Professional Documents
Culture Documents
The Combination Progressive-Spinning Method (Fig. 3), As The Name Implies, Combines The Progressive and
Uploaded by
Ahmad Falah TfsOriginal Description:
Original Title
Copyright
Available Formats
Share this document
Did you find this document useful?
Is this content inappropriate?
Report this DocumentCopyright:
Available Formats
The Combination Progressive-Spinning Method (Fig. 3), As The Name Implies, Combines The Progressive and
Uploaded by
Ahmad Falah TfsCopyright:
Available Formats
In one technique, a rotating flame head is used for internal spin hardening of odd-shape parts that would
present handling problems if the parts themselves were rotated. Each part is positioned by a simple handling
device, and the flame head rotates inside the part.
In contrast to the progressive method, in which acetylene is usually used (because of its high flame
temperature and rapid heating rates), satisfactory results can be obtained in spin hardening with natural
gas, propane, or manufactured gas. The choice of gas depends on the shape, size, and composition of the
workpiece and on the depth of case required, as well as on the relative cost and availability of each gas.
A wide choice of quenchants also is possible in the spin hardening method. Because the flame is extinguished
or withdrawn before the part is quenched, any appropriate quenchant may be used for immersion quenching.
In spray quenching, the quenchant is usually water, a water-based liquid such as soluble oil, or a simulated oil
in the form of a polymer-base quenchant; air also has been used.
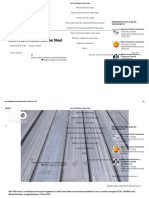
The combination progressive-spinning method (Fig. 3), as the name implies, combines the progressive and
spinning methods for hardening long parts such as shafts and rolls. The workpiece is rotated as in the
spinning method; but, in addition, the heating heads traverse the roll or shaft from one end to the other.
Only a narrow circumferential band is heated progressively as the flame head moves from one end of the
work to the other. The quench follows immediately behind the heating head, either as an integral part of the
head or as a separate quench ring.
Fig. 3 Combination progressive-spinning flame hardening
This method provides a means of hardening large surface areas with relatively low gas flows. Progressive-
spinning units designed to handle a broad range of diameters and lengths are available commercially.
Fuel Gases
Several different fuel gases are used in flame hardening. In selecting a fuel gas for a given application, the
required rate of heating and the cost of the gas must be considered, along with the initial cost of equipment
and maintenance costs.
Flame hardening does not alter the composition of the base metal if done properly. Carburizing, neutral, and
oxidizing flames can be used. Oxidizing flames have high oxygen ratios and can be detrimental because they
produce extremely hot temperatures that can cause decarburization and overheating. A carburizing flame
can prevent some decarburization but can also introduce unwanted carbon into the surface. For best results,
neutral or slightly carburizing flames should be used (Ref 1).
A comparison of the heating rates of fuel gases can be made when certain fundamental properties of usable
mixtures with oxygen are known. A parameter that correlates well with actual heating speed is combustion
intensity, or specific flame output. This is the product of the normal velocity of burning multiplied by the net
heating value of the mixture of oxygen and fuel gas. A knowledge of these two properties often permits the
selection of the most suitable fuel gas for a specific hardening speed and depth of case. The fuels of
greatest commercial interest are ranked by combustion intensity (at metallurgically suitable ratios of
mixture with oxygen) in the following order: acetylene, MAPP (methylacetylene propadiene), propane,
methane. Values of normal burning velocity and the heating values of metallurgically suitable mixtures are
listed in Table 1.
Table 1 Fuel gases used for flame hardening
(a) Product of normal velocity of burning multiplied by heating value of oxy-fuel gas mixture.
(b) Varies with heating value and composition
The time required for heat penetration is another good criterion for judging the heating qualities of a fuel
provided that all other variables remain constant. Figure 4 shows comparative heating times for stabilized
MAPP, acetylene, and propane, using an efficient coupling distance for each fuel. These curves show that a
greater depth of hardness can be obtained with MAPP in a shorter length of time (Ref 2).
