Professional Documents
Culture Documents
Instantaneous Frac. Yield Overall Fractional Yield: Max. Mix. Model
Uploaded by
Elena TodorovskaOriginal Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
Instantaneous Frac. Yield Overall Fractional Yield: Max. Mix. Model
Uploaded by
Elena TodorovskaCopyright:
Available Formats
=
0
= 1
0
=
0
0
=
0
=1
=0
=0
0
=
1
1 +
= =
1
=
0
0
=
0
=
0
/
ln(
1
) =
(
1
1
1
2
))
=
1 +
=
2
4
2
Reactors in Series and Parallel
PFR CSTR
Series Parallel Series, Equal Size Unequal Size
Treat as single PFR with
V=VTOTAL
Treat as single PFR with V = VTOTAL
if V/F = constant line
[(
)
1/
1]
=
1
0
=
1
()
Recycle: R = ratio of fluid returned to reactor, to volume of fluid leaving the system
1
= (
+ 1
)
0
=
0
= ( +1)
(
+1
)
| =
0
0
= ( + 1)
0
+
+1
|
=0
First Order, CD
+ 1
= ln[
0
+
( + 1)
] Second Order 2A->R, CD
+ 1
=
0
(
0
(
0
+
)
Reactions in Series: irreversible A -> R -> S [CA + CR + CS = CA0]
PFR CSTR
0
= (
2
)
2
,
=
ln (
2
/
1
)
2
1
0
=
1
[(
2
/
1
)
1/2
+ 1]
2
,
=
1
0
=
1
1 +
1
|
1
2
1 +
2
Instantaneous Frac. Yield Overall fractional yield Reactions in Parallel: A -> R; A -> S [R is desired product]
=
CSTR
=
=
1
= (
0
)
Residence Time Distribution F(t) = Integral of E(t) from 0 to t Segregation Model
() =
()
()
= ()
0
2
= (
)
2
()
0
3
=
1
3/2
(
)
3
()
()()
0
Normalised RTD Ideal CSTR Ideal PFR Tracer Balance LamFlow Rea. n Tanks in Series
=
()
= ()
() =
1
/
() = ( ) () =
0
/
() =
2
2
3
if
2
() =
1
( 1)!
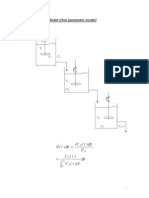
Max. Mix.
Model
= life
expectancy
0
+
()
1 ()
As INF, X=0 or CA=CA0; Integrate numerically, starting at large and
ending with final conversion at =0
T-X
Charts:
Desired conversion must intersect locus of a rate curve actual rate found by accounting for initial concentration.
Slope of line linking this point to optimum temperature on x-axis is given by heat capacity divided by heat of reaction.
For a given
conversion:
)
T is the temperature at locus from above, and Tio is the temperature
reactant must be cooled to, to maintain optimum temperature.
To determine the volume of a PFR,
numerical integration is required:
Use conversions that correspond to each locus, and the associated corrected
rate, up to the maximum temperature; and a best guess rate at zero conversion.
