yet
- Lab 02 - Preparation of Smears and Gram StainingDocument10 pagesLab 02 - Preparation of Smears and Gram StainingVincent ReyesNo ratings yet
- A. Laboratory Test For GO (Method, Principle, Equipment, and Procedure)Document4 pagesA. Laboratory Test For GO (Method, Principle, Equipment, and Procedure)AprilNo ratings yet
- Microbio Prac Write Up 2Document7 pagesMicrobio Prac Write Up 2TADIWANASHE TINONETSANANo ratings yet
- Acute Kidney InjuryDocument26 pagesAcute Kidney InjuryMichelle ErikaNo ratings yet
- Procedures in The Event of Bomb ThreatDocument2 pagesProcedures in The Event of Bomb ThreatMichelle ErikaNo ratings yet
- Fluids Lecture Notes Part IDocument22 pagesFluids Lecture Notes Part IMichelle ErikaNo ratings yet
- Acute Dehydration Nursing Care PlanDocument2 pagesAcute Dehydration Nursing Care PlanMichelle ErikaNo ratings yet
- 12 AthrosDocument3 pages12 AthrosMichelle ErikaNo ratings yet
- NCA DelivDocument2 pagesNCA DelivMichelle ErikaNo ratings yet
- Fluids 5Document2 pagesFluids 5Michelle ErikaNo ratings yet
- Ateneo de Zamboanga University: College of Nursing Nursing Skills OutputDocument3 pagesAteneo de Zamboanga University: College of Nursing Nursing Skills OutputMichelle ErikaNo ratings yet
- rHEUMATOID ARTHRITISDocument3 pagesrHEUMATOID ARTHRITISMichelle ErikaNo ratings yet
- 2 Gram StainingDocument3 pages2 Gram StainingMichelle ErikaNo ratings yet
- MEJIA, Michelle Erika F. BSN Iii-B NCM103 (RESPIDocument1 pageMEJIA, Michelle Erika F. BSN Iii-B NCM103 (RESPIMichelle ErikaNo ratings yet
- MEJIA, Michelle Erika F. BSN Iii-B NCM103 (RESPIDocument1 pageMEJIA, Michelle Erika F. BSN Iii-B NCM103 (RESPIMichelle ErikaNo ratings yet
- Drugs Affecting Cardiac and Renal Systems LecDocument10 pagesDrugs Affecting Cardiac and Renal Systems LecMichelle ErikaNo ratings yet
- Drugs To Treat Endocrine ProblemsDocument26 pagesDrugs To Treat Endocrine ProblemsMichelle ErikaNo ratings yet
- Pathophysiology Concepts of Altered Health StatesDocument59 pagesPathophysiology Concepts of Altered Health StatesMichelle ErikaNo ratings yet
- Parenting StylesDocument21 pagesParenting StylesMichelle ErikaNo ratings yet
- Small Intestine BiopsyDocument3 pagesSmall Intestine BiopsyMichelle ErikaNo ratings yet
- MEJIA, Michelle Erika F. BSN Iii-B NCM103 (RESPIDocument1 pageMEJIA, Michelle Erika F. BSN Iii-B NCM103 (RESPIMichelle ErikaNo ratings yet
- I. Description: Ateneo de Zamboanga UniversityDocument3 pagesI. Description: Ateneo de Zamboanga UniversityMichelle ErikaNo ratings yet
- I. Description: Ateneo de Zamboanga UniversityDocument3 pagesI. Description: Ateneo de Zamboanga UniversityMichelle ErikaNo ratings yet
- Nursing Care ManagementDocument51 pagesNursing Care ManagementMichelle ErikaNo ratings yet
- Computer Components GuideDocument20 pagesComputer Components GuideMichelle ErikaNo ratings yet
- Fluids 4Document4 pagesFluids 4Michelle ErikaNo ratings yet
- Greek Tragedy by Rowil SantinloDocument43 pagesGreek Tragedy by Rowil SantinloMichelle ErikaNo ratings yet
- Presentation 1Document1 pagePresentation 1Michelle ErikaNo ratings yet