Gas
Heating Valve
Flame Temperatur
Usual
ratio of
oxygen
to fuel
gas
Heating
Normal
velocity of
burning
Combustion
intensity
(a)
Usual
ratio
of air
to fuel
gas
with Air with Oksigen
MJ/m
3
Btu/ft
3
o
C
o
F
o
C
o
F MJ/m
3
Btu/ft
3
mm/s in/s
mm/s x
MJ/m
3
in./s
Btu/ft
3
Acetylene 53.4 1433 3105 5620 2325 4125 1.0 26.7 716 535 21 14,284 15,036 12
City gas
11.2-
33.5
300-
900
2540 4600 1985 3605
(b) (b) (b) (b) (b) (b) (b) (b)
Natural gas 37.3 1000 2705 4900 1875 3405 1.75 13.6 364 280 11 3,808 4,004 9.0
Propane 93.9 2520 2635 4775 1925 3495 4.0 18.8 504 305 12 5,734 6,048 25.0
MAPP 90 2406 2977 5301 1760 3200 3.5 20.0 535 381 15 7,62 8,025 22
Fig. 4 Comparison of heating times for MAPP, acetylene, and propane. Flame velocity, 170 m/s (550 ft/s);
port size, No. 69 drill (0.74 mm, or 0.0292 in.); coupling distance, 9.5 mm ( 3/8 in.); material, 1036 steel.
Oxygento-fuel ratios: MAPP, 5.0; acetylene, 1.33; propane, 4.5
The ratio of oxygen to fuel is very important in obtaining maximum heating efficiency from the fuel.
However, oxygento-fuel ratios should not be confused with oxygen and fuel consumption rates, which vary
with flame velocity, port size, and heating time. Stoichiometrically, acetylene requires 2 mols of oxygen
per mol of gas for complete combustion, MAPP requires 4 mols, and propane requires 5 mols. With acetylene,
however, a range of only 1 to 1.5 volumes of oxygen is provided directly, with the remainder being drawn
from the surrounding atmosphere. Neither MAPP nor propane has sufficient heat for flame hardening unless
more oxygen is supplied, normally at a rate of four parts of oxygen per part of fuel. MAPP burns over a
rather wide range of oxygen-to-fuel ratios, however, and thus permits a wider range of heat output while
still providing high heat generation when necessary. The use of MAPP increases oxygen consumption, and fuel
is more expensive than oxygen.
Bulk systems of supply for oxygen and fuel gases greatly reduce their cost, but of greater importance is the
elimination of cylinder handling and of the residual losses usually attendant upon the use of gases in
cylinders. Bulk systems also provide a more nearly constant supply of gas at uniform pressure. A
disadvantage of acetylene is that it cannot be stored in bulk, thereby requiring manifolds of cylinders.
Depth of Heating. Shallow hardness patterns (less than 3.2 mm, or 0.125 in., deep) can be attained only with
oxy-gas fuels. The high-temperature flames obtained with oxy-gas fuels provide the fast heat transfer
necessary for effective localization of the heat pattern. Deeper hardness patterns permit the use of either
oxy-gas fuels or air-gas fuels. Oxy-gas fuels will localize the heat, but care is required in their application to
avoid overheating the surface during the development of the deeper-seated heat. Air-gas fuels, with their
slower rates of heat transfer (lower flame temperatures), minimize or eliminate surface overheating but
generally extend the heat pattern beyond the desired hardness pattern. For this reason, air-gas flame
hardening is generally limited to steels of shallow hardenability. In this manner, the hardness pattern is
controlled by the quench rather than by the heating. The deeper-seated heat produced by air-gas flames
may preclude the use of air-gas mixtures because excessive distortion may occur. In consideration of these
factors, the use of air-gas heating will depend primarily on the shape of the part insofar as the configuration
favors heat localization and a lower rate of heat transfer.