aaaasdasd
Optimum Recycle Ratio
Best multiple reactor
scheme, when no
recycling occurs
Best reactor scheme,
when recycling occurs
Rate Equations obtained using the Integral Method of Analysis for a Constant Volume Batch Reactor
+(
+(
)
n
th
Order -r = kC
n
|
1
0
1
= ( 1)|
1
Zero-Order
<
0
=
0
=
0
= 0
Homogeneous Catalysed
A -> R
A + C -> R + C
ln (
0
) = ln(1
) = (
1
+
2
) =
Autocatalytic Reactions A + R -> R + R ln (
+
(1
)
) = ln (
0
(
0
(
0
0
)
) =
0
( +1) = (
0
+
0
)
Irreversible Parallel
Reactions
A -> R
A -> S
ln(
0
) = (
1
+
2
)
0
=
1
2
First Order Reversible A <-> R ln(1
) = ln (
) =
+ 1
+
1
Second Order Reversible
A + B <-> R + S
2A <-> R + S
2A <-> 2R
|ln (
(2
1)
) = 2
1
(
1
1)
0
|
0
=
0
||
0
=
0
=0
Steady State Conservation of Energy. Second CP term is CPROD - CREACTANT
)] = 0
=
0
CSTR
w/exchanger:
=
(
1
2
)
ln[(
1
)/(
2
)]
{(
1
) [1
]}
PFR: = (
Integrals
=
1
+1
+1
+
=
1
ln ( +)
( +)
2
=
1
+
( +)
=
( +)
+1
(( +1) )
( +1)( +2)
( + )
=
( +)
+1
+1
=
1
= (
2
)
( +)
2
=
+
+ln ( +)
ln() = ()
ln()
=
1
2
(ln())
2
()( ) = ()
2
+
2
=
1
2
ln (
2
+
2
)
Laplace Transforms
()
()
1
1
+1
2
2/3
()
1
!
( )
+1
( )
()
( )
()
1
()
1
2
1 3(2 1)
+1/2
()
0
()
()
()
()
() (0)
()
2
() (0) (0)
!
1
+1
1
( )( )
( )( )
!
1
( )
+1
Half-Life: CA = CA0/2 Integration by Parts Differential Equation
1/2
=
2
1
1
( 1)
0
1
= []
+ =
+
Variable-Volume Batch Reactor
Zero Order
ln(
0
) =
0
ln (1 +
) =
First Order ln(1
) = ln(1
0
) =
Second Order
(1 +
ln(1
) =
0
Performance Equations for n
th
-order kinetics, for constant density: A = 0
PFR or (Batch at CD) CSTR (Mixed Flow)
General Case
=
0
= |
0
|
=0
=
0
= |
=0
n = 0; -r = k
0
=
0
=
n = 1; -r = kC = (1 +
) ln(1
(1 +
)
1
= |
=0
n = 2; -r = kC
2
0
= 2
(1 +
) ln(1
) +
+(
+ 1)
2
0
=
(1 +
)
2
(1
)
2
n = n; -r = kC
n
|( 1)
0
1
= (
0
)
1
1 = (1
)
1
1|
=0
0
1
=
(1 +
(1
n = 1, rev.; CR0 =
0
A<->rR
1
=
+
+
[(1 +
) ln(1
(1 +
You might also like
- Time Response AnalysisDocument151 pagesTime Response AnalysisTushar GuptaNo ratings yet
- Transient Response Analysis: Test Signals: Impulse Step Ramp Sin And/or CosDocument38 pagesTransient Response Analysis: Test Signals: Impulse Step Ramp Sin And/or Cosomar9aNo ratings yet
- Math Formula ListDocument3 pagesMath Formula ListKumaravel PadmaroopaNo ratings yet
- DT DP RT R: Constant-Volume Batch ReactorDocument20 pagesDT DP RT R: Constant-Volume Batch Reactorxx_aleksa_hrvatska_xxNo ratings yet
- Chapter 2Document10 pagesChapter 2floriscalcNo ratings yet
- Foca 3Document11 pagesFoca 3jsvarnikachhaviNo ratings yet
- Lecture 8Document21 pagesLecture 8sreeNo ratings yet
- Ch-5 Time Res WebpageDocument48 pagesCh-5 Time Res WebpageTushar GuptaNo ratings yet
- Chapter 3 - MatlabDocument59 pagesChapter 3 - MatlabZe SaNo ratings yet
- Computational Fluid Dynamics IntroductionDocument18 pagesComputational Fluid Dynamics IntroductionthuielNo ratings yet
- EL-4701 Modelos de Sistemas: FormularioDocument9 pagesEL-4701 Modelos de Sistemas: FormularioEmmanuel AcostaNo ratings yet
- CRE 1-3 Unit (2016-2017) PDFDocument56 pagesCRE 1-3 Unit (2016-2017) PDFgouthamNo ratings yet
- Compressible Flow PDFDocument90 pagesCompressible Flow PDFOmer TokhNo ratings yet