You might also like
- Afrox Product CatalogueDocument92 pagesAfrox Product CataloguedhurushaNo ratings yet
- Mold and Die - PPTDocument110 pagesMold and Die - PPTThaloengsak Kucharoenpaisan100% (2)
- Gas Consumption, Time, and Speeds. Gas Consumption in Flame Hardening Varies With The Thickness of TheDocument4 pagesGas Consumption, Time, and Speeds. Gas Consumption in Flame Hardening Varies With The Thickness of TheAhmad Falah TfsNo ratings yet
- Fuel Savings For Slab Reheating Furnaces Through Oxyfuel CombustionDocument6 pagesFuel Savings For Slab Reheating Furnaces Through Oxyfuel CombustionДимитър СлавовNo ratings yet
- Brocal BordaDocument10 pagesBrocal BordaFarah Jane GiltendezNo ratings yet
- Oxyfuel Cutting - Process and Fuel GasesDocument6 pagesOxyfuel Cutting - Process and Fuel Gasesunknown8787No ratings yet
- Cutting Processes Application of Oxyfuel CuttingDocument5 pagesCutting Processes Application of Oxyfuel CuttingMehmet SoysalNo ratings yet
- Flame StabilityDocument11 pagesFlame StabilityMir Reza Negahban100% (1)
- AWS Abbreviations Oxyfuel Cutting - OFC Oxyacetylene Cutting - OFC-A Oxyfuel Cutting - Process and Fuel GasesDocument8 pagesAWS Abbreviations Oxyfuel Cutting - OFC Oxyacetylene Cutting - OFC-A Oxyfuel Cutting - Process and Fuel GasesahmedNo ratings yet
- E0512029038 PDFDocument10 pagesE0512029038 PDFHeber Farid Fabrica QuispeNo ratings yet
- Burners For Glass Melting Furnaces FinalDocument9 pagesBurners For Glass Melting Furnaces FinalDavid WattsNo ratings yet
- 1oxyacetylen Cutting and WeldingDocument67 pages1oxyacetylen Cutting and WeldingJayendra Kanani100% (1)
- Glass International 2010 - Air Liquide16742Document3 pagesGlass International 2010 - Air Liquide16742HardikNo ratings yet
- Solid Rocket MotorsDocument10 pagesSolid Rocket MotorsAnonymous Nwwc9Jn2RNo ratings yet
- Hong AnalysisDocument38 pagesHong AnalysisleovenuNo ratings yet
- Coal Drying - Paper 1Document12 pagesCoal Drying - Paper 1Arshad RangwalaNo ratings yet
- Gas-Phase and Catalytic Combustion in Heat-Recirculating BurnersDocument29 pagesGas-Phase and Catalytic Combustion in Heat-Recirculating BurnersAlex CoțNo ratings yet
- Specify Better Low NOx Burners For FurnacesDocument4 pagesSpecify Better Low NOx Burners For Furnacesyogitadoda100% (1)
- Research Work Week 10Document2 pagesResearch Work Week 10jonas lintagNo ratings yet
- An Important Challenge To Overcome For The Direct Use of Ammonia As A Fuel Is ItsDocument4 pagesAn Important Challenge To Overcome For The Direct Use of Ammonia As A Fuel Is ItsDANIEL MONISNo ratings yet
- Selection of FiredDocument5 pagesSelection of Firedahmed hossamNo ratings yet
- Combustion FuelDocument59 pagesCombustion FuelBabu Aravind100% (2)
- Design of Exhaust Gas Heat ExchangerDocument56 pagesDesign of Exhaust Gas Heat ExchangerAnkit saxena100% (11)
- Atomic Absorption and Atomic Fluorescence SpectrometryDocument54 pagesAtomic Absorption and Atomic Fluorescence SpectrometryMuhammad SaadNo ratings yet
- 1.2.1 - Different Types of Gasifiers and Their Integration With Gas TurbinesDocument11 pages1.2.1 - Different Types of Gasifiers and Their Integration With Gas TurbinesRene GonzalezNo ratings yet