- Chapter 2.2 Response Ist Order SystemsDocument30 pagesChapter 2.2 Response Ist Order SystemsSyed AliNo ratings yet
- Peretmuan 12 Laplace in CircuitsDocument56 pagesPeretmuan 12 Laplace in CircuitsSando CrisiasaNo ratings yet
- Tie Line Bias ContriolDocument19 pagesTie Line Bias ContriolKornepati SureshNo ratings yet
- Hacettepe University: Nem 473-Assignment I Nuclear Fuel & Materials Due To:19.12.2011 BAŞAK FALAY (20726895)Document9 pagesHacettepe University: Nem 473-Assignment I Nuclear Fuel & Materials Due To:19.12.2011 BAŞAK FALAY (20726895)Başak FalayNo ratings yet
- Gevorderde SysteemidentificatieDocument243 pagesGevorderde SysteemidentificatiefonsverbiestNo ratings yet
- Math Stats Booklet 1Document20 pagesMath Stats Booklet 1Koh Boon HaoNo ratings yet
- UO2016F Slide 1 - Basic Relations and Equations of Heat ConductionDocument20 pagesUO2016F Slide 1 - Basic Relations and Equations of Heat ConductionSushil KumarNo ratings yet
- Experiment ReportDocument11 pagesExperiment ReportSouravmeenaNo ratings yet
- 15.1 (A) For Example:: Problem SolutionsDocument46 pages15.1 (A) For Example:: Problem SolutionsLuis AntonioNo ratings yet
- Ch. 1: Review of ProbabilityDocument19 pagesCh. 1: Review of ProbabilityKyusang ParkNo ratings yet
- Formula Overview (Aeronautics)Document8 pagesFormula Overview (Aeronautics)Aishwarya RaviNo ratings yet
- AssDocument7 pagesAssMichael Busayo OlajideNo ratings yet
- Kinetics & Reactor Design IDocument75 pagesKinetics & Reactor Design Ianon_864813890No ratings yet
- Https Courseworks - Columbia.edu Access Content Group PHYSC1401 001 2013 3 Final Exam Physics 1401 Formula Sheet FinalDocument3 pagesHttps Courseworks - Columbia.edu Access Content Group PHYSC1401 001 2013 3 Final Exam Physics 1401 Formula Sheet FinalSahir JaggiNo ratings yet
- Aproximacion de Born-OppenheimerDocument9 pagesAproximacion de Born-OppenheimerDavid ReyesNo ratings yet
- Assignment 8Document17 pagesAssignment 8Shawn DeolNo ratings yet
- Taller Ingenieria de Las Reacciones - Determinacion Del Orden de ReaccionDocument15 pagesTaller Ingenieria de Las Reacciones - Determinacion Del Orden de ReaccionJesus JulioNo ratings yet
- Table ComplexDocument5 pagesTable ComplexRavinder RangaNo ratings yet
- Fourier, Laplace, and Z TransformDocument10 pagesFourier, Laplace, and Z TransformHandi RizkinugrahaNo ratings yet
- Control Systems Formula SheetDocument12 pagesControl Systems Formula SheetliamhrNo ratings yet
- SOV ConductionDocument47 pagesSOV ConductionRanadip AcharyaNo ratings yet
- C3 Forced Vibration BGDocument11 pagesC3 Forced Vibration BGLâm KhanhNo ratings yet
- Stationary Time SeriesDocument21 pagesStationary Time SeriesJay AydinNo ratings yet
- EL-4701 Modelos de Sistemas: FormularioDocument9 pagesEL-4701 Modelos de Sistemas: FormularioAngel RamirezNo ratings yet
- Summary of EquationsDocument6 pagesSummary of EquationsMasood Ahmed KhanNo ratings yet
- Modeling: ∞ −st 1 s 1 s n n! s −at 1 s+a ω s +ω s s +ωDocument4 pagesModeling: ∞ −st 1 s 1 s n n! s −at 1 s+a ω s +ω s s +ωjameelahmadNo ratings yet
- Chapter 2-Mass Reactor Model (102 P)Document102 pagesChapter 2-Mass Reactor Model (102 P)shardulkaviNo ratings yet
- T Ax T By: Properties of Continous Time Fourier Series Fourier Transform Properties Properties of Laplace TransformDocument4 pagesT Ax T By: Properties of Continous Time Fourier Series Fourier Transform Properties Properties of Laplace TransformRavinder RangaNo ratings yet
- Mechanics of Solids Week 7 LecturesDocument15 pagesMechanics of Solids Week 7 LecturesFlynn GouldNo ratings yet
- 01b-Fourier Series RevisionDocument37 pages01b-Fourier Series RevisionGeorges YoussefNo ratings yet
- CHE3161 - Semester1 - 2011 - SolutionsDocument12 pagesCHE3161 - Semester1 - 2011 - Solutionsvenkiee50% (2)
- Eq SheetDocument1 pageEq Sheetuama87No ratings yet
- W Z Is The Adaptive: CSP401 Test #10Document3 pagesW Z Is The Adaptive: CSP401 Test #10Jason GonzalezNo ratings yet
- Homework Set 3: Chemical Reaction Engineering, CHE 625: Problem 1,2Document5 pagesHomework Set 3: Chemical Reaction Engineering, CHE 625: Problem 1,2Bankole 'Layeni' SamsondeenNo ratings yet
- Irreversible First-Order Reaction in SeriesDocument8 pagesIrreversible First-Order Reaction in SeriesElena TodorovskaNo ratings yet
- Ten-Decimal Tables of the Logarithms of Complex Numbers and for the Transformation from Cartesian to Polar Coordinates: Volume 33 in Mathematical Tables SeriesFrom EverandTen-Decimal Tables of the Logarithms of Complex Numbers and for the Transformation from Cartesian to Polar Coordinates: Volume 33 in Mathematical Tables SeriesNo ratings yet
- Answers to Selected Problems in Multivariable Calculus with Linear Algebra and SeriesFrom EverandAnswers to Selected Problems in Multivariable Calculus with Linear Algebra and SeriesRating: 1.5 out of 5 stars1.5/5 (2)
- Fundamentals of Electronics 3: Discrete-time Signals and Systems, and Quantized Level SystemsFrom EverandFundamentals of Electronics 3: Discrete-time Signals and Systems, and Quantized Level SystemsNo ratings yet
- Logical progression of twelve double binary tables of physical-mathematical elements correlated with scientific-philosophical as well as metaphysical key concepts evidencing the dually four-dimensional basic structure of the universeFrom EverandLogical progression of twelve double binary tables of physical-mathematical elements correlated with scientific-philosophical as well as metaphysical key concepts evidencing the dually four-dimensional basic structure of the universeNo ratings yet
- Application of Derivatives Tangents and Normals (Calculus) Mathematics E-Book For Public ExamsFrom EverandApplication of Derivatives Tangents and Normals (Calculus) Mathematics E-Book For Public ExamsRating: 5 out of 5 stars5/5 (1)
- CHPR4405 Sample Exam2Document7 pagesCHPR4405 Sample Exam2Elena TodorovskaNo ratings yet
- CHPR4405 Sample ExamDocument26 pagesCHPR4405 Sample ExamElena TodorovskaNo ratings yet
- CHPR5501 Adv. Reaction Eng. Part 1Document40 pagesCHPR5501 Adv. Reaction Eng. Part 1Elena TodorovskaNo ratings yet
- GENG5507 StatisticalTablesDocument6 pagesGENG5507 StatisticalTablesElena TodorovskaNo ratings yet