- Ecp57vol7 008Document8 pagesEcp57vol7 008Samanway DasNo ratings yet
- Performance and Thermal Efficiency Parametric Design of An Energy Efficient Rotary FurnaceDocument12 pagesPerformance and Thermal Efficiency Parametric Design of An Energy Efficient Rotary FurnacealiNo ratings yet
- Selected Topics On Combustion ProcessesDocument27 pagesSelected Topics On Combustion ProcessesAbu Izzan Al BunyNo ratings yet
- Eit-M: Thermal Power PlantDocument13 pagesEit-M: Thermal Power PlanthayelomNo ratings yet
- IJEDR1703010Document8 pagesIJEDR1703010Belay AyalewNo ratings yet
- Combustion Kinetics of Coal Chars in Oxygen-Enriched EnvironmentsDocument20 pagesCombustion Kinetics of Coal Chars in Oxygen-Enriched EnvironmentsLukman HakimNo ratings yet
- Steam Generator PerformanceDocument7 pagesSteam Generator Performancervkumar61No ratings yet
- Xyfuel Combustion of Low Calorific Blast Furnace Gas For Steel Reheating FurnacesDocument9 pagesXyfuel Combustion of Low Calorific Blast Furnace Gas For Steel Reheating FurnacesGangadharKasinathSastryNo ratings yet
- Design and Analysis of Downdraft Biomass Gasifier Using Computational Fluid DynamicsDocument7 pagesDesign and Analysis of Downdraft Biomass Gasifier Using Computational Fluid DynamicsjosephutomoNo ratings yet
- CFD Investigation On The Effect of Angled Secondary Air To The Air Flow and Coal Combustion of A 660 MW Tangential-Fired Utility BoilerDocument21 pagesCFD Investigation On The Effect of Angled Secondary Air To The Air Flow and Coal Combustion of A 660 MW Tangential-Fired Utility Boilersaravan1891No ratings yet
- Diesel Engine Exhaust Gas Recirculation A Review On Advanced and Novel Concepts 2004 Energy Conversion and ManagementDocument18 pagesDiesel Engine Exhaust Gas Recirculation A Review On Advanced and Novel Concepts 2004 Energy Conversion and ManagementShikhaRajNo ratings yet
- 8.2 Experimental Research On Pre-Chamber Jet Ignition in Rapid Compression Machine and Natural Gas EngineDocument18 pages8.2 Experimental Research On Pre-Chamber Jet Ignition in Rapid Compression Machine and Natural Gas EnginehdjdbNo ratings yet
- AUTO 303 PPT 1 Combustion EngineeringDocument69 pagesAUTO 303 PPT 1 Combustion EngineeringpatiencetinotendajengwaNo ratings yet
- Boiler Heat Transfer Theory-02Document18 pagesBoiler Heat Transfer Theory-02Sai SwaroopNo ratings yet
- Project N1 Combine Cycle Power Plant FinalDocument18 pagesProject N1 Combine Cycle Power Plant FinalHilburnNo ratings yet
- EGR ENGINE FinalDocument26 pagesEGR ENGINE Finalmeysam1215No ratings yet
- Boiler Efficiency and CombustionDocument12 pagesBoiler Efficiency and Combustionhafidhrahadiyan2No ratings yet
- Development of Radiant Burner Methane-Pure OxygenDocument8 pagesDevelopment of Radiant Burner Methane-Pure OxygenLTE002No ratings yet
- Mechanics Experiment Afjustable Inclined PlaneDocument7 pagesMechanics Experiment Afjustable Inclined PlaneMuhammad Aftab SarwarNo ratings yet
- Annular Shaft Kiln For Lime Burning With Kiln Gas Recirculation PDFDocument8 pagesAnnular Shaft Kiln For Lime Burning With Kiln Gas Recirculation PDFFerNo ratings yet