- CHPR5501 Adv. Reaction Eng. Part 2Document26 pagesCHPR5501 Adv. Reaction Eng. Part 2Elena TodorovskaNo ratings yet
- A Collection of Formulas For GENG5507 (Version 9 5 14) : AcronymsDocument12 pagesA Collection of Formulas For GENG5507 (Version 9 5 14) : AcronymsElena TodorovskaNo ratings yet
- CHPR4406 LectureNDocument15 pagesCHPR4406 LectureNElena TodorovskaNo ratings yet
- CHPR4406 LectureRDocument9 pagesCHPR4406 LectureRElena TodorovskaNo ratings yet
- Different Types of CulturesDocument2 pagesDifferent Types of CulturesElena TodorovskaNo ratings yet
- CHPR4406 Tutorial01 SolutionsDocument14 pagesCHPR4406 Tutorial01 SolutionsElena TodorovskaNo ratings yet
- CHPR4406 Tutorial02Document3 pagesCHPR4406 Tutorial02Elena TodorovskaNo ratings yet
- CHPR4406 Tutorial02 SolutionsDocument16 pagesCHPR4406 Tutorial02 SolutionsElena TodorovskaNo ratings yet
- General Graphical Design Procedure For Single Reaction: (Chapter 9, Levenspiel)Document19 pagesGeneral Graphical Design Procedure For Single Reaction: (Chapter 9, Levenspiel)Elena TodorovskaNo ratings yet
- CHPR4406 Tutorial01Document2 pagesCHPR4406 Tutorial01Elena TodorovskaNo ratings yet
- CHPR4406 LectureTDocument9 pagesCHPR4406 LectureTElena TodorovskaNo ratings yet
- CHPR4406 LectureSDocument17 pagesCHPR4406 LectureSElena TodorovskaNo ratings yet
- CHPR4406 Reactions Lecture 1Document16 pagesCHPR4406 Reactions Lecture 1xx_aleksa_hrvatska_xxNo ratings yet
- CHPR4406 Notes HBrMechanismDocument4 pagesCHPR4406 Notes HBrMechanismElena TodorovskaNo ratings yet
- Irreversible First-Order Reaction in SeriesDocument8 pagesIrreversible First-Order Reaction in SeriesElena TodorovskaNo ratings yet
- CHPR4406 Notes PreventRunawayReactionsDocument6 pagesCHPR4406 Notes PreventRunawayReactionsElena TodorovskaNo ratings yet
- Steady State Plug Flow Reactor: On Accumulati RXN by Nce Disappeara Output InputDocument10 pagesSteady State Plug Flow Reactor: On Accumulati RXN by Nce Disappeara Output InputElena TodorovskaNo ratings yet
- N K V N K C K C K R: Iii) First Order Reversible ReactionDocument5 pagesN K V N K C K C K R: Iii) First Order Reversible ReactionElena TodorovskaNo ratings yet
- Sample ResumeDocument2 pagesSample ResumeElena TodorovskaNo ratings yet
- ESA Essential Facts 2013Document16 pagesESA Essential Facts 2013witcher2No ratings yet
- R R R A: Autocatalytic ReactionDocument6 pagesR R R A: Autocatalytic ReactionElena TodorovskaNo ratings yet
- CHPR4406 AssignmentDocument2 pagesCHPR4406 AssignmentElena TodorovskaNo ratings yet
- CHPR4406 AssignmentDocument2 pagesCHPR4406 AssignmentElena TodorovskaNo ratings yet
- Concise Selina Solutions Class 9 Maths Chapter 15 Construction of PolygonsDocument31 pagesConcise Selina Solutions Class 9 Maths Chapter 15 Construction of Polygonsbhaskar51178No ratings yet
- Abas Drug Study Nicu PDFDocument4 pagesAbas Drug Study Nicu PDFAlexander Miguel M. AbasNo ratings yet
- DG Oil SpecificationDocument10 pagesDG Oil SpecificationafsalmohmdNo ratings yet
- Febrile Neutropenia GuidelineDocument8 pagesFebrile Neutropenia GuidelineAslesa Wangpathi PagehgiriNo ratings yet
- Bichelle HarrisonDocument2 pagesBichelle HarrisonShahbaz KhanNo ratings yet