- Coal Based Power Plant Using Oxy-Combustionfor CO2 Capture - Pressurized Coal CombustionDocument8 pagesCoal Based Power Plant Using Oxy-Combustionfor CO2 Capture - Pressurized Coal CombustionJahangir MalikNo ratings yet
- Improving of Refinery Furnaces Efficiency Using Mathematical ModelingDocument6 pagesImproving of Refinery Furnaces Efficiency Using Mathematical Modelingusman_hafeez86No ratings yet
- Gas Cleaning and Cooliing in Fluidized Bed Gasifier For Shaft Power ApplicationDocument6 pagesGas Cleaning and Cooliing in Fluidized Bed Gasifier For Shaft Power ApplicationDeepak KumarNo ratings yet
- Fundamentals of Gas Welding and CuttingDocument85 pagesFundamentals of Gas Welding and CuttingScott TrainorNo ratings yet
- Optimize Fired Heater Operations To Save MoneyDocument8 pagesOptimize Fired Heater Operations To Save Moneyyogitadoda100% (2)
- Increasing Operational Stability in Low No GT Combustor by A Pilot FlameDocument10 pagesIncreasing Operational Stability in Low No GT Combustor by A Pilot FlameGilles CabotNo ratings yet
- Ref11 - SP10 - Danloy1 - Essen - New PDFDocument3 pagesRef11 - SP10 - Danloy1 - Essen - New PDFCofe MilkNo ratings yet
- Applied Energy: Stanislaw Szwaja, Arkadiusz Jamrozik, Wojciech TutakDocument11 pagesApplied Energy: Stanislaw Szwaja, Arkadiusz Jamrozik, Wojciech TutakEderNo ratings yet
- Recover Heat From Waste Inciniration PDFDocument4 pagesRecover Heat From Waste Inciniration PDFcvolkan1100% (1)
- Plate Type CFBC HotDocument192 pagesPlate Type CFBC HotManoj PaneriNo ratings yet
- Fluidized Bed Combustion (FBC) in Coal Based Thermal Power PlantsDocument10 pagesFluidized Bed Combustion (FBC) in Coal Based Thermal Power PlantsShambhu MehtaNo ratings yet
- 04 Combustion ChamberDocument6 pages04 Combustion ChamberprasannabalajiNo ratings yet
- Fundamentals of Gas Welding and CuttingDocument73 pagesFundamentals of Gas Welding and CuttingDilipNo ratings yet
- Esl Ie 85 05 120Document10 pagesEsl Ie 85 05 120losmoscasbrNo ratings yet
- Acetylene, the Principles of Its Generation and UseFrom EverandAcetylene, the Principles of Its Generation and UseNo ratings yet
- Acetylene, the Principles of Its Generation and Use: A Practical Handbook on the Production, Purification, and Subsequent Treatment of Acetylene for the Development of Light, Heat, and PowerFrom EverandAcetylene, the Principles of Its Generation and Use: A Practical Handbook on the Production, Purification, and Subsequent Treatment of Acetylene for the Development of Light, Heat, and PowerNo ratings yet
- Strengthening The Mechanical Properties of 20MnCr5 Steel by Developing Martensite Structure Through Deep Cryogenic TreatmentDocument3 pagesStrengthening The Mechanical Properties of 20MnCr5 Steel by Developing Martensite Structure Through Deep Cryogenic TreatmentInternational Journal of Innovative Science and Research TechnologyNo ratings yet
- PM 6 - ApplicationsDocument21 pagesPM 6 - ApplicationsShivraj ChouguleNo ratings yet
- Boq For 160 KLD Arvindo Hospital, Jaitpura, Shibu Enterprises.Document10 pagesBoq For 160 KLD Arvindo Hospital, Jaitpura, Shibu Enterprises.Anshika RaiNo ratings yet