- Tenses English Grammar PresentationDocument14 pagesTenses English Grammar PresentationMaz Gedi60% (5)
- Chapter 4: Thermal ComfortDocument16 pagesChapter 4: Thermal ComfortWengelNo ratings yet
- Thermal Physics Lecture 1Document53 pagesThermal Physics Lecture 1Swee Boon OngNo ratings yet
- ACC030 Comprehensive Project April2018 (Q)Document5 pagesACC030 Comprehensive Project April2018 (Q)Fatin AkmalNo ratings yet
- VRARAIDocument12 pagesVRARAIraquel mallannnaoNo ratings yet
- Study Notes - Google Project Management Professional CertificateDocument4 pagesStudy Notes - Google Project Management Professional CertificateSWAPNIL100% (1)
- SL Generator Ultrasunete RincoDocument2 pagesSL Generator Ultrasunete RincoDariaNo ratings yet
- P01 - PT in Building & Its AdvantagesDocument11 pagesP01 - PT in Building & Its AdvantagesPartha Pratim RoyNo ratings yet
- Unit-Ii Syllabus: Basic Elements in Solid Waste ManagementDocument14 pagesUnit-Ii Syllabus: Basic Elements in Solid Waste ManagementChaitanya KadambalaNo ratings yet
- Tax Havens IMF PDFDocument59 pagesTax Havens IMF PDFClassic PhyXNo ratings yet
- Landscape ArchitectureDocument9 pagesLandscape Architecturelisan2053No ratings yet
- Localization On ECG: Myocardial Ischemia / Injury / InfarctionDocument56 pagesLocalization On ECG: Myocardial Ischemia / Injury / InfarctionduratulfahliaNo ratings yet
- Revenue Memorandum Circular No. 55-2016: For ExampleDocument2 pagesRevenue Memorandum Circular No. 55-2016: For ExampleFedsNo ratings yet
- Operating Instructions: Rotary Lobe PumpDocument77 pagesOperating Instructions: Rotary Lobe PumpRuslan SlusarNo ratings yet
- Painters Rates PDFDocument86 pagesPainters Rates PDFmanthoexNo ratings yet
- Pyromet Examples Self StudyDocument2 pagesPyromet Examples Self StudyTessa BeeNo ratings yet
- DHA - Jebel Ali Emergency Centre + RevisedDocument5 pagesDHA - Jebel Ali Emergency Centre + RevisedJam EsNo ratings yet
- Rapp 2011 TEREOS GBDocument58 pagesRapp 2011 TEREOS GBNeda PazaninNo ratings yet
- Ubicomp PracticalDocument27 pagesUbicomp Practicalvikrant sharmaNo ratings yet
- Tec066 6700 PDFDocument2 pagesTec066 6700 PDFExclusivo VIPNo ratings yet
- (Problem Books in Mathematics) Antonio Caminha Muniz Neto - An Excursion Through Elementary Mathematics, Volume III - Discrete Mathematics and Polynomial Algebra (2018, Springer)Document647 pages(Problem Books in Mathematics) Antonio Caminha Muniz Neto - An Excursion Through Elementary Mathematics, Volume III - Discrete Mathematics and Polynomial Algebra (2018, Springer)Anonymous iH6noeaX7100% (2)
- Less Homework More TroubleDocument7 pagesLess Homework More Troubleg697a0mw100% (1)
- Properties of WaterDocument23 pagesProperties of WaterNiken Rumani100% (1)
- 6int 2008 Dec ADocument6 pages6int 2008 Dec ACharles_Leong_3417No ratings yet
- Assessing Apical PulseDocument5 pagesAssessing Apical PulseMatthew Ryan100% (1)



















































![Mathematical Tables: Tables of in G [z] for Complex Argument](https://imgv2-1-f.scribdassets.com/img/word_document/282615796/149x198/febb728e8d/1714993295?v=1)