- Topic 2 Property Enhancing and Surface Processing Operations 2017Document87 pagesTopic 2 Property Enhancing and Surface Processing Operations 2017lim50% (2)
- Ramp-Up and Long-Term Performance of The Albion Process™ Plant at Geopromining Gold ArmeniaDocument11 pagesRamp-Up and Long-Term Performance of The Albion Process™ Plant at Geopromining Gold ArmeniaLevent ErgunNo ratings yet
- Single Extruder Screw and BarrelDocument11 pagesSingle Extruder Screw and BarrelLogesh kumarNo ratings yet
- AIMOL Cement IndustryDocument57 pagesAIMOL Cement IndustryAdrianNo ratings yet
- Optimization of Surface Roughness in Honing of Engine Cylinder Liners With Sic Honing StonesDocument7 pagesOptimization of Surface Roughness in Honing of Engine Cylinder Liners With Sic Honing StonesHoang LamNo ratings yet
- 3.various Units of IOCL Paradip: 3.1. Avu/Srlpg UnitDocument8 pages3.various Units of IOCL Paradip: 3.1. Avu/Srlpg UnitAkankshya MishraNo ratings yet
- Woodcraft Magazine - AprilMay 2022Document68 pagesWoodcraft Magazine - AprilMay 2022Tibor KovácsNo ratings yet
- Solidification of Castings-FDocument7 pagesSolidification of Castings-FAshok PradhanNo ratings yet
- Pulp and Paper 2007 - Paper CGE Systems SDN BHDDocument10 pagesPulp and Paper 2007 - Paper CGE Systems SDN BHDHarald EbbersNo ratings yet
- Carbon Fibre Skinning Starter KitDocument8 pagesCarbon Fibre Skinning Starter KitcraigbonnymanNo ratings yet
- AISI 1045 Medium Carbon SteelDocument9 pagesAISI 1045 Medium Carbon SteelFatih BahşiNo ratings yet
- Distance From Addis Ababa (Capital), KMDocument21 pagesDistance From Addis Ababa (Capital), KMThaigroup CementNo ratings yet
- Recryst4lliz4tion and Melting Point Determin4ti0nDocument6 pagesRecryst4lliz4tion and Melting Point Determin4ti0nTimothy DrakeNo ratings yet
- BOSCHERT - CompactDocument2 pagesBOSCHERT - CompactNachoLarreaNo ratings yet
- Manufacturing-Defects-In-Corrugated-Board-Boxes-Their-Causes-And-Remedies 2Document22 pagesManufacturing-Defects-In-Corrugated-Board-Boxes-Their-Causes-And-Remedies 2Orvin ManlapidNo ratings yet
- 20MVA TransformerDocument12 pages20MVA TransformerMehul PatelNo ratings yet
- Asme Section Ii A-2 Sa-724 Sa-724mDocument4 pagesAsme Section Ii A-2 Sa-724 Sa-724mdavid perezNo ratings yet
- Asian Epoxy HB CoatingDocument1 pageAsian Epoxy HB CoatingNS2 Engineering and ConstructionNo ratings yet
- m2t2-1910 252Document4 pagesm2t2-1910 252Abd El-rahman MohamedNo ratings yet
- Recovery Boiler History and Future VakkilainenDocument14 pagesRecovery Boiler History and Future VakkilainennotengofffNo ratings yet
- DS Battery Cover Flyer enDocument2 pagesDS Battery Cover Flyer enPhung LucNo ratings yet
- UAS Bahasa Inggris - Ilham SaputraDocument3 pagesUAS Bahasa Inggris - Ilham SaputraIlham SaputraNo ratings yet
- Nitoseal PU 280 PDFDocument2 pagesNitoseal PU 280 PDFhelloitskalaiNo ratings yet
- TE-24100 AMP Read MeDocument2 pagesTE-24100 AMP Read Meayberkyurtsever22No ratings yet
- Welding Technology Solutions To Geothermal Energy Production Challenges - tcm153-574191Document5 pagesWelding Technology Solutions To Geothermal Energy Production Challenges - tcm153-574191JulioNo ratings yet